1. Introduction
Benign prostatic hyperplasia (BPH) is a common non-malignant condition in older men, characterized by excessive stromal and epithelial proliferation that leads to lower urinary tract symptoms (Barry, 2001). More than 50% of men over the age of 50 and nearly 80% of those over 80 exhibit prostate enlargement (Roehrborn, 2008). Enlargement contributes to voiding difficulty, urinary frequency, and urinary retention, thereby reducing quality of life. Without appropriate management, BPH may progress to urinary tract infection or renal impairment, underscoring the need for continuous monitoring and treatment (Seok and Yi, 2017). BPH development involves hormonal imbalance, chronic inflammation, oxidative stress, and impaired apoptosis (Untergasser et al., 2005). Testosterone is converted to dihydrotestosterone (DHT) by 5α-reductase, which activates androgen receptors (ARs) and drives prostate cell proliferation, making androgen metabolism a central pathway in BPH pathogenesis (Carson and Rittmaster, 2003). Elevated interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and cyclooxygenase-2 (COX-2) levels are strongly associated with prostatic proliferation (De Nunzio et al., 2011; Naiyila et al., 2023). In addition, reduced Bax and caspase-3 expression and elevated Bcl-2 levels promote abnormal cell survival (Ho and Habib, 2011). Stromal-epithelial interactions, NF-κB activation, and oxidative stress-driven inflammatory amplification further contribute to
BPH progression (Inamura and Terada, 2024; Kaltsas et al., 2025; Xu et al., 2024). Stromal cells are considered major regulators of BPH, as stromal AR activation and cytokine secretion influence epithelial proliferation and androgen sensitivity. Therefore, WPMY-1 stromal cells, a well-established prostate fibroblast-like line, were used in this study to examine stromal-derived androgenic and inflammatory responses (Cunha et al., 2004). Standard BPH treatments, including α-blockers, 5α-reductase inhibitors (PDE5) inhibitors, can cause sexual dysfunction and hormonal disturbances, and symptoms frequently recur after discontinuation (Abubakar et al., 2023; Gratzke et al., 2015; McConnell et al., 2003). As a result, interest has grown in natural products that offer fewer adverse effects and improved long-term applicability (Csikós et al., 2021; Stewart and Lephart, 2023).
Allium ampeloprasum (Amaryllidaceae) is phytochemically similar to garlic and contains sulfur-based compounds such as alliin and allicin (Brewster, 2008; Hughes and Lawson, 1991). It exhibits antioxidant, immunomodulatory, antidiabetic, and anti-adipogenic activities (Figliuolo et al., 2001; Lee et al., 2020; Lu et al., 2011). Organosulfur compounds found in Allium species, including allicin and S-allyl cysteine, can modulate NF-κB signaling, reduce oxidative stress, and influence hormone-related pathways (Alam et al., 2023; Gorrepati et al., 2024; Iwar et al., 2024). Despite these well-known bioactivities, the effects of A. ampeloprasum on prostate health remain unclear. Therefore, this study investigated whether Allium ampeloprasum extract (AAE) mitigates DHT-induced hyperplasia-related changes in WPMY-1 cells and assessed its potential as a functional food ingredient for prostate health.
2. Materials and methods
Allium ampeloprasum was purchased from Gangjin-gun (Jeollanam-do) local market and used for experiments. After removing stems and peels and washing, 300 mL of 70% ethanol was added to 100 g of Allium ampeloprasum and extracted by immersion for one week at room temperature in the dark. The extract was passed through filter paper (No. 2, Hyundai Micro, Seoul, Korea) and subsequently concentrated using a rotary evaporator (EYELA N-1300, Tokyo Rikakikai, Tokyo, Japan). The concentrated solution was then subjected to freeze-drying with a freeze dryer (MP-05K, Mareuda, Gwangju, Korea) to obtain the powdered extract. Following freeze-drying, 4.7 g of powdered extract was obtained, corresponding to a yield of approximately 4.7%. The extract powder was reconstituted in phosphate-buffered saline (PBS) and subsequently passed through a 0.2 μm syringe filter (Sartorius, Göttingen, Germany) prior to use.
WPMY-1 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% antibiotic-antimycotic (Gibco) at 37°C, 5% CO2. To confirm protective effects against BPH, WPMY-1 cells were pretreated with AAE at various concentrations for 2 h, then treated with 10 nM DHT for 24 h.
WPMY-1 cells were seeded in 96-well plates at a density of 1×104 cells/well concentration and cultured for 24 h. To assess the effect of the extract on cell viability, the culture medium was removed and the cells were subsequently treated with AAE at 0, 50, 100, 300, 500, 700, and 1,000 μg/mL for 24 h. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/mL; Sigma-Aldrich Co., St. Louis, MO, USA) was diluted to 0.5 mg/mL, added to each well, and incubated at 37°C for 4 h to allow formazan formation. The supernatant was discarded, and the insoluble formazan crystals were dissolved in dimethyl sulfoxide (DMSO). Absorbance was then measured at 540 nm using a microplate reader (Versamax, Molecular Devices, San Jose, CA, USA) to determine cell viability relative to the untreated control.
For pretreatment, WPMY-1 cells were incubated with AAE at varying concentrations for 2 h, after which they were treated with DHT (10 nM) for 24 h. Cell lysate and medium were collected. 5α-reductase and PSA concentrations were measured according to the manual provided by enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, Inc. San Diego, CA, USA). Concentrations in each treatment group were calculated by confirming concentrations in medium after AAE and DHT treatment.
For pretreatment, WPMY-1 cells were incubated with AAE at varying concentrations for 2 h, after which they were treated with DHT (10 nM) for 24 h. Medium was collected and the concentrations of IL-6, IL-1β and TNF-α concentrations were measured according to the manual provided by ELISA kit (Abcam, Cambridge, UK). Concentration in each treatment group were calculated by confirming concentrations in medium after AAE and DHT treatment.
Following treatment of WPMY-1 cells with AAE and DHT, cellular proteins were extracted using radioimmunoprecipitation assay (RIPA) buffer supplemented with 1% protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA), and homogenized to obtain total protein lysates. Protein concentrations were determined by a BCA assay kit (Thermo Fisher Scientific, Waltham, MA, USA), and equal amounts of protein were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) for electrophoresis. Separated proteins were subsequently transferred onto polyvinylidene fluoride (PVDF) membranes (Roche Diagnostics, Mannheim, Germany), which were blocked with EveryBlot blocking buffer (Bio-Rad, Hercules, CA, USA) for 10 min at room temperature. The membranes were incubated overnight at 4°C with primary antibodies (Cell Signaling Technology, Danvers, MA, USA) diluted at 1:1000-1:4000, followed by three washes with TBS-T (Tris-buffered saline containing 1% Tween-20) for 15 min each. Thereafter, HRP-conjugated secondary antibodies (Cell Signaling Technology) diluted at 1:1000-1:2500 were applied for 2 h at room temperature, and the membranes were again washed three times with TBS-T. Protein bands were visualized using an enhanced chemiluminescence (ECL) substrate (Thermo Fisher Scientific) and detected with a digital imaging system (MicroChemi 4.2, DNR Bio-Imaging Systems, Neve Yamin, Israel).
3. Results and discussion
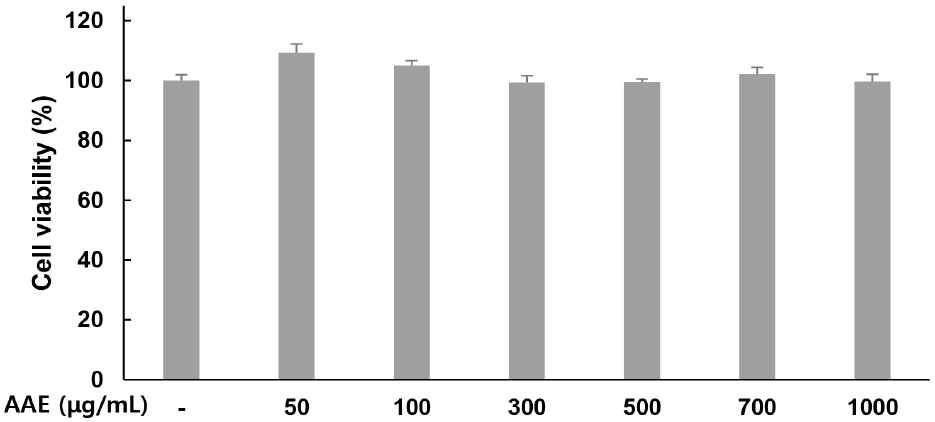
Cytotoxicity of AAE in WPMY-1 cells was assessed using the MTT assay. Treatment with AAE at 0, 50, 100, 300, 500, 700, and 1,000 μg/mL for 24 h resulted in cell viability values of 100, 109.27, 105.04, 99.34, 99.44, 102.18, and 99.64% respectively, confirming the absence of cytotoxicity (Fig. 1). Therefore, only non-toxic concentrations were used in subsequent experiments.
To evaluate the effect of AAE on inflammatory cytokine production (IL-6, IL-1β, and TNF-α), WPMY-1 cells were pretreated with the extract at different concentrations for 2 hand then exposed to DHT (10 nM) for 24 h. Cytokine levels were then quantified by ELISA.
For IL-6, the untreated group showed 32.74 pg/mL while the 10 nM DHT alone treatment group showed 304.81 pg/mL, indicating increased production. AAE treated groups at 50, 100, and 200 μg/mL showed 275.08, 210.04, and 148.59 pg/mL respectively, with decreased production levels, confirming 9.75%, 31.09%, and 51.25% reduction in IL-6 compared to the only DHT-treated group (Fig. 2A). For IL-1β, the untreated group showed 53.02 pg/mL while the DHT alone treatment group showed 595.07 pg/mL, indicating increased production. AAE treated groups at 50, 100, and 200 μg/mL showed 475.20, 410.02, and 231.92 pg/mL respectively, with decreased production levels, confirming 20.14%, 31.10%, and 61.03% reduction in IL-1β compared to the only DHT-treated group (Fig. 2B). For TNF-α, the untreated group showed 69.61 pg/mL while the 10 nM DHT alone treatment group showed 297.11 pg/mL, indicating increased production. AAE treated groups at 50, 100, and 200 μg/mL showed 210.33, 170.16, and 153.91 pg/mL respectively, with decreased production levels, confirming 29.21%, 42.73%, and 48.20% reduction in TNF-α compared to the only DHT-treated group (Fig. 2C).
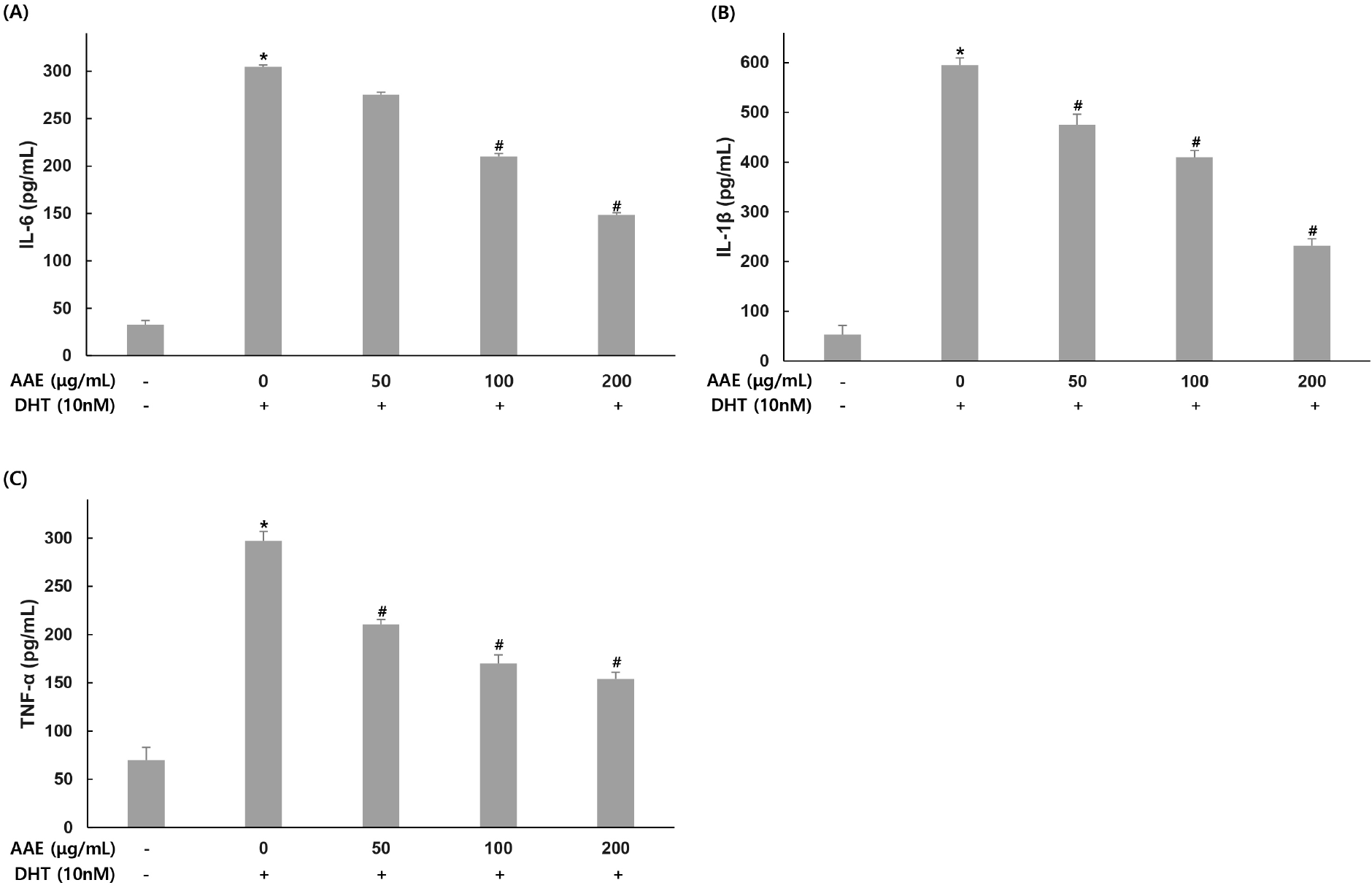
IL-6, IL-1β and TNF-α are major pro-inflammatory cytokines that create an inflammatory microenvironment in prostatic tissue and activate signaling pathways involved in proliferation and apoptosis (Kwon et al., 2014). In this study, DHT significantly increased the secretion of IL-6, IL-1β, and TNF-α in WPMY-1 cells, consistent with inflammatory patterns in BPH (Kramer and Marberger, 2006; Roehrborn, 2008). Pretreatment with AAE markedly reduced these cytokines, indicating that the extract effectively suppresses androgen-driven inflammation. These results suggest that AAE may inhibit BPH progression partly through downregulation of IL-6, IL-1β, and TNF-α. Similar findings were reported by Kim et al. (2023), who demonstrated that hesperidin reduced DHT-induced increases in IL-6, IL-1β, and TNF-α. Their study proposed that modulation of androgen-related inflammatory signaling plays a central role in mitigating BPH pathogenesis, which aligns with the inhibitory effects of AAE observed here.
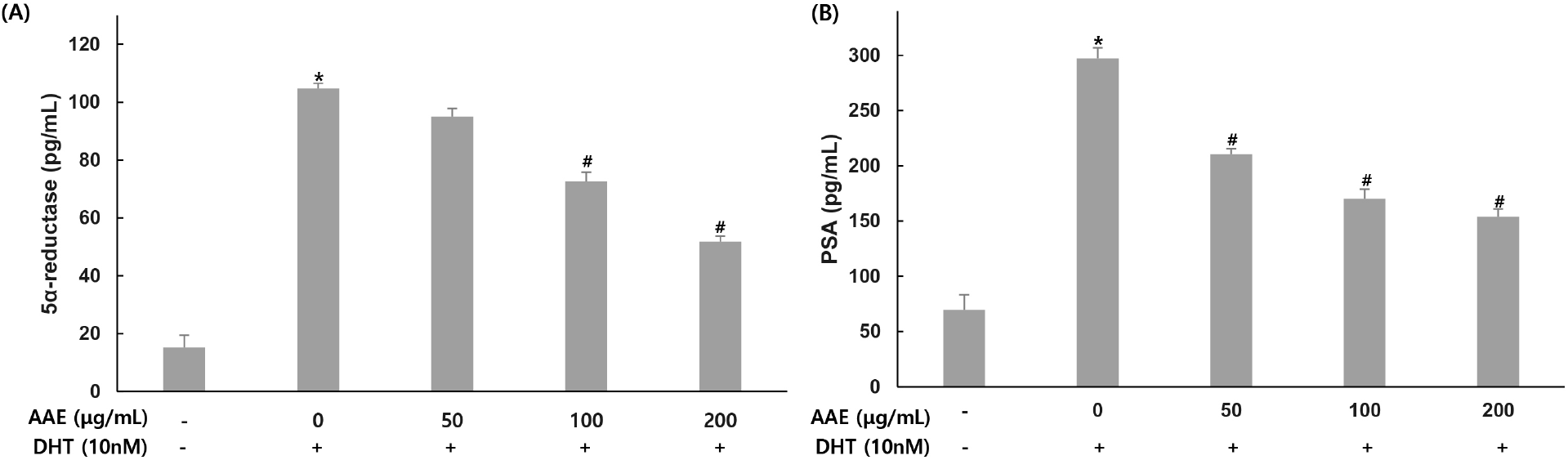
To evaluate the effect of AAE on 5α-reductase and PSA production, WPMY-1 cells were pretreated with the extract at different concentrations for 2 h, followed by stimulation with DHT (10 nM) for 24 h. 5α-reductase and PSA were then quantified by ELISA.
For 5α-reductase, the untreated group showed 15.17 pg/mL while the 10 nM DHT treatment group showed 104.81 pg/mL, indicating increased production. AAE treated groups at 50, 100, and 200 μg/mL showed 95.05, 72.65, and 51.79 pg/mL respectively, with decreased production levels, confirming 9.31%, 30.68%, and 50.59% reduction in 5α-reductase compared to the only DHT-treated group (Fig. 3A). For PSA, the untreated group showed 69.61 pg/mL while the 10 nM DHT treatment group showed 297.11 pg/mL, indicating increased production. AAE treated groups at 50, 100, and 200 μg/mL showed 210.33, 170.16, and 153.91 pg/mL respectively, with decreased production levels, confirming 29.21%, 42.73%, and 48.20% reduction in PSA compared to the only DHT-treated group (Fig. 3B).
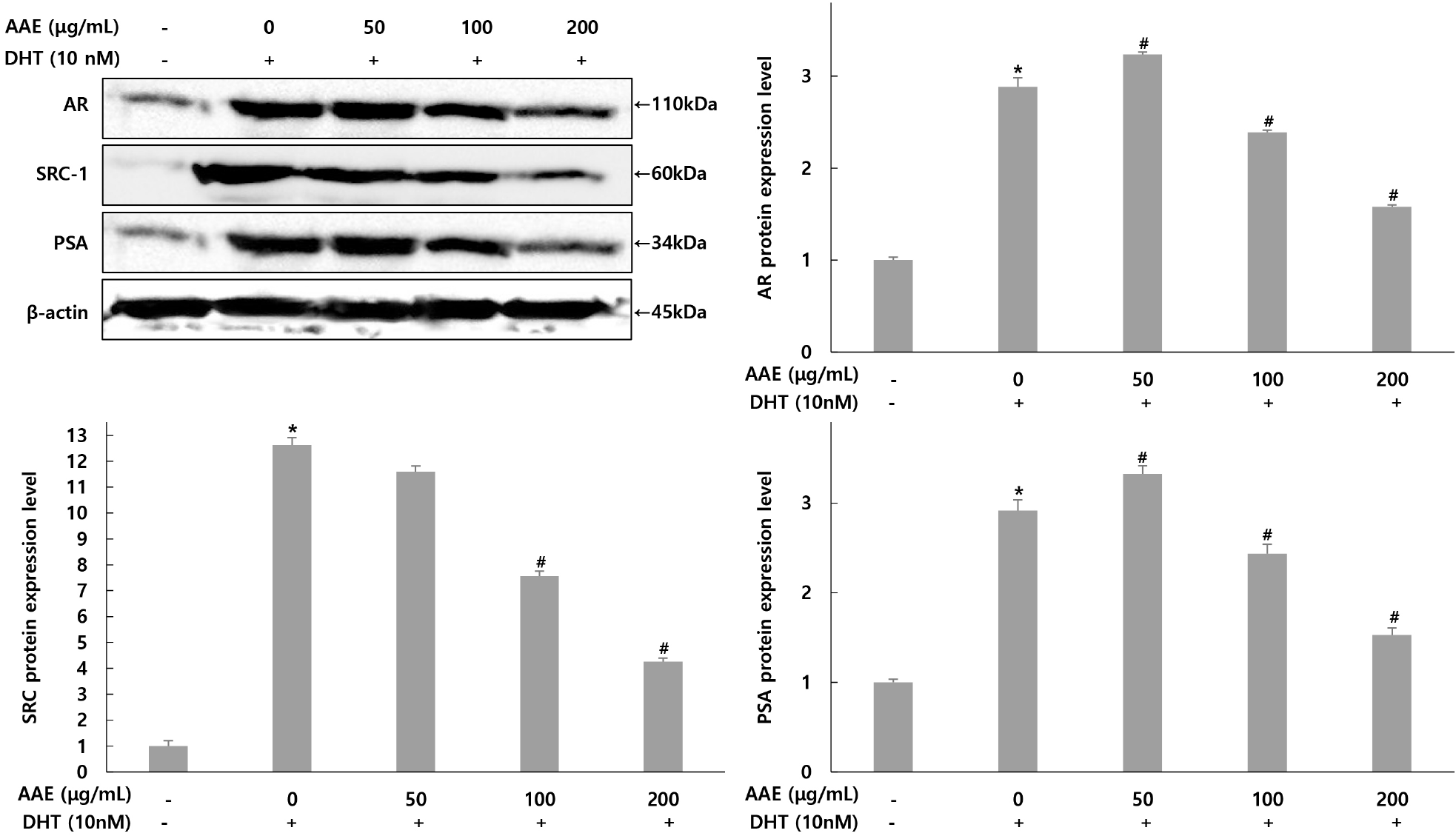
Expression changes by AAE (50, 100, 200 μg/mL) treatment were confirmed for factors involved in AR signaling: AR, SRC-1, and PSA (Fig. 4). For AR expression, The only DHT-treated group showed a 2.88-fold increase compared to the untreated group, while AAE treated groups at 50, 100, and 200 μg/mL showed 3.23-, 2.38-, and 1.57-fold increases, confirming a 12.15% increase, 17.36% and 45.49% decrease compared to the only DHT-treated group. For SRC-1 expression, the only DHT-treated group group showed a 12.61-fold increase compared to the untreated group, while AAEtreated groups at 50, 100, and 200 μg/mL showed 11.59-, 7.56-, and 4.25-fold decrease, confirming 8.09%, 40.05%, and 66.3% decreases compared to the only DHT-treated group. For PSA expression, the only DHT-treated group showed a 2.91-fold increase compared to the untreated group, while AAE treated groups at 50, 100, and 200 μg/mL showed 3.32-, 2.43-, and 1.52-fold decrease, confirming a 14.09% increase, 16.49% and 47.77% decrease compared to the only DHT-treated group.
5α-Reductase catalyzes the conversion of testosterone to DHT and is a central mediator of BPH pathology. Pharmacological inhibitors such as finasteride and dutasteride reduce prostate volume and improve urinary symptoms by targeting this enzyme (McConnell et al., 2003; Roehrborn, 2008). DHT-induced AR activation also increases PSA, a widely used clinical indicator of BPH severity (Lilja, 2003). In the present study, DHT elevated both 5α-reductase and PSA levels in WPMY-1 cells, whereas AAE markedly reduced their expression. Because upregulation of 5α-reductase enhances AR signaling and subsequently promotes PSA induction, the reduction of both markers indicates that AAE suppresses androgen-dependent pathways (Tsai et al., 2006). These findings suggest that AAE may interfere with the testosterone-to-DHT conversion process and attenuate downstream AR signaling, thereby mitigating BPH-related changes.
AR and SRC-1 are also crucial regulators of prostatic growth, promoting the transcription of androgen-responsive genes such as PSA (Heinlein and Chang, 2004). Consistent with this, DHT increased AR, SRC-1, and PSA protein expression, whereas AAE reduced all three factors in a dose-dependent manner. This indicates that AAE may counteract androgen-mediated transcriptional activation and suppress hyperplastic signaling. Similar inhibitory patterns were reported by Kim et al. (2021), who showed that hazelnut extract attenuated DHT-induced increases in AR, SRC-1, and PSA, supporting the concept that phytochemicals can regulate prostatic growth by modulating AR signaling. The present results are consistent with these findings. Taken together, these data demonstrate that AAE modulates key androgen-related enzymes and signaling components in DHT-stimulated WPMY-1 cells. By reducing 5α-reductase and PSA production and suppressing AR and SRC-1 expression, AAE shows potential as a natural agent for managing BPH.
The expression changes of Bcl-2, Bax, and caspase-3 related to apoptosis in WPMY-1 cells were assessed using western blot analysis (Fig. 5). For Bcl-2, the group treated with only DHT exhibited a 2.52-fold increase compared to the untreated control. In contrast, the AAE treated groups demonstrated decreases in Bcl-2 levels to 2.52-, 1.67-, and 1.30-fold, representing reductions of 0.79%, 33.73%, and 48.41% relative to the only DHT-treated group. For Bax, the only DHT-treated group showed a 0.58-fold decrease compared to the untreated control, while the AAE treated groups experienced increases to 1.57-, 1.74-, and 1.85-fold, translating to rises of 170.69%, 200.00%, and 331.03% in Bax expression compared to the only DHT-treated group. Similarly, for caspase-3, the only DHT-treated group demonstrated a 0.68-fold decrease compared to the untreated control, whereas the AAE treated groups showed increases to 1.74-, 1.71-, and 1.93-fold, reflecting increases of 155.88%, 151.47%, and 183.82% compared to the only DHT-treated group.
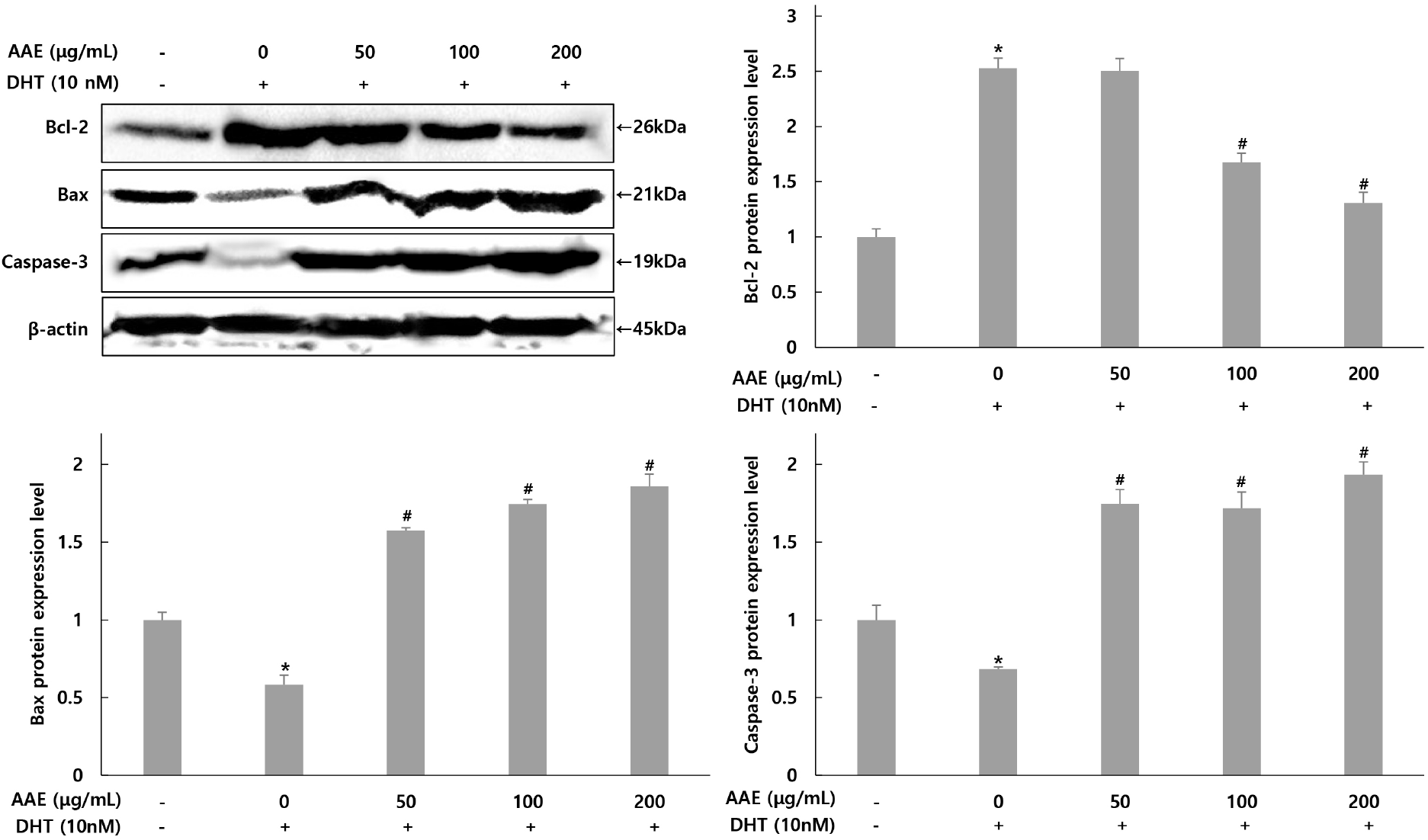
This study also evaluated the effects of AAE on apoptosis-related proteins in DHT-exposed WPMY-1 cells. Bax promotes mitochondrial cytochrome c release, and caspase-3 functions as a key executioner of apoptosis, whereas Bcl-2 acts as an anti-apoptotic regulator. Because Bax and Bcl-2 exert opposing functions, the Bax/Bcl-2 ratio is widely used as an indicator of apoptotic balance (Adams and Cory, 2007; Green and Llambi, 2015). Compared with untreated cells, DHT decreased the expression of Bax and caspase-3 while increasing Bcl-2 levels, indicating a shift toward an anti-apoptotic state. AAE pretreatment reversed these changes by increasing Bax and caspase-3 and reducing Bcl-2, thereby restoring a pro-apoptotic balance relative to the DHT-treated group. These findings are consistent with previous reports demonstrating that DHT promotes proliferation by enhancing anti-apoptotic signaling during BPH progression (Kramer and Marberger, 2006; Roehrborn, 2008). The AAE-induced shift toward apoptosis suggests that the extract may counteract DHT-driven cell survival. This interpretation is further supported by earlier studies showing that Allium-derived compounds modulate mitochondrial apoptotic pathways (Amagase, 2006; Guo et al., 2020). Collectively, these results highlight AAE as a potential functional ingredient capable of reactivating apoptosis pathways suppressed by DHT, thereby contributing to the mitigation of BPH progression.
4. Conclusions
The present study evaluated the protective effects of Allium ampeloprasum extract (AAE) in a DHT-stimulated WPMY-1 stromal cell model. AAE was non-cytotoxic within the tested concentration range and attenuated DHT-induced alterations in inflammatory cytokines, androgen-related signaling molecules, and apoptosis-associated markers. Although these findings suggest that AAE exerts biological activities relevant to prostate health, they should be interpreted with caution because they are derived from a single stromal cell line and a limited panel of molecular markers. AAE significantly reduced the secretion of IL-6, IL-1β, and TNF-α, consistent with previous reports showing that organosulfur compounds abundant in Allium species-such as allicin, S-allyl cysteine, and S-allyl mercaptocysteine-can suppress NF-κB activation and mitigate oxidative and inflammatory stress. DHT treatment also increased AR, SRC-1, and PSA expression, whereas AAE downregulated these factors in a concentration-dependent manner, suggesting potential modulation of androgen-dependent transcription. Furthermore, AAE restored the Bax/Bcl-2 ratio and enhanced cleaved caspase-3 and caspase-9 expression, indicating recovery of apoptosis pathways suppressed by DHT.
Despite these promising observations, several limitations should be acknowledged. First, the exclusive use of WPMY-1 stromal cells limits the extrapolation of these findings to the stromal-epithelial interactions that characterize the in vivo prostate microenvironment. Second, the absence of a pharmacological positive control (e.g., finasteride or dutasteride) restricts comparisons between AAE and established BPH therapeutics. Third, although the study demonstrates bioactivity, it does not include phytochemical profiling of AAE; thus, the specific active constituents responsible for the observed effects remain unidentified. Incorporating such analytical data in future investigations will be essential for clarifying the molecular contributors and enhancing mechanistic interpretation. Additionally, broader pathway analyses and in vivo studies will be necessary to substantiate the biological relevance of AAE.
In summary, AAE appears to mitigate key pathological processes associated with DHT-induced stromal activation-including inflammatory cytokine production, AR-associated signaling, and suppression of apoptosis. While these findings support the potential development of AAE as a functional ingredient for prostate health, comprehensive phytochemical characterization and multi-model mechanistic studies are required before definitive conclusions can be drawn.










