1. Introduction
Essential oils derived from plants are gaining widespread attention due to their natural, safe, and effective properties in various fields, including food, cosmetics, and pharmaceuticals (Bolouri et al., 2022). Among them, essential oils extracted from species of the Citrus family are considered one of the groups with the greatest application potential, thanks to their characteristic aroma, strong antioxidant capacity, and a wide spectrum of antibacterial activity (Palazzolo et al., 2013). Citrus species such as lemon (Citrus aurantiifolia), orange (Citrus sinensis), grapefruit (Citrus grandis), and calamondin (Citrus microcarpa) are commonly grown in Dong Nai province - one of the major Citrus growing areas in Southern Vietnam. Abundant raw materials and reasonable prices are favorable conditions for the exploitation and development of Citrus essential oils to serve green industries. Citrus essential oils are complex mixtures of many volatile compounds, in which monoterpenes such as limonene, β-pinene, γ-terpinene, and oxygenated compounds such as citral, linalool, or geraniol often account for a high proportion (González-Mas et al., 2019; Viuda-Martos et al., 2009). However, the chemical composition and biological activity of essential oils can vary greatly depending on the species, cultivation conditions, harvest season, and extraction method. Therefore, GC-MS analysis is an effective tool for identifying and quantifying the main compounds, thereby relating them to the biological capacity of each essential oil. In addition to the composition factors, physical characteristics such as color, density, refractive index, along with indicators reflecting antioxidant capacity (DPPH, ABTS, etc.) and antibacterial ability (for Gram-positive bacteria such as Staphylococcus aureus or Gram-negative bacteria such as Escherichia coli) are also considered important criteria to evaluate and compare the application effectiveness of essential oils (Hao et al., 2025). Many studies have shown that Citrus essential oils often contain a significantly high limonene content, demonstrating a more pronounced antibacterial effect.
This study aims to provide a comparative evaluation of four indigenous Citrus peel essential oils from Dong Nai, focusing on their physicochemical properties and biological activities, to support their effective utilization in functional food, bio-preservation, and natural therapeutics.
2. Materials and methods
Essential oils were extracted from the peels of grapefruit (CgEO, Citrus grandis), orange (CsEO, Citrus sinensis), calamondin (CmEO, Citrus microcarpa), and lemon (CaEO, Citrus aurantiifolia) grown in Dong Nai province, Vietnam, using steam distillation. After extraction, the essential oils were stored in dark glass bottles at room temperature to maintain stability, limit oxidation, and preserve the natural chemical and biological properties of the products.
Four bacterial strains were used in this investigation: two Gram-negative bacteria, Salmonella enteritidis (ATCC 13076) and Escherichia coli (ATCC 25922), and two Gram-positive bacteria, Staphylococcus aureus (ATCC 33591) and Bacillus cereus (ATCC 11778). These strains of bacteria were supplied by the Industrial University of Ho Chi Minh City’s Institute of Biotechnology and Food Technology.
The chemicals used in the study included 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma, St. Louis, USA), 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS, Sigma, St. Louis, USA), and dimethyl sulfoxide (DMSO, Nanjing, China). In addition, the culture and antibacterial testing media used include Mueller-Hinton agar (HiMedia, Thane, India) and nutrient broth (HiMedia, Thane, India). All other chemicals were of analytical grade.
Relative density, absolute density, freezing point, acid value, ester value, and saponification value were measured following the methods outlined in ISO 279:1998, ISO 1041:1973, ISO 1242:2023, and ISO 7660:1983, respectively.
Fragrance retention was measured following Quyen and Quoc (2024) with minor adjustments. Essential oil was diluted in 96% ethanol to make 20, 40, 60, 80, and 100% (v/v) mixes. For each mix, three drops were placed on scent-testing paper and allowed to spread for a few seconds. Researchers timed how long it took for the scent to vanish completely under ambient conditions to assess retention.
The chemical composition of EO was analyzed using gas chromatography-mass spectrometry (GC-2030, Shimadzu Co., Kyoto, Japan). An aliquot of approximately 1 μL of the sample was autosampled and injected into an Agilent 5977E MSD, linked to an Agilent 7890A GC. A 30-m Carbowax 20MTM column, fed with helium at a steady rate of 10 mL/min and a 10:1 split, was used for the separations. The injector was held at 250°C for the run. The temperature program began with a 2-min hold at 50°C, ramped to 250°C at 10°C/min, and then maintained this temperature for 5 minutes. It subsequently increased to 280°C, and was held for an additional 3 min. Mass spectra were taken in electron ionization (EI) mode at 70 eV.
The antioxidant capacity (AC) of EO was evaluated by the free radical scavenging activity (RSA) using the DPPH method, following the procedure described by Quyen and Quoc (2024), with minor modifications. The EO was dissolved in ethanol (96%) to create different concentrations. Then, 0.3 mL of EO solution was mixed with 2.7 mL of 0.1 mM DPPH solution and left to rest at room temperature in the dark for 30 min. The color loss of DPPH was measured using a spectrophotometer at 517 nm. Vitamin C was used as a control. The percentage of inhibition was calculated based on the EO concentration, from which the concentration required to achieve 50% inhibition (IC50) was estimated. The AC was calculated using the following formula:
where Acontrol denotes the absorbance of the DPPH solution, while Asample refers to the absorbance of the essential oil solution when the DPPH solution is present.
With a few minor adjustments, the experiments were carried out using the methodology outlined by Le et al. (2023). To standardize the ABTS solution, 2.45 mM potassium persulfate and 7 mM ABTS were dissolved in deionized water. After being combined in a 1:1 ratio, this service reacted for 16 hours at room temperature in the dark to produce ABTS+ radicals (ABTS radical cation). The ABTS solution was diluted with stored water after 16 hours, resulting in an absorbance of 0.70±0.02 at 734 nm. Next, 3 mL of the ABTS solution was combined with 0.1 mL of the EO solution, which had been prepared at various concentrations. Add alcohol to make a volume of five milliliters. The absorbance at 734 nm was measured after this solution was left at room temperature in the dark for six minutes. The essential oil concentration, used to estimate the concentration required to achieve 50% inhibition (IC50), was used to calculate the percentage of inhibition. The following formula was used to determine the AC:
where Acontrol denotes the absorbance of the ABTS solution, while Asample refers to the absorbance of the essential oil solution when the ABTS solution is present.
With some adjustments, the paper disc method outlined by Quyen and Quoc (2024) was used to calculate the antibacterial activity (AA). First, an inoculating loop was used to evenly distribute 100 μL of bacterial suspension (0.5 McFarland standard concentration, or roughly 1.5×108 CFU/mL) onto MHA medium. Using sterile paper discs (6 mm in diameter), 5 μL of the EO was inoculated, while the positive and negative controls were gentamicin (10 μg/disc) and dimethyl sulfoxide (DMSO) (5%, v/v), respectively. For twenty-four hours, the paper was compressed at 37°C. The diameter of the inhibition zone surrounding the paper disc was used to gauge the antibacterial activity.
The collected data were analyzed using one-way ANOVA to identify significant differences. The data were analyzed using the ANOVA technique and mean comparisons in the Stagraphics Centurion XV (StatPoint, Inc., Warrenton, Virginia, United States). The least significant difference (LSD) method was used to determine a 95% confidence level (p<0.05). The mean±standard deviation (mean±SD) is used to display the results. The principal components of the three oil types were characterized, and their compositional differences were clarified by applying principal component analysis (PCA) to the compound concentration data using covariance matrices. R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria) was used to conduct the analysis.
3. Results and discussion
Physicochemical properties, such as pH, density, acid value (AV), saponification value (SV), ester value (EV), and fragrance retention (FR) are important indicators that reflect the quality of essential oils, affecting stability, preservation, antibacterial properties and industrial applications (Turek and Stinzing, 2013). The results for pH, density, acid value, saponification, ester, and fragrance retention are presented in Table 1. Accordingly, four essential oil samples from Dong Nai - CsEO, CgEO, CaEO, and CmEO - showed significant differences in all the above parameters (p<0.05), with similar values were reported in other studies as well.
Regarding pH, essential oil samples in Vietnam ranged from 4.29 (CaEO) to 6.63 (CmEO). The CaEO sample had the lowest pH (4.29), indicating a significantly higher acidity compared to the other samples. In contrast, the CmEO sample had a pH of 6.63, which was close to neutral (Table 1). This result is comparable to the range of 5.2-6.1 reported in Citrus essential oils from Nigeria (Ibipiriene et al., 2022).
The highest density was reported in CmEO (RD=0.9261; AD=0.9233 g/mL), while CgEO showed the lowest (RD=0.8108, AD=0.8083 g/mL), indicating that CgEO is significantly lighter than the other essential oil samples. This low density is consistent with its high content of light monoterpenes, such as D-limonene (85.11%), and a minimal presence of heavier sesquiterpenes. In contrast, the higher density of CmEO may reflect the presence of heavier compounds, such as β-caryophyllene, α-thujene, and terpinen-4-ol. According to Cheniclet and Carde (1985), low density is often associated with oils rich in light monoterpenes.
In terms of acid value (AV), CaEO (5.84 mg KOH/g) and CmEO (5.55 mg KOH/g) were in the same range as the Sikkim Himalayan lemon essential oil (5.27-5.80 mg KOH/g) (Pradhan et al., 2019), and higher than the Malaysian sample (4.13 mg KOH/g) (Felicia et al., 2024). Compared to the Nigerian essential oil (6.96-12.93 mg KOH/g), CaEO showed a lower acid value (5.84 mg KOH/g), indicating better chemical stability and lower levels of hydrolytic degradation. In essential oils, a lower acid value reflects reduced formation of free fatty acids, which are typically generated by the breakdown of esterified compounds (Ibipiriene et al., 2022).
The saponification value (SV) indicates the amount of volatile fatty acids or esters in the essential oil (Tesfaye et al., 2016). In this study, SV was highest in CmEO (135.86 mg KOH/g) and lowest in CgEO (114.99 mg KOH/g). Compared to essential oils from other regions, CaEO (128.80 mg KOH/g) was comparable to that from Malaysia (121.67 mg/g) and fell within the middle range compared to Nigeria (122.74-158.42 mg KOH/g) and Sikkim, Himalaya (30.03-163.37 mg KOH/g). High SV generally reflects good foaming and solubility properties, which are suitable for cosmetic applications (Sharifi-Rad et al., 2017). Ester value (EV) - a volatile odor component - was also high in all samples, especially in CmEO (130.31 mg KOH/g) and CaEO (122.96 mg KOH/g). These values are comparable to those of the Malaysian sample (117.54 mg KOH/g, Felicia et al., 2024) and fall within the wide range of 21.70 to 157.54 mg/g reported from Sikkim, Himalayas (Pradhan et al., 2019). High EV indicates high aromatic ester content, which contributes to the characteristic aroma and long-lasting scent retention (Djojoputro and Ismadji, 2005).
The most notable feature of CaEO is its superior scent retention: at 100% essential oil, CaEO retains scent for up to 78.83 h, significantly higher than CsEO (63.82 h), CgEO (49.82 h) and CmEO (35.72 h). This exceptional fragrance longevity suggests that CaEO may be especially suitable for use in long-lasting perfumery formulations, air-freshening products, or natural deodorants. Moreover, its sustained aroma profile could enhance the effectiveness of food packaging films, herbal balms, and aromatherapy preparations that require prolonged sensory impact.
The analytical results showed that essential oils from Citrus species grown in Dong Nai have diverse physical and chemical properties, reflecting differences in chemical composition, molecular structure and growing conditions. When compared with studies in Malaysia, Nigeria, and India, the indicators such as acid value, saponification value, and ester value of Vietnamese samples are either equivalent or superior, indicating potential for international trade and application. These results provide a crucial scientific foundation for utilizing native Citrus fruit peel by-products to produce high-quality essential oils, thereby contributing to the strategy of developing natural, friendly, and sustainable raw materials in food and cosmetics.
GC-MS analysis revealed that all four Citrus peel essential oil (EO) samples-CsEO, CgEO, CaEO, and CmEO-were predominantly composed of monoterpene hydrocarbons, with D-limonene as the major constituent in all samples: CgEO (85.11%), CaEO (72.69%), CsEO (70.56%), and CmEO (68.98%) (Table 2). This composition pattern is consistent with previous studies on Citrus species and correlates with notable biological activities, including antimicrobial, antioxidant, anti-inflammatory, and anticancer properties (Anandakumar et al., 2021).
Despite this similarity in major components, the minor compounds showed clear variation and are key in determining the oils’ biological potential and aromatic diversity. CaEO contained high levels of α-pinene (22.53%) and γ-terpinene (2.27%), both of which are known to disrupt microbial membranes and exhibit strong antibacterial effects, particularly against Gram-positive bacteria (Borges et al., 2022). Additionally, the presence of terpinen-4-ol enhances its value as a natural preservative or antiseptic agent (Yadav and Rao, 2016). Although these compounds appear in small amounts in the Citrus essential oils, they may contribute significantly to their superior antibacterial potential and distinct aroma profile.
CsEO showed the most chemically diverse profile, with oxygenated compounds such as linalool (1.71%), geranial (1.71%), neral (0.63%), geraniol (0.01%), etc., contributing to both fragrance complexity and broad-spectrum antibacterial activity (Takoi et al., 2010). This suggests a higher potential for therapeutic and commercial applications.
In contrast, CgEO was highly concentrated in limonene (85.11%) with few minor components, making it an ideal candidate for limonene purification and industrial use in perfumery and cosmetic formulations (Fisher and Phillips, 2008).
Meanwhile, CmEO exhibited a distinct chemical profile rich in uncommon monoterpenes like α-thujene (13.32%), β-pinene (6.56%), sabinene (5.39%), and β-caryophyllene (1.84%), all of which have been associated with anti-inflammatory, antifungal, and anticancer activities (Valente et al., 2013). This indicates its potential in natural health and cosmetic products.
Comparison with previous reports showed that while Citrus EOs from Vietnam had similar D-limonene content (62.95%) to those in Thailand (Sreepian et al., 2021), they had much lower γ-terpinene (13.30%) and β-pinene (11.41%) levels. Algerian samples, meanwhile, had a more diverse minor profile, including citral (2.7%), indicating a regional influence on the phytochemical makeup (Boughendjioua and Djeddi, 2017). The variability in minor compounds plays a pivotal role in determining the bioactivity and potential uses of Citrus EOs, beyond their shared high limonene content.
Principal component analysis (PCA) was performed to evaluate the chemical composition differences among four Citrus peel essential oils (CsEO, CgEO, CaEO, CmEO). The PCA plot of the samples showed that the four essential oil samples were clearly distributed in two-dimensional space, with the first principal axis (Dim 1) and the second principal axis (Dim 2) explaining 48.80% and 37.30% of the total variance, respectively (Fig. 1A).
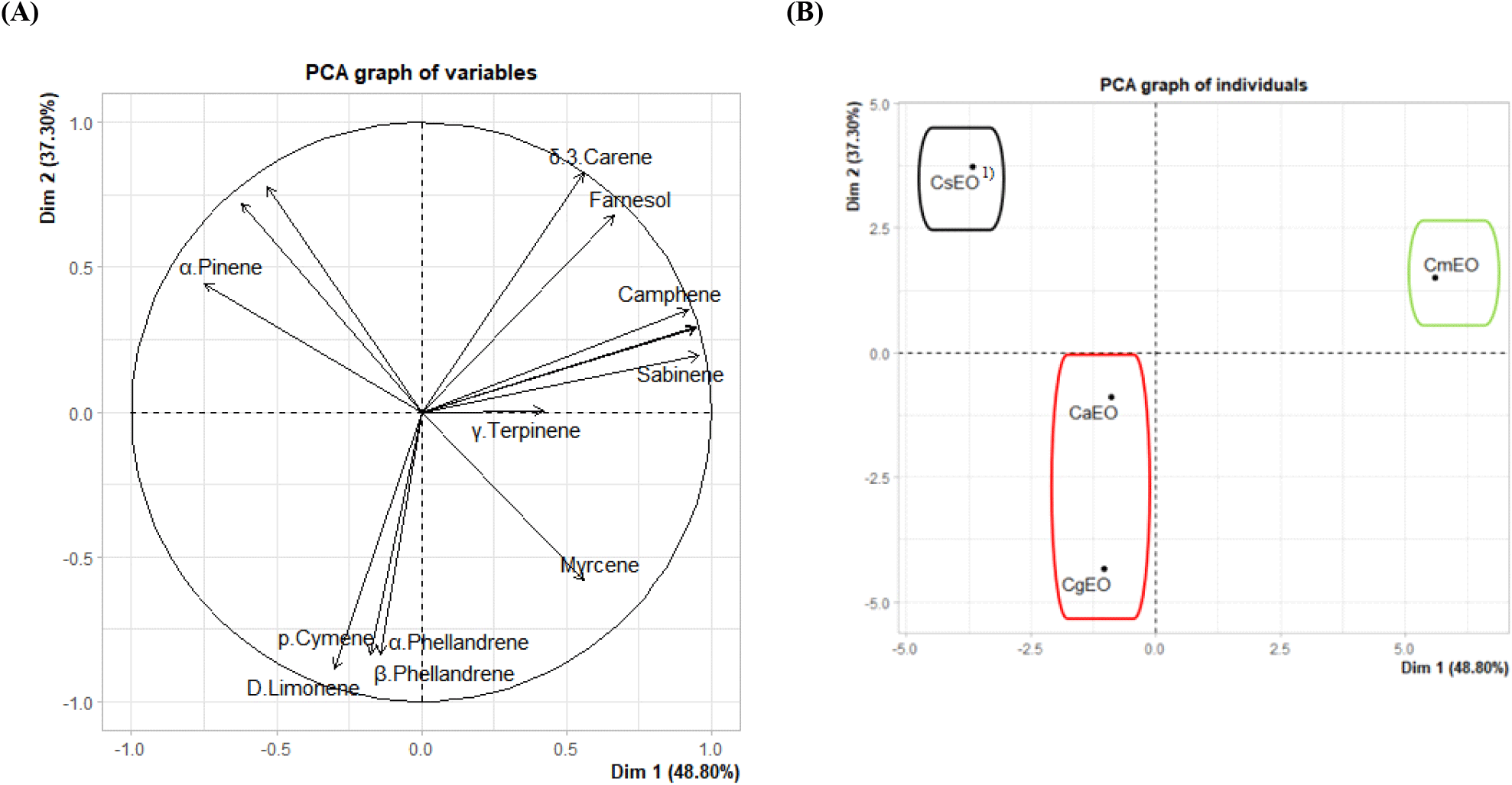
The CmEO sample is isolated on the right side of the PCA plot, reflecting a clear difference in chemical composition. This result is consistent with the GC-MS analysis, which showed that CmEO is rich in β-pinene (6.56%), sabinene (5.39%), and α-thujene (13.32%) compounds, which are absent or present in very low amounts in the other samples. CaEO and CgEO are located close to each other, with D-Limonene being the dominant component (72.69% and 85.11%), and exhibiting low levels of other monoterpenes. Meanwhile, CsEO forms a separate cluster due to its high content of α-pinene (24.81%) and D-limonene (70.56%).
The PCA variable plot (Fig. 1B) shows that α-pinene, D-limonene, γ-terpinene, sabinene, myrcene and camphene are the main compounds contributing to the sample separation, especially α-pinene and D-limonene (long, well-directed vectors).
In general, the PCA results are consistent with the GC-MS data, suggesting that the differences between samples primarily stem from the uneven distribution of monoterpene hydrocarbons compounds related to the antibacterial, antioxidant, and aromatic properties of essential oils.
Antioxidant capacity (AC) is one of the important biological properties of natural essential oils, which helps neutralize harmful free radicals and prevent lipid oxidation in foods, pharmaceuticals and cosmetics (Abeyrathne et al., 2021). In this study, the essential oil samples (CsEO, CgEO, CaEO, and CmEO) all demonstrated the ability to scavenge DPPH and ABTS radicals; however, the potency differed significantly at p<0.05 (Table 3).
With the DPPH assay, the IC50 value of CsEO was 183.53 mg/mL, which was better than that of CgEO (343.74 mg/mL), CaEO (292.48 mg/mL) and CmEO (485.81 mg/mL). Although this activity was still significantly lower than that of vitamin C (IC50=4.62 μg/mL), it demonstrated that CsEO had the highest potential activity among the tested samples. In the ABTS method, all samples showed stronger activity, with CsEO achieving IC50=4.74 mg/mL, lower than CaEO (7.53 mg/mL), CmEO (9.49 mg/mL) and close to vitamin C (6.61 μg/mL), indicating better ABTS radical neutralization ability than DPPH, which is common for compounds with flexible terpene structures (Valantina and Neelamegam, 2015).
Importantly, although CsEO had a relatively lower concentration of D-limonene, its enhanced antioxidant performance may stem from synergistic interactions between D-limonene and minor oxygenated monoterpenes such as linalool, geranial (neral), and terpinene-4-ol. According to Dev and Joseph (2025) study on Citrus essential oils, it has similarly suggested that the antioxidant and antimicrobial efficacy is not solely attributable to D-limonene but arises from the combined effects of both major and minor constituents.
When compared with other studies, orange essential oil from Malaysia had IC50-DPPH=7.73±2.00 mg/mL (Felicia et al., 2024), showing significantly better activity than the CsEO from Vietnam (183.53 mg/mL). In another study, CsEO from Korea had IC50-DPPH=86.17-3025.67 mg/mL and IC50-ABTS= 0.16-12.08 mg/mL, which are consistent with the values obtained from the four samples obtained in this study (Yang and Park, 2025).
Notably, essential oil samples from Ilorin, Nigeria showed stronger AC with IC50-DPPH ranging from only 27.29-33.00 μg/mL in four Citrus species (C. limon, C. aurantiifolia, C. sinensis and C. grandis) - markedly higher than those from Dong Nai (Ameen et al., 2021). This difference may be due to variations in the content and type of major antioxidant compounds, such as D-limonene, γ-terpinene, β-pinene, or soluble flavonoids, contained in the extracts.
Thus, although the Citrus samples from Dong Nai showed weaker AC than those from other regions, especially in the DPPH assay, they still exhibited significant values in the ABTS assay. This suggests the potential for exploiting indigenous Citrus essential oils as complementary natural antioxidants, especially in bio-packaging, traditional medicine or personal care products, when combined with enhancing agents such as polyphenols or vitamin E.
The antibacterial activity (AA) of Citrus peel essential oil is one of the important biological properties, which is evaluated through the diameter of the zone of inhibition against pathogenic microorganisms. The results showed that the essential oil samples from Dong Nai were all capable of inhibiting some Gram-negative and Gram-positive bacteria. However, the effectiveness varied depending on the bacterial species and the type of essential oil (Fig. 2).
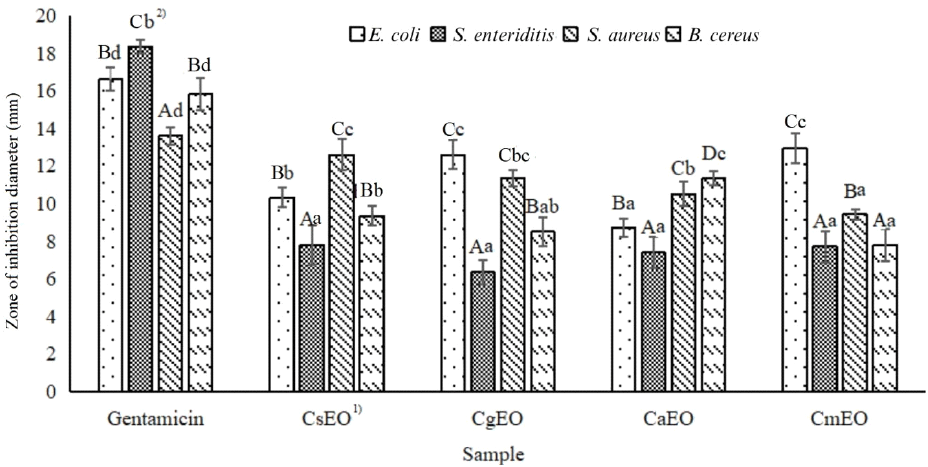
Among the tested strains, E. coli was the bacterium most strongly inhibited by CgEO (12.63±1.04 mm) and CsEO (10.34±0.62 mm), while CaEO (8.74 mm) showed lower effectiveness. This result is similar to the report by Felicia et al. (2024) in Malaysia, where CsEO inhibited E. coli with a zone of inhibition of 11.0 mm, which is much lower than that of P. aeruginosa (17.7 mm) - indicating differences between bacterial species and essential oil sources.
For Gram-positive bacteria, S. aureus was significantly inhibited by CsEO (12.63 mm), followed by CgEO (11.36 mm), and CaEO (10.54 mm). This level is almost equivalent to that reported in Perak, Malaysia, where C. hystrix essential oil showed strong efficacy against S. aureus (19.3±1.5 mm) and S. epidermidis (19.3±0.6 mm), while the efficacy on E. coli was only 8.3 mm (Mohideen et al., 2022). Thus, it can be seen that the essential oil from Citrus peel tends to have a stronger inhibitory effect on Gram-positive bacteria, due to the simpler cell membrane structure, which is easily disrupted by monoterpenes such as limonene, α-pinene, and γ-terpinene (Borges et al., 2022).
With Bacillus cereus, a Gram-positive bacterium commonly found in spoiled foods, CaEO showed the best inhibitory ability (11.36 mm), while other samples ranged from 7.82-9.36 mm. For Salmonella enteritidis, a Gram-negative bacterium that causes diarrhea, all samples showed lower efficiency, especially CmEO (7.73 mm). This is consistent with the observation that Gram-negative bacteria are often more resistant due to their thick outer membrane and antibiotic efflux system (Wei et al., 2019).
The antibacterial mechanism of Citrus peel essential oil is primarily related to the presence of monoterpene compounds, including D-limonene, α-pinene, sabinene, and γ-terpinene. These molecules are able to penetrate and disrupt the structure of bacterial cell membranes, causing leakage of ions and intracellular components, leading to osmotic imbalance and cell death (Liu et al., 2022). Compared to the standard antibiotic gentamicin (zone of inhibition, 16.63-18.36 mm), the essential oil exhibits a significantly lower efficacy, but still achieves an acceptable level of inhibition, particularly in applications such as antibacterial packaging, or biological preservatives.
4. Conclusions
The research results show that Citrus peel essential oils in Dong Nai possess significant biological and chemical properties, especially high levels of D-limonene and monoterpenes, such as α-pinene, γ-terpinene and sabinene. The differences in chemical composition between the samples are clearly shown through PCA analysis, in which CmEO shows a clear separation from the other three samples. In terms of biological activity, the samples exhibit certain antioxidant and antibacterial properties, particularly against Gram-positive bacteria. Combined with physical properties such as stability, aroma retention, and high saponification value, these Citrus essential oils show strong potential for application in the production of functional foods, natural cosmetics, and biological preservatives. At the same time, the exploitation of essential oils from fruit peel by-products contributes to increasing the value of agricultural products and moving towards sustainable development. However, this study has some limitations. Only in vitro antioxidant and antibacterial assays were conducted, and no specific mechanisms of action were explored. Future studies should include in vivo models, mechanism-based investigations, and broader sample sets from different harvest periods and geographical regions to confirm and extend the current findings.










