Introduction
The Wnt/β-catenin signaling pathway mediates key cell-cell signaling events during embryogenesis as requirement for adult tissue maintenance, and is mandatory in adipogenesis (1). Wnt ligands are secreted proteins that act through autocrine and paracrine mechanisms to influence the differentiation of many different cell types (2). The Wnt/β -catenin pathway plays important physiological roles including embryonic development and cellular proliferation. Large number of studies have suggested that the Wnt/β-catenin pathway plays a role in adipose cell communication and the inhibition of adipogenesis (3,4). The understanding of molecular and cellular events regulating adipogenesis is crucial for designing rational therapies for prevention or treatment of obesity and metabolic syndromes (5).
Ricinus communis is a soft-wood small tree which is widespread throughout tropics and warm temperature regions of the world. The by-products this plant, such as leaf and root have been used for treatment of inflammation (6,7). In addition, oils from R. communis have been reported to have potential antimicrobial, anticarcinogenic, and antioxidant activities (8,9). However, compared to many pharmacological studies, the effect of R. communis extract on regulating adipogenesis as therapeutic drug for the treatment of obesity has not been reported. In this study, we investigated the effect of R. communis extract on inhibition of the adipocyte differentiation through activating the Wnt/β-catenin signaling pathway.
Materials and Methods
R. communis (whole plant) extract was purchased from Plant Extract Bank (http://extract.pdrc.re.kr; Daejeon, Korea) The extract obtained from 30-40 g of crude sample was mixed with 200 mL of methanol using a solvent extraction equipment (ASE300 Accelerated Solvent Extractor, DIONEX Corporation, Sunnyvale, CA, USA) at 50℃, and 1,500 psi for 20 min and dried (Modul spin 40, Biotron corporation, Alberta, Canada) at 40℃ for 24 h.
The 3T3-L1 preadipocytes (10) were cultured in Dulbecco's modified Eagle's medium (DMEM) with high glucose concentration, 110 mg pyruvate/L supplemented with thermally inactivate 10% (v/v) calf serum (Gibco, Waltham, MA, USA), 100 μg/mL penicillin, and 100 μg/mL streptomycin in a CO2 incubator at 37℃. To induce adipocyte differentiation, 3T3-L1 cells were cultured with DMEM plus 10% (v/v) heat inactivated fetal bovine serum (FBS) (Gibco) containing 520 μM isobutylmethylxanthine, 1 μM dexamethasone and 167 nM insulin. After 2 days, the media was then changed, and 167 mM of insulin in distilled water was added in a 1:1,000 dilution. On day 4, medium was replaced with DMEM containing 10% FBS, and changed with fresh identical medium every 2 days. To measure the anti- differentiation effects of each drug, 3T3-L1 preadipocytes were induced to differentiate in the presence of different concentrations of R. communis extract and LiCl (Lithum Chloride).
HEK 293-TOP cells (3×104) containing pTOPFlash with Tcf4 response element-luciferase gene were seeded into 96-well plates and incubated in DMEM medium with 10% FBS in a CO2 incubator at 37℃ for 1 day. The cells were then treated with RCE (1, 10, 100 μg/mL) for 24 h. Total cell lysates were extracted with 25 μL 1x reporter lysis buffer (Promega, Madison, WI, USA) per each well and luciferase activities were measured by adding 25 μL luciferin (USB, Cleveland, OH, USA) per each well using a Microplate Luminometer (BMG Labtech, Offenburg, Germany).
Dye solution was prepared as follows: 150 mg Oil Red O was dissolved in 30 mL of isopropanol. Then, the precipitate was removed by filtration and the supernatant was stored at room temperature after addition of 20 mL of distilled water. Adipocyte cell layers were washed with PBS, fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, stained with the Oil Red O dye solution for 1 h, and then washed with distilled water. Cells were checked by the bright-field optical microscope (Nikon TE-200U, Tokyo, Japan).
Cells were fixed with 4% (v/v) paraformaldehyde in PBS for 15 min at room temperature and washed with PBS. For permeabilization, cells were treated with 0.1% (v/v) Triton X-100 for 15 min at room temperature. And, cells were blocked with 5% (v/v) BSA and 1% (v/v) normal goat serum in PBS for 30 min at room temperature and successively incubated with mouse anti-β-catenin antibody (BD transduction laboratory, Lexington, KY, USA, 1:100) overnight at 4℃. Cells were rinsed with PBS, incubated with Alexa Fluor 488-conjugated goat anti-mouse antibody (Molecular Probes, Eugene, OR, USA, 1:400) for 1 h at room temperature, counterstained with 4'-6- Diamidino-2-phenylindole (DAPI) (Boehringer Mannheim, Mannheim, Germany, 1:5,000), and examined under a confocal microscope, LSM510 META (Carl Zeiss, Gottingen, Germany).
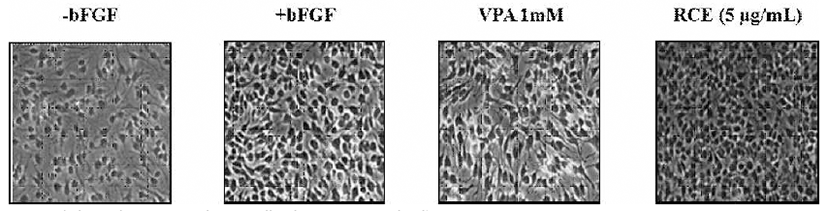
Primary rat neural stem cells were isolated from the cerebral cortex of E14 Sprague-Dawley (SD) rats (KOATECH, Namyangju, Korea). The isolated cells were plated in dishes coated with 15 μg/mL poly-L-ornithine and 10 μg/mL fibronectin (Sigma-Aldrich, St. Louis, MI, USA), grown in N2 medium [DMEM:F12 (1:1) (Invitrogen) containing 100 μM putrescine, 30 nM selenite, 20 nM progesterone, 1.55 mg/mL D-(+)-glucose, 25 μg/mL insulin, 0.1 μg/mL apotransferrin (Sigma-Aldrich), 0.5 mM Glutamax, 100 IU/mL penicillin, and 100 μg/mL streptomycin] containing 10 ng/mL bFGF (basic Fibroblast Growth Factor; Invitrogen, Carlsbad, CA, USA), or valproic acid (VPA) as an inducer of neural tube defects and incubated in 5% (v/v) CO2 at 37℃. To monitor toxicity of RCE, the cells were plated at 2×105 cells per each well in a 6-well plate and treated with 5 μg/mL RCE for 48 h. The animal experimental procedures were approved by the committee for the Care and Use of Laboratory Animals, Yonsei University College of Medicine and were performed in accordance with the Committee’s Guidelines and Regulations for Animal Care (IRB No. 09-013).
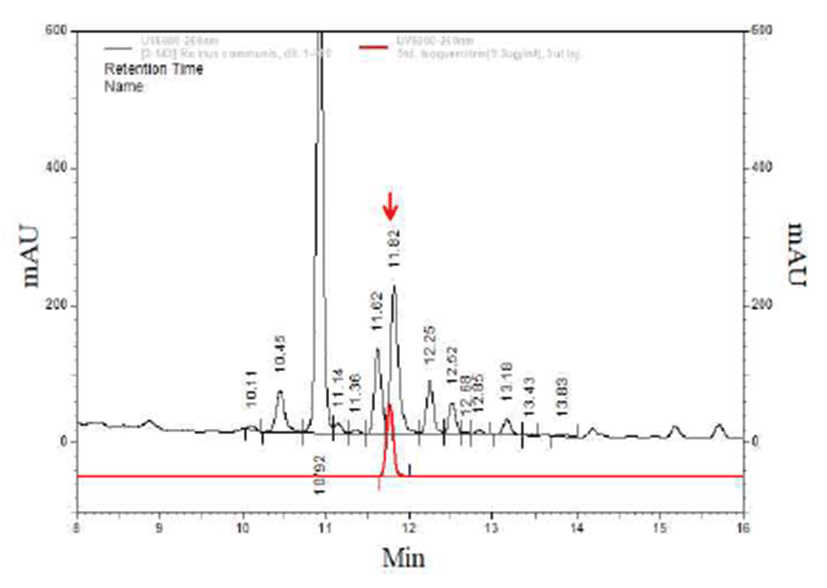
The HPLC analysis was carried out in Agilent 1260 HPLC-DAD system as following conditions: injection volume, (10 μL); Capcell pak C18, UG, 5um, column 4.6×250 mm, (Agilent Technologies Inc, Santa Clara, CA, USA), column temperature at 35℃, mobile phase A being acetonitrile/acetic acid/water (3/0.5/96.5,v/v/v) and mobile phase B acetonitrile /acetic acid/water (50/0.5/49.5,v/v/v), linear gradient elution from 72.5% A/27.5% B (v/v) to 65% A/35% B (v/v) during 0-10 min, from 65% A/35% B (v/v) to 20% A/80% B (v/v) during 10-35 min, from 20% A/80% B (v/v) to 0% A/100% B (v/v) during 35-40 min; mobile phase flow rate was 1 mL/min. The samples were monitored at 360 nm. Flavonoids and their derivatives in the tested sample were determined by comparing the retention times and peak areas with those of authentic reference compounds (Fig. 5). The identified compounds were confirmed by internal standard (11).
Results and Discussion
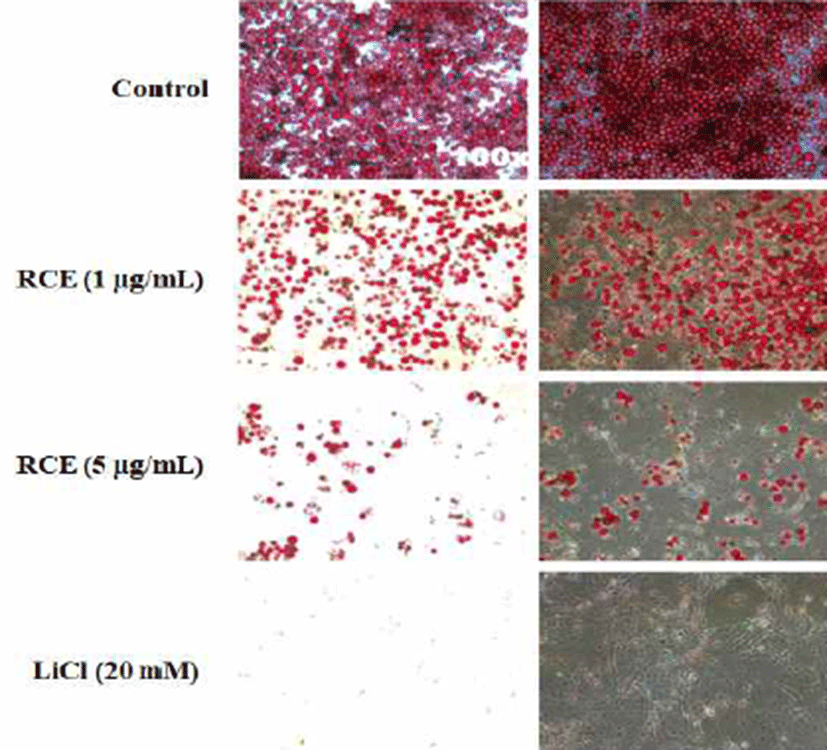
The differentiation of cells monitored by Oil Red O staining was totally abolished by the treatment of 20 mM of LiCl, and the GSK3β inhibitor activated the Wnt/β-catenin signaling. RCE subsequently inhibited the adipocyte differentiation of 3T3-L1 cells in concentration-dependent manner (Fig. 1). These results suggest that RCE inhibited the differentiation of 3T3-L1 cells which indicated the role of the Wnt/β-catenin activator in the adipocyte differentiation.
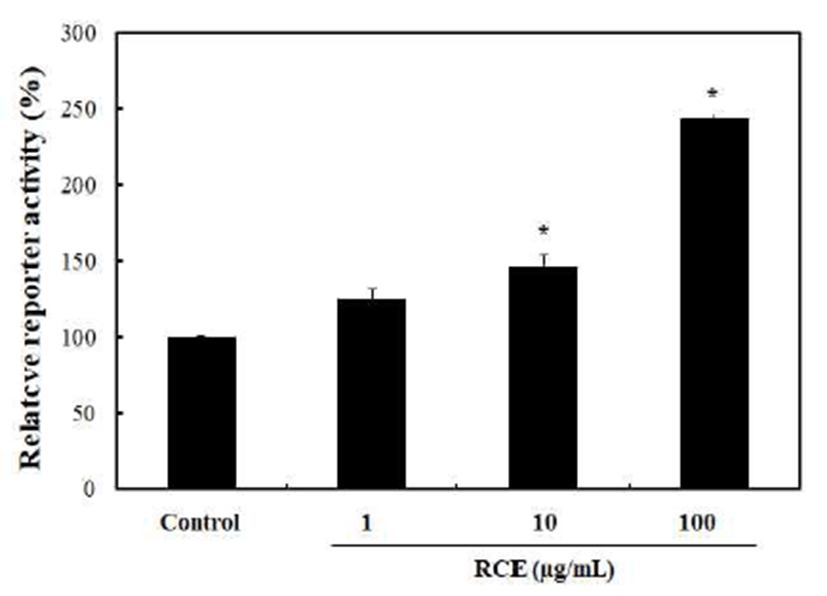
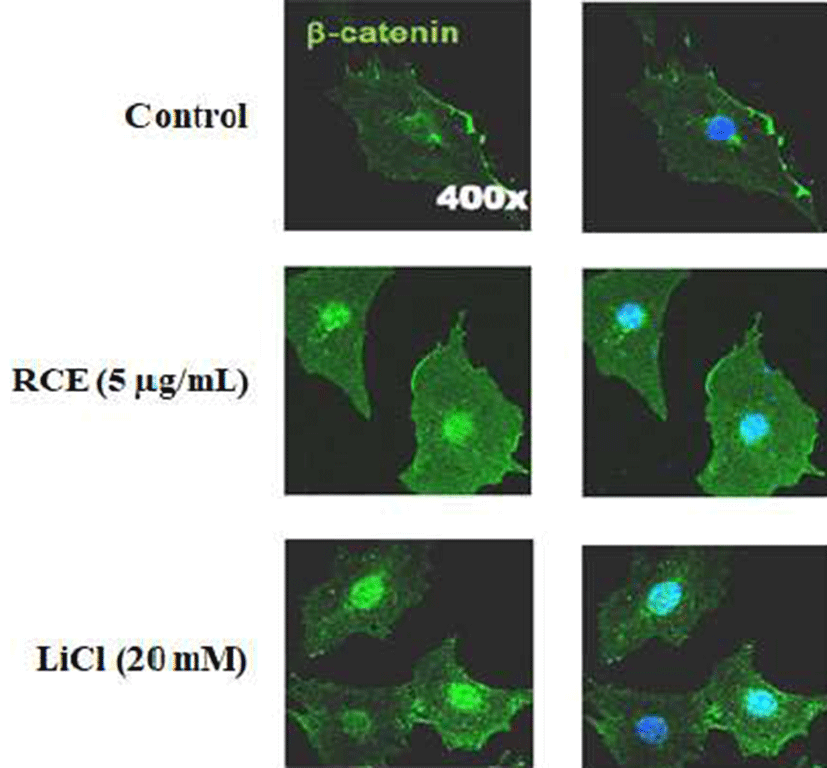
The luciferase activity of HEK 293-TOP cells specifically increased approximately 2-folds by the treatment of RCE at concentrations of 100 μg/mL, as compared to the non-treated control (Fig. 2). Activation of the Wnt/β-catenin pathway by RCE was further confirmed by immunocytochemical analysis which shows an increment of nuclear localization of β-catenin (Fig. 3). Here, β-catenin similarly increased and nuclear localized as shown by the treatment of LiCl, which was used as the positive control. These results indicated that the activation of the Wnt/β-catenin signaling by RCE was specific (12). The Wnt/β-catenin signaling plays a role in the suppression of the differentiation of pre-adipocyte cells during the adipocyte differentiation of 3T3-L1 cells. Therefore, the Wnt/β-catenin pathway could be an attractive target for monitoring development adipocytes differentiation (13).
Neural stem cells have been used to screen compound toxicity (14). To monitor toxicity of RCE, we examined the effect of RCE in rat neural stem cells. The cells were treated with 5 μg/mL RCE for 48 h in the presence of 10 ng/mL bFGF. Cytotoxicity of RCE was not detected in the rat neural stem cells (Fig. 4).
Several peaks were monitored in the HPLC profile of RCE (Fig. 5). One peak was qualitatively identified based on retention times of 15 HPLC reference compounds used, and the other compounds were not identified owing to lack of authentic references. Among the identified flavonoids, isoquercitrin was the most abundant. There were several reports on the mechanism for the anti-obesity effects of flavonoids, such as isorhamnetin and quercetin (15), and silibinin and polyphenol, such as epigallocatechin 3 gallate, genestin, resveratrol (16), curcumin (17), isoflavone (18) and baicalin (19). Especially, in our previous report, it was shown that isoquercitrin increased the Wnt/β‐catenin signaling reporter activity (12). Therefore, these results indicated that the adipocyte differentiation was critically reduced by isoquercitrin in R. communis. This study investigated whether natural plant extract may regulate the Wnt/β catenin signaling and adipogenesis. R. communis, a soft wood small tree found throughout tropics and warm temperature regions of the world, was identified as an activator of the Wnt/β catenin signaling. The RCE subsequently inhibited the adipocyte differentiation of 3T3 L1 cells. In this regard, RCE may be an appropriate material for the treatment of obesity.