Introduction
Conjugated linoleic acid (CLA) is a mixture of cis and trans geometrical isomers of linoleic acid (LA) that have conjugated double bonds. The abundantly found isomers of CLA have double bonds at positions 8,10; 9,11; 10,12; and 11,13 (1,2). In the past, before CLA was first identified, it was consumed naturally and adequately as part of the general diet. However, since the 1970s, changes in dietary habits and livestock breeding have led to a decrease in the natural intake of CLA (3). Michael Pariza and his research team from the University of Wisconsin first described CLA in 1985. CLA as an anti-mutagenic agent was isolated from fried ground beef during research on anti-cancer activity (4,5). It has since been detected in many dairy foods and the flesh of ruminants.
CLA has been actively researched (6-8) since the late 1990s. During that period, human clinical trials were conducted addressing the effects of CLA on body fat reduction, leading to an explosive growth of CLA as the first diet food product in the USA (9). CLA has been associated with a multitude of effects including anti-cancer (10), anti-diabetic (11), cholesterol-lowering (12), immune enhancement (13), and antioxidant (14) effects, and effects on women's health (15). CLA-containing foods include milk products such as milk and cheese, which are synthesized from linoleic acid by microorganisms living in the rumen of mammals (5,16,17). In addition, CLA is present in beef, chicken, eggs, and safflower seed oil (18-20).
CLA is necessary to develop high value-added and high function dairy products that meet modern dairy industry standards and to develop valuable dairy products that can be imported and exported. CLA production methods involve either microbial fermentation or chemical synthesis. Linoleic acid, present in safflower seed oil and sunflower oil, is used for CLA production by chemical methods (21). The chemical synthesis method is only used due to the residual substances derived from the chemical used in synthesis (21,22). It is, however, desirable to synthesize CLA by more natural means, via microbial synthesis especially from probiotic bacteria that can be grafted with dairy products (23,24). Naturally occurring CLA produced by microbial fermentation has been attributed with beneficial effects on human health. Lactobacillus, Bifidobacteria, and Propionibacteria have the ability to convert linoleic acid to CLA (25). The main disadvantage, however, has been the low yield obtained from microbial methods when compared to high-yielding chemical methods. Therefore, it is essential to develop new and efficient techniques for the increase of the fermentation products. The purpose of this study was to develop a high quality functional dairy product with increased CLA content through an eco-friendly fermentation method, utilizing probiotic lactic acid bacteria and vegetable oil materials.
Materials and methods
Raw milk for production of fermented milk was obtained an acidity of 0.15% and a pH of 6.8 from the Holstein-Friesian breed of dairy cattle of the Imsil area (Korea). Soybean oil, corn oil, sunflower oil, grape seed oil, canola oil, olive oil, rice oil, sesame oil, and perilla oil were purchased from Sajo Company (Seoul, Korea). Pumpkin seed oil, walnut oil, safflower seed oil, and flaxseed oil were purchased from Jeongwoodang Company (Seoul, Korea). Lactic acid bacteria for the production of fermented milk included commercial strain YF-L812 (Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus), standard strain Bifidobacterium breve KCTC 3419, and Lactobacillus sakei subsp. LJ011 (isolated from kimchi). These strains were cultured twice in sterilized 10% skim milk, and starter stocks were prepared by mixing the three bacterial strains in a ratio of 4:3:3. The reagents used for extraction and instrumental analysis were above reference grade and purchased from Sigma chemical Co. (St. Louis, Mo, USA).
In this study, we followed the analytical methods described by Ledoux et al. (26), Werner et al. (27), and Kim and Liu (28) to establish optimal analysis conditions of CLA fatty acids. Gas chromatography (GC, Agilent, USA) for fatty acid analysis was performed using a Supelcoax-10 fused capillary GC column (Supelco, 60 m×0.32 mm, 0.25 μm). Analysis conditions were as follows: helium gas 1 mL/min, split ratio 50:1, injector temperature 250℃, detector temperature 260℃, and column oven temperature 190℃ at 4.5℃/min maintained for 15 min. Analysis of each fatty acid was compared with the retention time of each standard fatty acid.
Thirty different strains of CLA-producing standard lactic acid bacteria and six strains of Lactobacillus species, isolated from fermented kimchi, were cultured in MRS medium supplemented with linoleic acid (2 mM) and tested for production patterns of CLA isomers by fermentation. Kimchi for isolation of lactic acid bacteria was obtained from different manufacturing sources at Jeonju City, Imsil County of Jeollabukdo, Gwangyang City of Jeollanamdo, and Kyonggido. We estimated the total amount of CLA produced, as well as the amount and ratios of CLA isomers, including cis9; trans11 CLA, to test for efficient CLA production by using methods mentioned above.
The ratio of commercial strains to standard lactic acid bacteria and lactic acid bacteria isolated from kimchi were varied and tested for stock production of a high efficiency CLA conversion starter. The amount of CLA (c9,t11) produced was specifically estimated and compared. To check efficiency of CLA conversion starter stock on milk, 0.05% linoleic acid was added to either 10% skim milk powder, low-fat milk (fat content 2%), or raw milk. This was inoculated with a 3% starter stock (that had been cultured in MRS for 24 h) and further incubated at 37℃ for 48 h. The CLA production was then estimated to check efficiency.
The novel efficient CLA conversion starter stock described in this study was prepared by mixing the commercial strains YF-L812 (Streptococcus thermophilus, Lactobacillus delbrueckii subsp. Bulgaricus), Bifidobacterium breve KCTC3419, and Lactobacillus sakei subsp LJ011 strain in a ratio of 4:3:3 and incubating it in MRS medium for 24 h. The cultured starter stock (3%) was then taken and added to raw milk and further incubated at 37℃ for 48 h
The above mentioned 14 types of vegetable oil were individually added to 0.05% raw milk and then incubated with 3% cultured lactic acid bacteria (previously cultured in MRS medium for 24 h) at 37℃ for 9 h, after which CLA content was measured. A similar procedure was followed to check efficiency of CLA production under varying amounts of safflower seed oil, which varied from 0.01-0.5%.
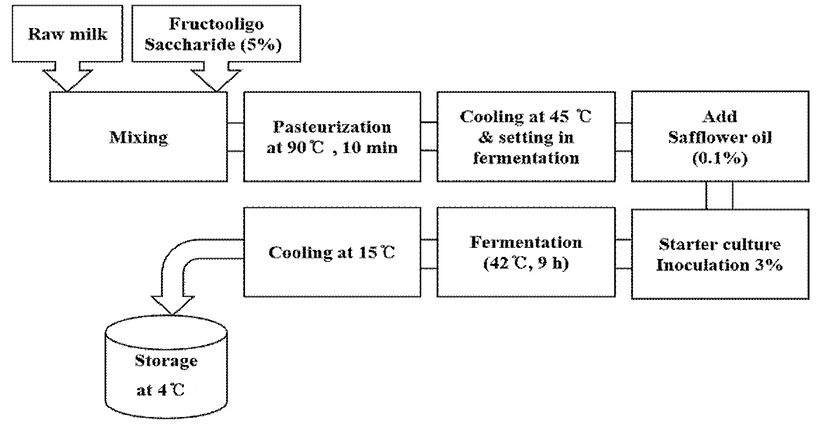
Fermented milk was prepared by adding 5% fructooligosaccharide to raw milk, followed by sterilization at 90℃ for 10 min and cooling to 45℃. Then, 0.1% safflower seed oil and starter stock were inoculated at 3% and cultured at 42℃ for 9 h until a pH of 4.6 was achieved, which indicates fermented milk containing a high CLA concentration. The fermented milk was then supplemented with CLA (mg/g), homogenized, and cooled to 15℃. Fermented milk was stored in a closed container at 4℃ (Fig. 1).
Changes in pH, titratable acidity, and number of lactic acid bacteria were measured during the fermentation process at 3 h intervals for 24 h. The pH level was measured using a pH meter (UB-10, Denver, USA). The titration was carried out by adding 10 mL of distilled water to 10 mL of sample to obtain a suspension. The suspension was titrated to pH 8.3 by adding NaOH, and the weight (g) of the specimen was divided to obtain the titratable acidity (%). The change in lactic acid bacteria count was obtained by taking 1 mL of the sample, diluting it in sterilized saline by the decimal dilution method, and culturing it at 37℃ for 48 h in BCP agar medium. The number of yellow colonies was measured and expressed as CFU (colony forming unit)/mL. Changes in pH, titratable acidity, and number of lactic acid bacteria were measured during storage for 28 days at weekly intervals while refrigerated at 4℃. For fermented milk containing CLA, the degree of change in CLA content during storage was checked by GC analysis for 28 days from 7 days after the manufacture of the product.
Data has been expressed as mean±SD. Statistical analysis for single comparisons was performed using Duncan’s multiple range test. Each experiment was repeated at least three times to yield comparable results. Null hypotheses of no difference were rejected if p-values were less than 0.05.
Results and discussion
Among the standard lactic acid bacteria tested, three strains of Lactobacillus acidophilus, one strain of Lactobacillus casei, two strains of Lactobacillus rhamnosus, two strains of Streptococcus thermophilus, two strains of Bifidobacterium bifidum, two strains of Bifidobacterium breve, and two strains of Bifidobacterium longum were found to produce CLA (Table 1). Among these Bifidobacterium breve, KCTC3419 yielded highest CLA conversion. However, fermentation using the different strains reduced upon addition of substrate (10-20 μg/mL of CLA conversion). CLA consists of several possible isomers c9,t11; t10,c12; t9,t11; t10,t12; c9,c11; t9,c11; c10,c12; and c10,t12. The isomer associated with important physiological activity in the human body is the cis9,trans11 CLA (29). Gorissen et al. (30) reported that Bifidobacteria produced high amounts of CLA and CLA isomers, and Bifidobacterium breve LMG 13194 strain produced large amounts of c9,t11-CLA. Similar results were obtained in this study. We, therefore, selected the strain Bifidobacterium breve KCTC3419 to use in the preparation of starter stock culture for efficient CLA conversion.
Among the lactobacilli isolated from kimchi, Lactobacillussakei subsp. LJ011, Lactobacillus sakei subsp. LI033, and Lactobacillus sakei subsp. LGy039 converted the substrateinto CLA (Table 1). The CLA conversion rate of Lactobacillus sakei subsp. LJ011 was found to be the highest at 67 μg/mL. Therefore, this strain was selected and used in the preparation of starter stock culture for the high efficiency CLA conversion. Renes et al. (31) reported that co-cultures of Lactobacillus strains are effective for increasing CLA conversion. In this study, we tried to further increase the CLA conversion rate by mixing the standard Bifidobacterium breve KCTC3419 strain and Lactobacillus sakei subsp. LJ011 strain isolated from kimchi.
Fermentation speed, quality, and sensory characteristics are important considerations for the starter stocks used in fermented milk (32). Lee et al. (33) reported that when fermented milk is prepared by mixing commercial starter with functional lactic acid bacterium, quality could be improved with the desired functionality. We, therefore, mixed the commercial strains YF-L812 (Streptococcus thermophilus, Lactobacillus delbrueckii subsp. Bulgaricus), Bifidobacterium breve KCTC3419, and Lactobacillus sakei subsp LJ011 strain in varying proportions and cultured it in MRS medium. This mixture was then added to substrate containing linoleic acid and tested for efficient CLA conversion. In our study, a ratio of 4:3:3 of YF-L812, Bifidobacterium breve KCTC3419 and Lactobacillus sakei subsp LJ011 strain yielded the highest CLA conversion rate (Table 2).
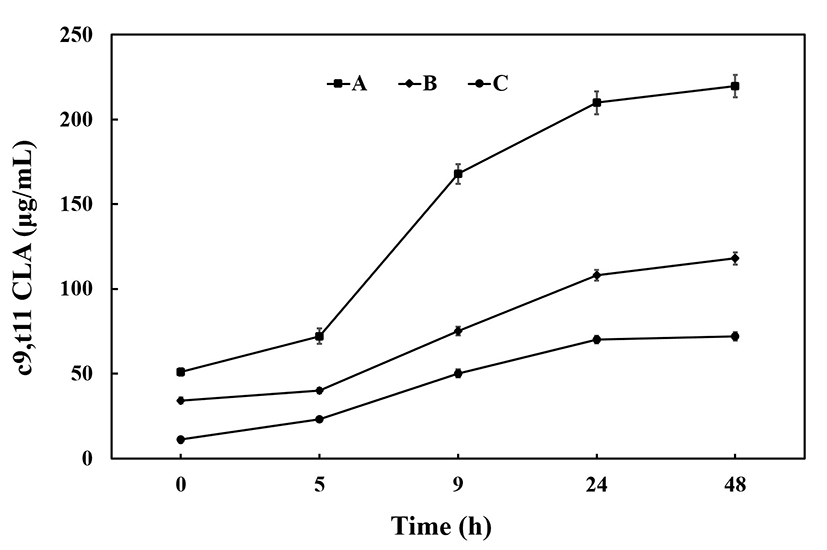
To characterize the CLA conversion rates of the starter stock, depending on the media used, we added linoleic acid to 10% skim milk, low-fat milk (2% fat content), or raw milk and then incubated it with the CLA conversion starter stock. We compared the production of CLA (c9,t11) among the three media used. The amount of CLA production in each medium tended to increase until 24 h after fermentation, after which no significant increase was observed till 48 h (Fig. 2). CLA production was found to be highest in raw milk medium, with 167 μg/mL being produced after 9 h of fermentation, and 207 μg/mL after 24 h of fermentation (Fig. 2), suggesting that higher fat content was associated with higher CLA production. Trigueros and Sendra (34) performed fermentation of high-fat, full-fat, and low-fat milk types to compare CLA content and reported a production of 93.33 μg/mL from high fat milk, which is only half the amount that we could produce with our starter stock. Thus, the starter stock generated in our study is capable of efficiently increasing CLA production.
To further establish the characteristics of the use of CLA conversion starter stock, we inoculated raw milk with 3% starter stock and cultured it at 37℃ for 48 h. We then measured the pH of the fermented milk and viable cell counts. The pH level of the fermented milk cultured in starter stock for CLA conversion was found to decrease in proportion to incubation time with the pH measuring 4.72 after 9 h of fermentation. Additionally, the number of lactic acid bacteria was found to be about 109 CFU/mL after 9 h of fermentation (data not shown). This result indicates that the starter stock may indeed have excellent applicability to produce functional fermented milk with highly efficient CLA conversion.
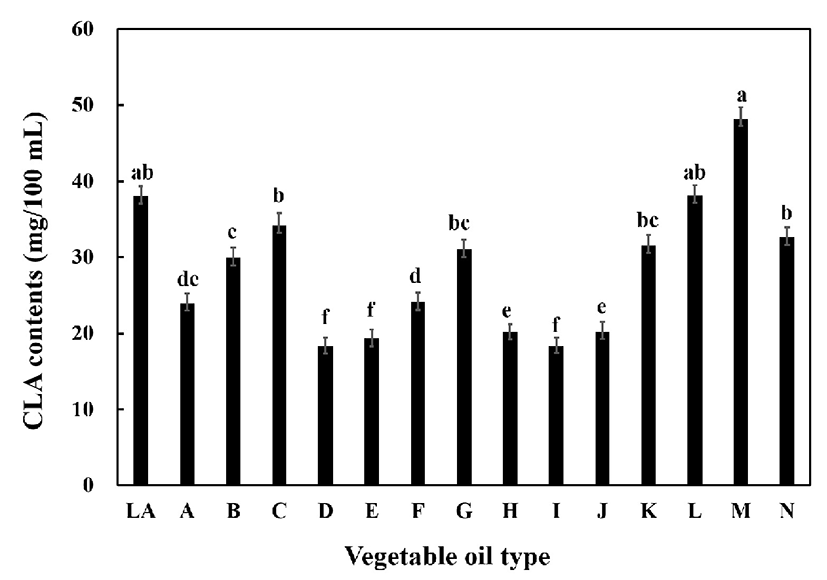
To confirm the CLA conversion rate of vegetable oil during fermentation by CLA conversion starter stock, 14 species of vegetable oil were individually added to raw milk and inoculated with the starter stock. The results are shown in Fig. 3. The CLA production from pure linoleic acid was 38.07 mg/100 mL. The conversion rate, as estimated from the CLA content, was found to be high in safflower seed oil (48.24 mg/100 mL), walnut oil (38.18 mg/100 mL), and sunflower oil (34.21 mg/100 mL). In fact, safflower seed oil showed higher production than linoleic acid. Thus, safflower seed oil, which is cheaper than linoleic acid, can be used as a substrate in the production of CLA fermented milk. Safflower seed oil has a high content of linoleic acid (35,36) and Ivan et al. (37) reported that the CLA production capacity of safflower seed oil is high. Therefore, in this study, we decided to use safflower seed oil as a substrate for the production of CLA during milk fermentation.
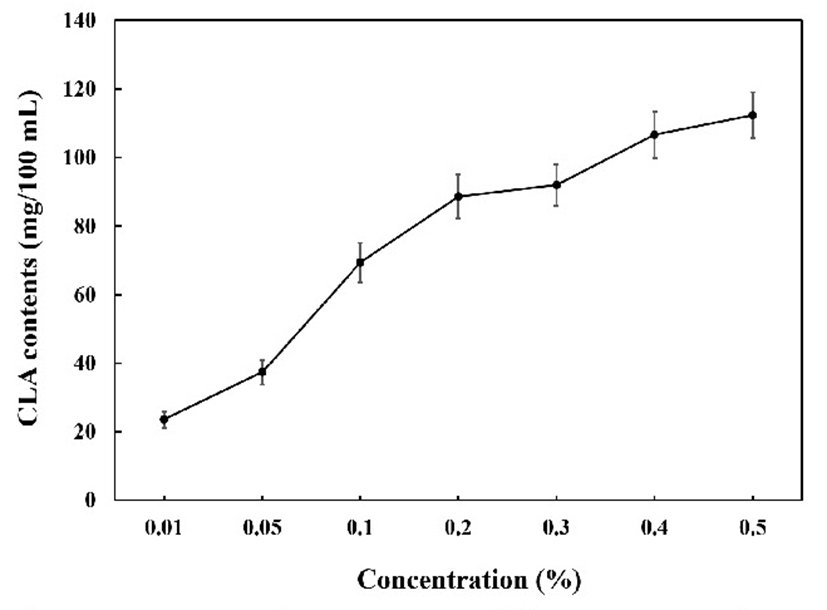
We next estimated the amount of safflower seed oil that can be used efficiently during fermentation by CLA conversion starter stock. The CLA content was measured after fermentation of raw milk using 3% starter stock, and supplementation with 0.01 to 0.5% safflower seed oil. We observed that CLA production increased with increasing concentrations of safflower seed oil in the fermented milk (Fig. 4). Conversion of CLA by 0.1% safflower seed oil added to the sample was found to be the most efficient. Therefore, in the production of fermented milk, we used a 0.1% concentration of safflower seed oil.
Fermented milk was prepared (as described in the methods) to investigate the possibility of producing fermented milk using starter stock for CLA conversion. We analyzed the quality of the fermentation process by estimating pH, titratable acidity, and number of lactic acid bacteria in fermented milk, during fermentation (Table 3). Fermented milk reached a fermentation end point at pH 4.6 within 9 h of fermentation, with a titratable acidity value of 0.85%, and lactic acid bacteria count of 1.2×109 CFU/mL. There was no significant difference observed in the quality characteristics of fermented milk in presence or absence of safflower seed oil. This is consistent with previous report in which addition of flower oil did not affect characteristics of fermented milk (38). Therefore, it is possible to manufacture fermented milk using starter stock for CLA conversion after addition of safflower seed oil as a substrate for CLA conversion.
Changes in pH, titratable acidity, number of lactic acid bacteria, and CLA content were also measured during storage for 28 days (Table 4). The pH level of fermented milk without added safflower seed oil decreased by 0.33% from 4.61 to 4.28 after 28 days of storage, whereas the titratable acidity increased by 0.32% from 0.85 to 1.20. Changes in lactic acid bacteria counts were not significant. These results suggest that addition of safflower seed oil does not affect the overall quality of fermented milk. Serafeimidou et al. (39) measured the CLA content of cow fermented milk and sheep fermented milk during storage at 5℃ for 14 days. They observed that CLA content of fermented cow milk decreased while that of fermented sheep milk increased. In our study, the contents of CLA were maintained at 0.1% of the total CLA content during the storage period (2 weeks).