1. Introduction
Red snow crab (Chionoecetes japonicus) has an innate reddish color and is one of the most popular crab species because of its desirable taste and relatively low price (Lee et al., 2022). This species inhabits soft grey mud or sandy floors at depths of 400-2,000 m in the East Sea of the Korean coast (Jun et al., 2019) and its annual production is approximately 25,000-30,000 tons, except during the spawning season of about two months (KFA, 2023). Of the total catch, approximately 70% is processed into cooked and frozen crab meat products and exported mainly to Japan, United States of America (USA) and Europe (Kim et al., 2015).
The conventional processing of the crab typically involves boiling and cooling steps to prevent rapid spoilage and to facilitate the separation of meat from the shell. However, there has been a growing demand for quality improvement, as these thermal processes often result in the loss of flavor and nutritional components (Jun et al., 2017). Because crab muscle is firmly attached to the shell, it is difficult to separate the meat without inducing protein denaturation in the subcuticular membrane (Dima et al., 2012). A novel meat separation technique that induces protein denaturation through freezing and sequential thawing was developed in our previous study, resulting in a reduction of nutritional loss to less than 20% compared to over 60% loss typically observed in conventional boiling-based processing (Jun et al., 2019).
Compared to fish, crustaceans contain higher levels of free amino acids and non-protein nitrogenous compounds in muscle tissue, which leads to a faster spoilage rate during storage (Lorentzen et al., 2016). Although the overall mechanisms of post-mortem deterioration differ between crustaceans and teleost fish, endogenous enzymes and microbial activity are the primary contributors in both cases (Gonçalves and de Oliveira, 2016). In teleost fish, metabolic energy is stored as creatine phosphate, which acts as a readily available energy reservoir to regenerate adenosine triphosphate (ATP) during muscle activity through the reversible reaction catalysed by creatine kinase (CK). After death, the depletion of ATP and the progressive inactivation of CK lead to the irreversible degradation of creatine phosphate into creatine, contributing to the decline of energy charge in muscle tissue (Watabe et al., 1991). Similarly, muscle glycogen is degraded into pyruvate and then metabolized into lactate or other organic acids via the tricarboxylic acid (TCA) cycle (Huss, 1995). In contrast, crustaceans store energy in the form of arginine phosphate, which is irreversibly converted to arginine and ATP during post-mortem metabolism. Arginine is further degraded into ornithine and urea through the urea cycle as freshness declines (Huang et al., 2020). Crustaceans are also susceptible to melanosis caused by polyphenol oxidase (tyrosinase), which catalyzes the oxidation of aromatic amino acids such as tyrosine (Gonçalves and de Oliveira, 2016). The development of melanosis causes undesirable discoloration and reduces the commercial value of crab meat, though this reaction also occurs in live crabs as a healing response to injury (Lian et al., 2018). Therefore, post-mortem changes in crustaceans are closely associated with activities of enzymes, such as arginase, tyrosinase, and alkaline phosphatase (ALPase), as well as their related metabolites.
Although some studies have analyzed endogenous enzyme activity in crab, studies investigating its simultaneous changes with related biochemical parameters during refrigerated storage are still limited. Therefore, the aim of this study was to investigate the biochemical mechanisms underlying quality deterioration in non-thermally processed red snow crab leg meat during refrigerated storage. Specifically, changes in pH, volatile basic nitrogen (VBN), bacterial counts, and the activities of key endogenous enzymes such as arginase, tyrosinase, and ALPase were analyzed, along with their related metabolites including arginine, ornithine, urea, and ATP-related compounds. To better understand the biochemical changes occurring under refrigerated conditions, the non-hermally processed group was compared with frozen and microwave-heat-treated leg meats, which served as reference groups with reduced enzymatic and microbial activity. The results are expected to provide fundamental insights into the post-mortem metabolic processes of crustaceans and contribute to the development of effective quality control strategies for non-thermally processed crab products.
2. Materials and methods
Thirty live red snow crabs (Chionoecetes japonicus, male; 448.8±36.5 g) were purchased from the Jumunjin Fishery Market (Gangneung, Korea) in October 2023 and used for leg meat preparation. The crabs were immediately transported to laboratory on ice and rinsed with tap water to remove surface contaminants. Leg meat was separated according to the freeze-thaw-pressing muscle separation method described in our previous study (Jun et al., 2019). Briefly, each crab was individually packed in a polyethylene bag and frozen at −20°C for 12 h. For leg meat separation, only the leg parts were collected from the frozen crabs and then thawed in ice water (0°C) for 30 sec. The edges of the merus parts were trimmed, and the leg meat was separated from the shell using a roller-type machine (YMC-103; YongMa Machinery, Daegu, Korea).
The proximate composition of the leg meat was analyzed according to the AOAC (2005) methods. Moisture content was determined by the atmospheric heating and drying method (method 950.46), crude protein content by the semi-Kjeldahl method using an automated apparatus (method 976.05), crude lipid content by the solvent extraction method with petroleum ether (method 991.36), ash content by the direct ashing method (method 923.03). The carbohydrate content was calculated by subtracting the sum of moisture, crude protein, crude lipid, and ash contents from 100.
The separated leg meat (approximately 50 g) was placed in petri dishes (90 mm in diameter, three pieces of the merus per dish) and vacuum-packaged using a multilayer film composed of low-density polyethylene, nylon, and polyethylene terephthalate (80 μm in thickness) to prevent cross-contamination from external environments during storage. The packaged samples were stored at −20°C (freezing group; F) or 4°C (raw group; Raw). In addition, microwave heating was selected as the heat source to investigate the effect of heat treatment on the biochemical parameters of crab meat. The heating condition was set to 700 W for 45 sec using a household microwave, based on preliminary trials that determined the ready-to-eat state of the leg meat. For the microwave-treated group (M), the separated leg meat was packaged under aseptic conditions in the same manner as described above after microwave treatment and stored at 4°C. Samples were collected on days 0, 1, 2, 3, 5, and 8 for the analysis of the biochemical parameters including pH, VBN, bacterial counts, and enzyme activities. Free amino acids were analyzed only on days 0, 2, and 5, while ATP-related compounds and visual appearance were evaluated on days 0, 2, 5, and 8.
For pH determination, 3 g of minced leg meat was mixed with 27 mL of deionized water (DW), homogenized (10,000 rpm for 2 min), and centrifuged at 8,000 ×g for 15 min at 4°C. The pH of the supernatant was determined using a pH meter (SevenEasy S20K; Mettler-Toledo, Schwerzenbach, Switzerland).
The VBN value was determined according to the general analytical method of the Korean Food Code (MFDS, 2024). Five grams of minced leg meat were mixed with 40 mL DW and homogenized (10,000 rpm for 1 min). After adding 5 mL of 20% trichloroacetic acid, the sample was homogenized again under same conditions. The homogenate was centrifuged at 10,000 ×g for 15 min at 4°C, and the supernatant was filtered through an Advantec No. 5C filter paper (Advantec Toyo Kaisha Ltd., Tokyo, Japan). The filtered sample was used as the test solution, and the VBN value was determined by the microdiffusion method using a Conway unit.
The total bacterial count was determined according to the standard plate count method described by Jun et al. (2017). Ten grams of sample were mixed with 90 mL of sterilized 0.1% peptone-buffered water (pH 7.2) and homogenized using a stomacher at 250 rpm for 5 min. The sample was serially diluted with the same diluent, and appropriate dilutions were spread onto plate count agar (Difco, Becton Dickinson and Co., Sparks, MD, USA). The plate was incubated at 35°C for 24 or 48 h, and colonies ranging from 20 to 200 were counted. The results were expressed as log CFU/g.
Alkaline phosphatase (ALPase), arginase, and tyrosinase were selected as representative enzymes involved in the biochemical degradation and quality deterioration of crab meat. To determine enzyme activities, 3 g of sample was ground with 15 mL of 1.5% sodium chloride solution (4°C) and centrifuged at 8,000 ×g for 30 min at 4°C. The supernatant was collected for subsequent enzyme activity assay. The activities were quantified using commercial colorimetric assay kits according to the manufacturer’s protocols. Specifically, ALPase activity was measured with a colorimetric assay kit (Cat. No. K412- 00; BioVision Inc., Milpitas, CA, USA), tyrosinase activity with kit K742-100 (BioVision Inc.), and arginase activity with kit K755-100 (BioVision Inc.). Enzyme activities were expressed as units per gram of sample (U/g).
Free amino acids (tyrosine, phenylalanine, arginine, and ornithine) and nitrogenous compound (urea) associated with arginase and tyrosinase activities were analyzed using a high-speed amino acid analyzer (L-8800; Hitachi High-technologies Co., Tokyo, Japan) equipped with an ion-xchange resin column (#2622, 5 μm, 4.6×60 mm; Hitachi High-technologies Co.). Ten grams of the sample were homogenized (10,000 rpm for 2 min) with 100 mL of 75% ethanol and centrifuged at 10,000 ×g for 15 min at 4°C. The extraction was repeated twice on the residues. After collecting the supernatants, ethanol was removed using a rotary evaporator, and the samples were diluted with DW. The samples were filtered through a 0.45 μm mixed cellulose ester (MCE) syringe filter unit before analysis. The analytical conditions were based on the methods described by Kim et al. (2016). A 10 μL aliquot was injected and flowed at 0.35 mL/min with the lithium citrate buffer containing ninhydrin reagent. The column oven temperature was programmed from 30 to 70°C (0.5°C/min), and the reaction coil temperature was maintained at 135°C. The detection wavelength was set to 570 nm.
ATP-related substances were analyzed using High performance liquid chromatography-diode array detector (HPLC-DAD) (Agilent 1200; Agilent Technologies, Santa Clara, CA, USA) according to the method of Nam et al. (2022). To minimize changes in ATP-related compounds, the leg meats were quickly and evenly minced, and 5 g of sample was immediately mixed with 20 mL of 0.6 M perchloric acid. The sample was homogenized (10,000 rpm for 2 min), and the pH was adjusted to approximately 4.0 using 5 M potassium hydroxide. After centrifugation (10,000 ×g for 20 min at 4°C), the supernatant was collected and appropriately diluted with the mobile phase (1% triethylamine-phosphoric acid, pH 6.5). The sample was filtered through a 0.20 μm MCE syringe filter unit before analysis. The analysis was achieved with a μBondapak C18 column (10 μm, 3.9×300 mm; Waters Co., Milford, MA, USA) using the mobile phase described above at a flow rate of 1.0 mL/min (isocratic elution). A 5 μL aliquot of the sample was injected, and detection wavelength was set to 245 nm. Standard substances, including hypoxanthine (Hx), inosine (HxR), inosine 5′-monophosphate disodium salt (IMP), adenosine 5′-monophosphate sodium salt (AMP), adenosine 5′-diphosphate sodium salt (ADP), and adenosine 5′-triphosphate disodium salt (ATP) (Sigma-Aldrich Inc., St. Louis, MO, USA), were used for identification and quantification. The identification was based on comparison of retention times with those of the standards analyzed under identical conditions, and quantification was performed using a three-point external standard calibration (50, 100, and 500 μg/L).
The appearance of the samples was recorded photographically using a phone camera (iPhone SE2, Apple Inc., Cupertino, CA, USA) under consistent lighting and background conditions.
All experimental results were expressed as the mean± standard deviation (SD). Significant differences among the mean values were determined using one-way analysis of variance (ANOVA) followed by Tukey’s test (p<0.05), performed using SPSS Statistics 20 (IBM Corp., Armonk, NY, USA).
3. Results and discussion
The proximate composition (n=3) of red snow crab leg meat was 83.71±0.29% moisture, 13.09±0.38% crude protein, 0.82±0.17% crude lipid, 1.78±0.12% ash, and 0.60±0.37% carbohydrate, indicating that protein was the predominant component (80.36%, dry basis), followed by ash (10.93%, dry basis) (data not shown). To better understand the biochemical mechanisms underlying quality deterioration in non-thermally processed crab meat (Raw group) during refrigerated storage, changes in various biochemical parameters were monitored and compared with those in frozen (F group) and microwave-heat treated crab meat (M group).
Fig. 1 shows the changes in the pH and VBN value, and total bacterial count. During storage, organic acid production or the accumulation of non-protein nitrogenous compounds from protein degradation can influence pH change in crab meat (Laly et al., 2021). The pH value of non-thermally processed leg meat was 7.22±0.08 and increased to 7.57±0.08 after microwave treatment (Fig. 1A). This increase may be attributed to the denaturation of proteins caused by heating, which leads to the destruction of free acidic groups and consequently results in an increase in the pH (Dericioglu et al., 2019). During 8 days of storage, the pH value of the F and M groups remained relatively stable, whereas the pH of the Raw group increased after day 3 and showed a significant difference on day 5 (p<0.05).
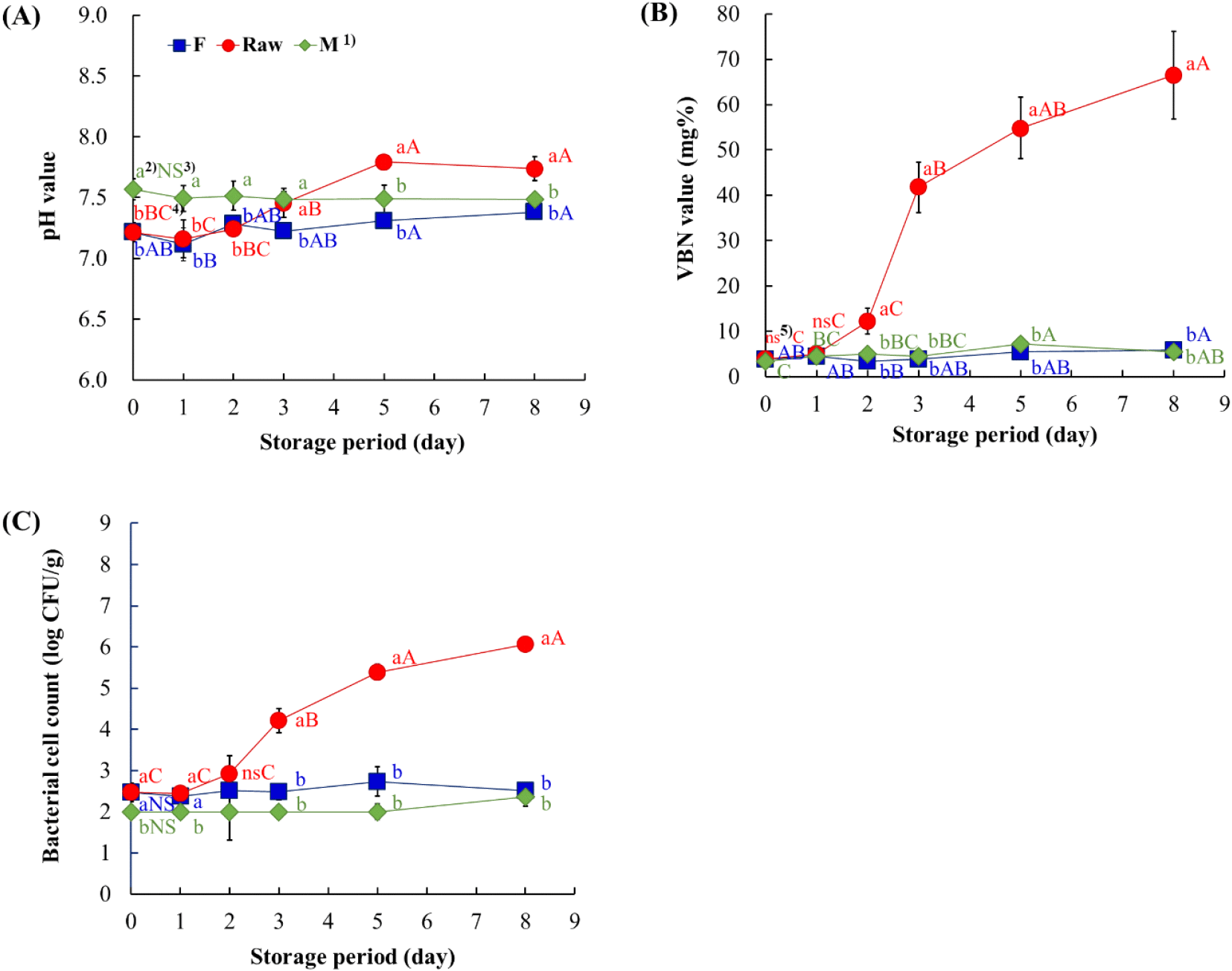
The VBN value in the Raw group increased sharply after day 3 (41.80±5.53 mg%) and reached to 66.54±9.66 mg% on day 8, whereas those of the F and M groups remained relatively constant throughout storage (Fig. 1B). In general, an increase in VBN value indicates a decline in freshness in fish muscle. Although the threshold can vary on fish species or post-processing, values below approximately 10-15 mg% are regarded as fresh, while values above 25-35 mg% are considered the onset of spoilage (Laly et al., 2021; Min et al., 2019). Based on this criterion, the VBN value of the Raw group exceeded the spoilage level after 3 days of storage.
The bacterial count of non-thermally processed leg meat was 2.47±0.23 log CFU/g, which decreased to below the detection limit (2.0 log CFU/g) after microwave treatment (Fig. 1C). During 8 days of storage, both the F group and M group maintained their initial bacterial count, whereas the Raw group showed a rapid increase from day 3 and reached 5.39±0.19 log CFU/g on day 5 and 6.06±0.15 log CFU/g on day 8. In general, the spoilage threshold for fishery products ranges from 5.0 to 6.0 log CFU/g (Odeyemi et al., 2020; Xia et al., 2023). However, sensory unacceptability and spoilage may occur at lower bacterial levels; therefore, an appropriate safety margin should be considered.
Post-mortem biochemical changes in crustaceans, including autolysis and putrefaction, are primarily caused by endogenous enzymes and microbial activity, leading to quality deterioration such as protein degradation, lipid oxidation, and melanosis (Gonçalves and de Oliveira, 2016). As described in the introduction, ALPase, arginase, and tyrosinase are key enzymes involved in the post-mortem biochemical reactions of crustaceans, participating in the hydrolysis of phosphate esters, the conversion of arginine to ornithine and urea, and the development of melanosis, respectively (Chen et al., 2024; Gornik et al., 2008; Lian et al., 2018).
ALPase activity in the Raw group increased sharply after day 3 (9.62±2.52 U/g) and reached the highest value on day 8 (57.55±5.35 U/g; Fig. 2A). The increase may promote the hydrolysis of phosphomonoesters such as AMP and IMP during post-mortem storage (Chen et al., 2024). In contrast, the F and M groups maintained relatively low levels of ALPase activity throughout storage, ranging between 0.22-1.83 U/g and 2.08-3.81 U/g, respectively. Arginase activity in the M group showed markedly lower levels from the beginning of storage (0.03±0.01 U/g) with slight variation (Fig. 2B), whereas the F group maintained relatively high activities (0.09-0.14 U/g). In the Raw group, arginase activity was initially comparable to that of the F group, but it began to decline sharply after day 3 and decreased to undetectable level by day 8. Arginase derived from the hepatopancreas of the freshwater prawn (Macrobrachium rosenbergii) has been reported to exhibit optimal activity under alkaline pH conditions and to specific metal ions (Ehigie et al., 2010), suggesting that similar biochemical properties may be present in other crustaceans. However, in the present study, arginase in the Raw group declined during storage. This reduction may have resulted from the depletion of its primary substrate (arginine) structural instability of the enzyme, or interference by other proteolytic activities.
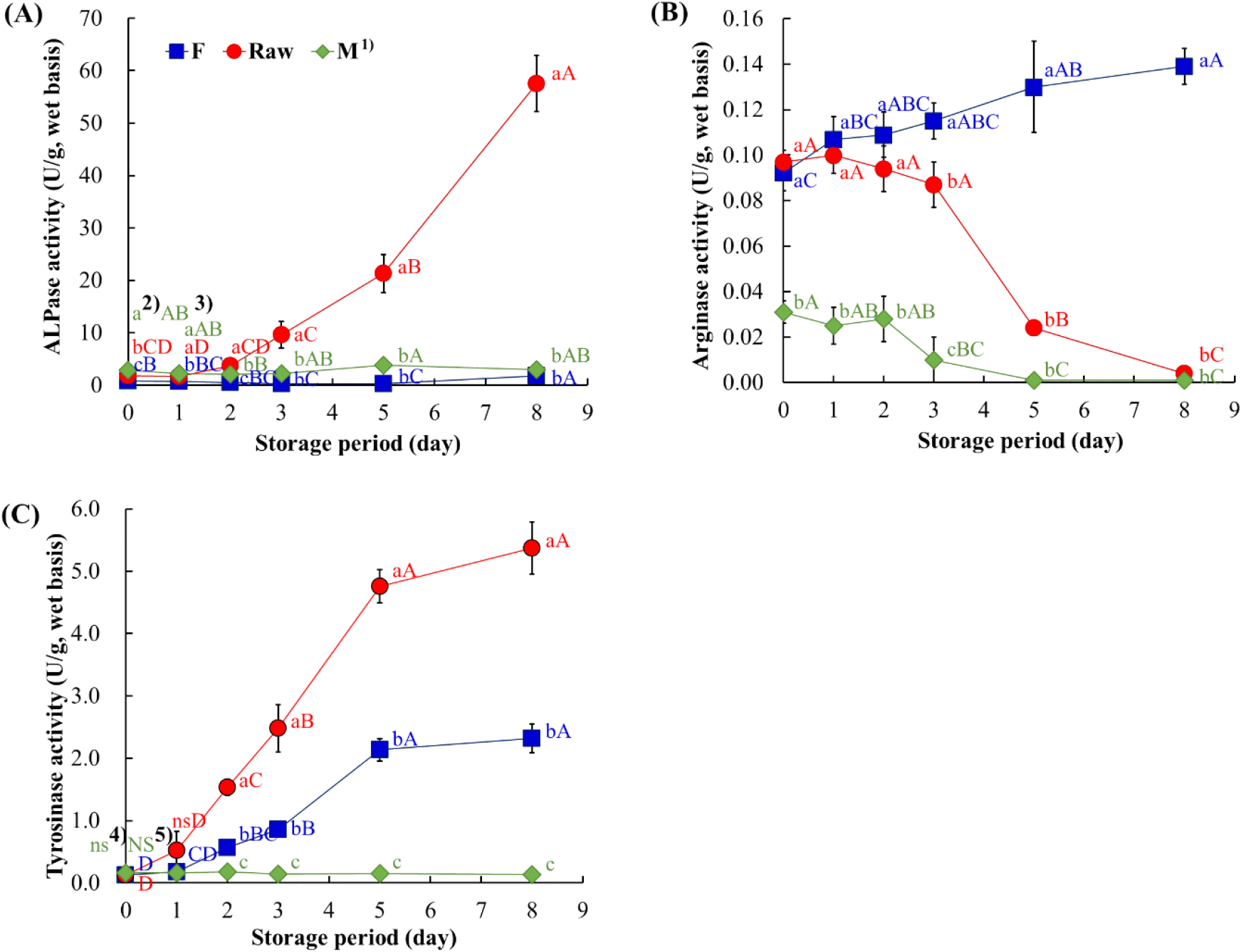
Tyrosinase is a polyphenol oxidase (PO) that is responsible for enzymatic browning in crustaceans (Lian et al., 2018). Although melanosis is harmless, it reduces consumer acceptance (Kim et al., 2000). Tyrosinase activity was initially low (approximately 0.13 U/g) in all groups but increased markedly in the Raw and F groups during storage. The activity in the Raw group increased sharply to 5.37±0.42 U/g by day 8, while the F group showed a gradual rise to 2.32±0.23 U/g. In contrast, the M group maintained its initial level throughout storage, indicating that the enzyme was inactivated by microwave heating. Previous studies have reported that thermal treatments in the range of 60°C to 80°C can increase PO activity in crab meat, while heating above 91°C effectively inactivates PO and delays the melanosis (Lian et al., 2018; Lorentzen et al. 2016).
The biochemical parameters of the non-thermally processed Raw group, including pH and VBN values as well as enzyme activities, changed markedly between days 2 and 5 of storage (Fig. 1 and 2). This period is presumed to correspond to an active phase of post-mortem metabolism and protein degradation, during which the accumulation of metabolites resulting from enzymatic activity can play a crucial role in quality deterioration (Nie et al., 2022). In particular, arginase and tyrosinase activities are associated with the urea cycle and melanosis, respectively (Gornik et al., 2020; Lian et al., 2018). These enzymatic pathways influence changes in related compounds such as arginine, ornithine, urea, tyrosine, and phenylalanine.
In this study, the changes in amino acids and nitrogenous compounds were analyzed on days 0, 2, and 5 of storage, which represented the periods showing the most distinct variations (Table 1). Among the three groups, the Raw group exhibited the most pronounced changes during storage. The arginine content significantly decreased from 366.4±15.4 mg% on day 0 to 11.0±5.4 mg% on day 5, whereas ornithine and urea levels significantly increased to 165.3±28.5 mg% and 65.0±4.3 mg%, respectively (p<0.05). These variations were consistent with the increased arginase activity observed during the same period (Fig. 2B), suggesting the conversion of arginine to ornithine and urea proceeded actively in the Raw group. In contrast, no significant changes were observed in the F and M groups, except for a slight increase in ornithine in the M group.
5) Means with different superscripts (a-c) within groups are significantly different (p<0.05) by Tukey’s test.
The phenylalanine and tyrosine contents showed a decreasing trend during storage in the Raw group, and tyrosine was not detected on day 5. In contrast, no significant changes were observed in the M group over 5 days of storage, suggesting that tyrosinase activity was suppressed by microwave heating. Previous studies have mainly focused on the changes in free amino acids and nitrogenous compounds during crab meat storage (Chiou et al., 2004; Jun et al., 2017), but their association with endogenous enzyme activity remains poorly understood. The present findings suggest that arginase and tyrosinase activities may be involved in modulating the levels of these compounds in non-thermally processed crab meat.
In fish muscle, ATP is sequentially degraded into ADP, AMP, IMP, HxR, and Hx during post-mortem storage through enzymatic reaction (Huss, 1995). The changes of these ATP-related substances are closely associated with the post-mortem metabolism and quality deterioration, in particular, IMP plays an important role in the manifestation of umami taste of fish (Hong et al., 2017). However, as IMP is further degraded to HxR and Hx, undesirable off-flavors and bitterness develop; thus the compositional changes of ATP-related compounds are widely used for evaluation of freshness decline (Guo et al., 2021). Furthermore, the degradation of AMP and IMP can be promoted by phosphatases such as ALPase, which is consistent with the increased ALPase activity observed in Fig. 2A.
Table 2 shows the changes in ATP-related substances in non-thermally processed leg meat during storage. At day 0, ATP and ADP were the predominant substances in all groups, but both gradually decreased during storage. In the Raw group, IMP and HxR temporarily increased on day 2 and then markedly decreased on day 5, accompanied by a distinct accumulation of Hx. The breakdown of IMP to HxR and Hx is associated with freshness loss and the development of undesirable off-flavors and bitterness in seafood (Hong et al., 2015). Interestingly, ADP remained relatively constant throughout storage, suggesting that the activities of ADP-degrading enzymes such as adenylate kinase were suppressed (Arai et al., 2020). In contrast, the F and M groups showed relatively slight changes in ATP and its degradation substances, and the IMP content remained comparatively stable during 8 days of storage. This tendency can be attributed to the inhibition of enzyme activity and microbial growth, which delayed the overall ATP degradation process. Lin et al. (2022) reported that ATP degradation in post-mortem crab meat is related to microbial activity. However, the specific enzymes involved in this process have not been clearly identified. The association between increased ALPase activity and the breakdown of AMP and IMP suggests that ATP degradation may be mediated by both endogenous enzymes and those produced by spoilage microorganisms.
1) Hx, hypoxanthine; IMP, inosine monophosphate; HxR, inosine; AMP, adenosine monophosphate; ADP, adenosine diphosphate; ATP, adenosine triphosphate.
5) Means with different superscripts (a-c) within groups are significantly different (p<0.05) by Tukey’s test.
Fig. 3 shows the appearance of non-thermally processed leg meat in each group during storage. After microwave treatment, the leg meat exhibited a slight reduction in volume, and drip exudation was observed on the surface. However, no remarkable changes were observed in the M group during 8 days of storage. The F and M groups appeared similar on day 0, and no noticeable changes were observed in the F group throughout storage. In contrast, the Raw group began to lose its reddish color and developed surface browning after 5 days, which progressed to tissue disruption and dark discoloration by day 8. These changes correspond These corresponds to the dark coloration that appeared on days 5 and 8, when tyrosinase activity reached its maximum levels.
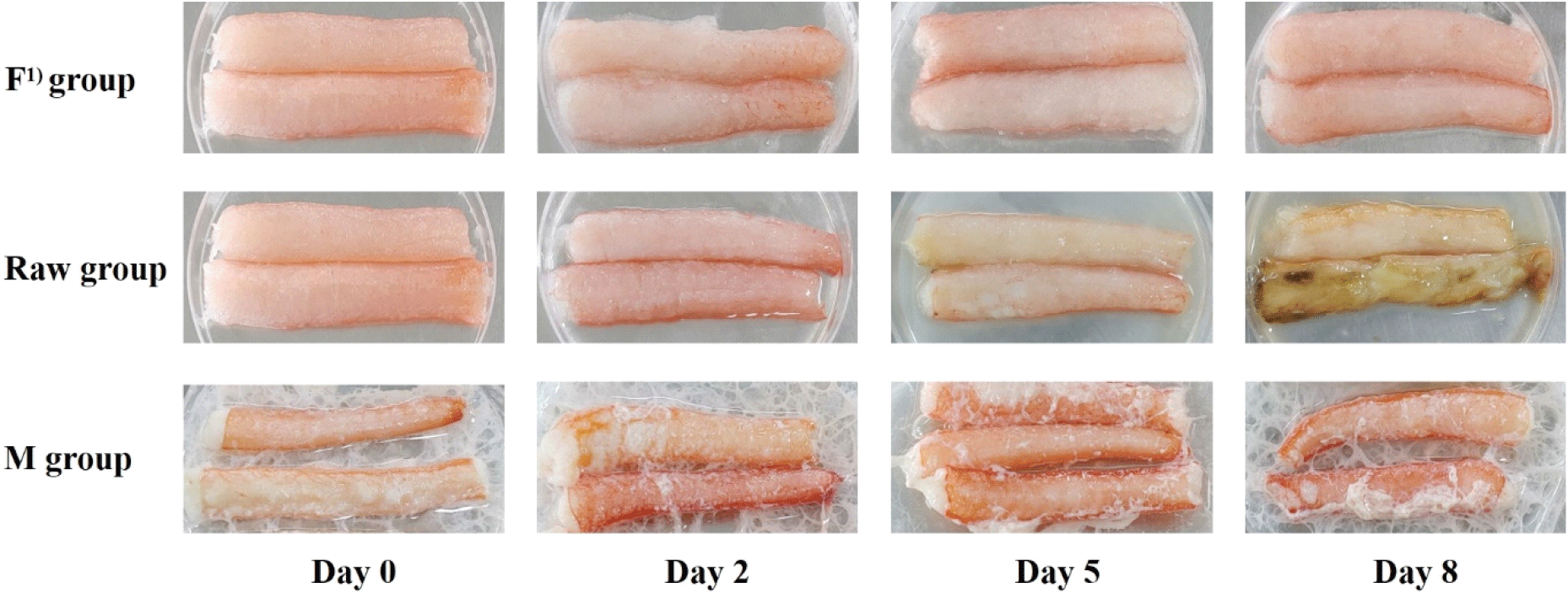
The tissue disruption observed in the Raw group could be attributed to protein degradation caused by enzymatic and microbial activities, as demonstrated by the results of the biochemical parameters. In particular, the sharp decrease in arginine and the accumulation of ornithine and urea indicate that active proteolysis and subsequent metabolic reactions occurred (Zheng et al., 2025). In contrast, the appearance of the F and M groups remained almost unchanged during storage, suggesting that enzyme and microbial activities were suppressed.
4. Conclusions
This study examined the biochemical changes associated with quality degradation in non-thermally processed red snow crab leg meat during refrigerated storage. The Raw group exhibited notable biochemical deterioration compared to the F and M groups. In particular, from day 3 of storage, a sharp increase in pH, VBN, and bacterial counts was observed with elevated ALPase and tyrosinase activities. In terms of ATP degradation, a decrease in IMP and an accumulation of Hx were detected. Additionally, the rapid depletion of arginine and the resulting accumulation of ornithine and urea indicated the active involvement of the arginase pathway, with enzyme activity subsequently reduced due to substrate limitation. Enzymatic and microbial reactions were closely related to the overall quality deterioration, as evidenced by discoloration and tissue disruption observed in the Raw group. In contrast, the F and M groups showed almost no visible changes during storage.
These findings provide biochemical evidence that quality deterioration in non-thermally processed red snow crab leg meat during refrigerated storage is driven by endogenous enzymes and microbial activity. Simultaneous monitoring of enzyme activities and related metabolites enables a clearer understanding of post-mortem biochemical changes. This study provides a basis for establishing objective indicators to assess freshness and manage the quality of non-thermally processed seafood during storage.










