1. Introduction
Cholesterol is an essential structural component of mammalian cell membranes and serves as a major precursor for the biosynthesis of steroid hormones, vitamin D, and bile acids. However, abnormally elevated cholesterol levels constitute a critical risk factor for various metabolic disorders, including cardiovascular disease (CVD) (Zhang et al., 2024). It is widely acknowledged that elevated cholesterol significantly contributes to the global morbidity and mortality associated with CVD, which remains one of the most prevalent metabolic diseases worldwide (Jung et al., 2022). Therefore, targeting cholesterol biosynthesis and lipid metabolism pathways represents a promising therapeutic strategy for the prevention and treatment of cardiovascular diseases.
Cholesterol biosynthesis primarily occurs through the enzymatic activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, followed by its secretion into the bloodstream and cellular uptake from low-density lipoproteins (LDL) (Luo et al., 2022). It is well established that cholesterol synthesis is tightly regulated by sterol regulatory element-binding proteins (SREBPs), which play essential roles in maintaining lipid homeostasis. Among the SREBP family members, SREBP-2 particularly initiates cholesterol biosynthesis by activating HMG-CoA reductase (HMGCR) through the modulation of 5’ adenosine monophosphate-activated protein kinase (AMPK) signaling (Lee et al., 2017; Nam et al., 2019). Based on these mechanisms, extensive research has focused on identifying HMG-CoA reductase inhibitors to effectively lower LDL cholesterol levels. However, prolonged use of these pharmacological agents (e.g., atorvastatin, pravastatin, lovastatin, and simvastatin) has been associated with several adverse effects, including hepatic injury, gastrointestinal disturbances, and myalgia (Golomb and Evans, 2008). In recent years, due to such adverse effects of conventional pharmaceutical drugs, considerable attention has been directed toward the development of functional foods and nutraceuticals that can safely improve cholesterol levels and prevent related chronic diseases.
Policosanol is a mixture of long-chain fatty alcohols derived primarily from sugarcane, sorghum, and corn (Gouni-Berthold and Berthold, 2002). Globally, Brazil, India, and China collectively account for over 65% of sugarcane production (Solomon, 2016); however, most research on policosanol has predominantly focused on Cuban sugarcane-derived policosanol. Several studies have reported that policosanol exerts inhibitory effects on cholesterol biosynthesis by reducing the enzymatic activity of HMG-CoA reductase and promoting the degradation of low-density lipoproteins (LDL), and increased bile acid excretion (Gouni-Berthold and Berthold, 2002; Nam et al., 2019; Singh et al., 2006). Additionally, beneficial properties such as inhibition of smooth muscle cell proliferation, platelet aggregation, LDL oxidation, and modulation of inflammatory pathways via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling have been documented (Arruzazabala et al., 1996; Thippeswamy et al., 2008). However, comprehensive mechanistic investigations into other lipid metabolism pathways and associated biomarkers affected by policosanol are limited.
In this study, we isolated policosanol (Reduchole22®) from Indian sugarcane using an ethanol extraction method and evaluated its lipid-lowering effects in a high-fat diet-induced mouse model. Unlike earlier studies that focused primarily on Cuban sugarcane-derived policosanol, our investigation aimed to assess the efficacy of an alternative source and to explore additional mechanistic pathways. Administration of policosanol significantly reduced cholesterol accumulation by enhancing bile acid synthesis and fecal excretion. Furthermore, it attenuated LDL cholesterol oxidation and downregulated the expression of key genes involved in cholesterol biosynthesis and lipid metabolism. These findings not only validate the cholesterol-lowering potential of Indian sugarcane-derived policosanol but also suggest its broader utility as a functional food ingredient for the dietary management of hypercholesterolemia and hyperlipidemia.
2. Materials and methods
Policosanol (Reduchole22®) from Saccharum officinarum was purchased from Ingex Botanicals (Bengaluru, India). Analytical-grade reagents, including 2-propanol, chloroform, and isopropyl alcohol (Merck, Mumbai, India); Milli-Q water (Merck, Mumbai, India); isoflurane (Raman & Weil Pvt Ltd, Mumbai, India); ethanol (CS Reagents, Mumbai, India); picric acid (HiMedia Labs, Mumbai, India); and thiopentone sodium (Thiosol) injection for anesthesia (Neon, Mumbai, India), were procured for experimental procedures. Additionally, lipid quantification Kit (Cell Biolabs, Inc., San Diego, CA, USA), cholesterol quantification kit (Sigma-Aldrich, St. Louis, MO, USA), cholesterol 7α-hydroxylase (CYP7A1, MyBiosource, San Diego, CA, USA) and oxidized low-density lipoprotein (oxLDL) ELISA Kit (Cell Biolabs, Inc., San Diego, CA, USA) were purchased and utilized for biomarker analyses. Other biochemical parameters used to evaluate the effects of policosanol in blood, feces, liver, and arterial tissues were measured using ELISA kits purchased from ELK Biotechnology (Shanghai, China). The biomarkers assessed included: 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), ATP-binding cassette sub-family G member 5 (ABCG5), acetyl-CoA acetyltransferase 1 (ACAT1), paraoxonase 1 (PON1), sterol regulatory element-binding protein 2 (SREBP2), low-density lipoprotein receptor (LDLR), lecithin-cholesterol acyltransferase (LCAT), farnesyl-diphosphate farnesyltransferase 1 (FDFT1), apolipoprotein A-1 (ApoA-1), apolipoprotein B (ApoB), and cholesteryl ester transfer protein (CETP).
Male C57BL/6 mice (6-8 weeks old, weighing approximately 20-25 g) purchased from AIIMS (New Delhi, India) were used in this study. Prior to experiments, animals underwent a general health examination and were acclimated to laboratory conditions for 7 days. Only animals showing no signs of illness or abnormality were included in the experiment. Each mouse was individually identified using picric acid marking and labeled with unique identification numbers, and cages were separately numbered to classify experimental groups. Animals were randomly assigned to experimental groups based on their body weights. Mice were housed under standard laboratory conditions in an air-conditioned room maintained at a temperature of 22±3°C, relative humidity of 30-70%, with a controlled photoperiod of 12-hour light/dark cycles. The animal room was provided with adequate air circulation and was disinfected daily. Animals were kept in sterile cages containing corncob bedding and had free access to rodent chow and purified GenPure RO drinking water ad libitum. Water quality was routinely monitored for microbial contamination, and drinking bottles and delivery tubes were regularly checked to ensure proper function. Normal control animals received a standard rodent chow diet (VRK Nutritional Solutions, Pune, India) containing 18% protein, whereas the experimental animals were fed a high-fat diet (HFD, 60% fat) throughout the duration of the study. All animal procedures were conducted in accordance with the guidelines established by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Fisheries, Animal Husbandry and Dairying (MoFAH&D), Government of India (Project approval number: RR/IAEC/120-2023). The entire study was conducted under the supervision of a study monitor and veterinarian. During the experimental period, animals were closely monitored, and no adverse effects, pain, or distress were observed. Animal experiment was conducted in accordance with the guidelines of committee for the control and supervision of experiments on animals (CCSEA Registration Number-1803/PO/RcBi/S/15/CCSEA).
A total of 60 male C57BL/6 mice were housed and acclimated for at least one week prior to initiating the experiments. Of these, 52 mice were fed a high-fat diet (HFD, 60%, VRK Nutritional Solutions, Pune, India) for 4 weeks to induce obesity, while the remaining 8 mice (normal control group) received a standard chow diet during the same period. After 4 weeks, animals were randomized into five groups (n=8 per group) based on their body weights. With the exception of the normal control (standard chow diet) and HFD control groups, all other groups were treated daily with either a reference standard or policosanol along with HFD for an additional 8 weeks. The experimental groups were designated as follows: Group 1: Normal control, receiving standard chow diet; Group 2: High-fat diet (HFD 60%) control; Group 3: Reference standard treatment, receiving Orlistat (10 mg/kg body weight/day); Group 4: Policosanol administered at 5 mg/kg body weight/day; Group 5: Policosanol administered at 10 mg/kg body weight/day; Group 6: Policosanol administered at 20 mg/kg body weight/day. Policosanol and orlistat were suspended in 0.5% carboxymethylcellulose (CMC) and administered orally (by gavage) once daily throughout the experimental period. The Normal control (Group 1) and HFD control (Group 2) groups received an equivalent volume of the 0.5% CMC vehicle by the same route.
During the treatment period, clinical signs of toxicity, including mortality, were monitored and recorded. Individual body weights were measured weekly from the first day until the conclusion of the treatment period. At the end of the experimental period, all animals were euthanized humanely using CO2 gas following isoflurane anesthesia. Immediately after euthanasia, necropsy was performed, and blood samples were collected for biochemical analyses. Serum and plasma were separated from blood samples and stored appropriately for subsequent biochemical assays. Organs were collected during necropsy and stored at −80°C until further analysis.
At the end of the experimental period, blood, feces, liver, and arterial tissues were collected for biochemical analyses. The collected tissues (liver, arteries, and feces) were thoroughly rinsed with ice-cold phosphate-buffered saline (PBS, pH 7.4) to remove residual blood, and then weighed prior to homogenization. Approximately 100 mg of each tissue sample was homogenized on ice in PBS using a glass homogenizer. The homogenates were further subjected to ultrasonication to ensure complete cell disruption, followed by centrifugation at 13,000 ×g for 15 min at 4°C. The resulting supernatants were collected and utilized for the assessment of selected biomarkers and lipid profiles according to the manufacturer’s instructions. Detailed information regarding the tissue sources and corresponding biomarkers analyzed is provided in the “Chemical” section.
Additionally, the atherogenic index (AI) was calculated using the following formula:
All data from the in vivo studies, including body weight, feed consumption, clinical chemistry parameters, and organ weights, were analyzed statistically using GraphPad Prism software (version 5.01). Results are presented as mean± standard deviation (SD). Significant differences between treatment groups and the pathological control group were determined using one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test.
3. Results and discussion
C57BL/6 mice were selected due to their well-characterized susceptibility to high-fat diet-induced hypercholesterolemia and atherosclerosis. This strain is widely used in preclinical studies evaluating lipid metabolism, reverse cholesterol transport, and cardiovascular risk, offering high translational relevance and compatibility with validated biomarkers for mechanistic exploration (Schreyer et al., 1998; Straniero et al., 2020). While species differences exist in cholesterol and bile acid metabolism between mice and rats, prior studies suggest that key lipid-regulatory mechanisms such as HMGCR inhibition and LDL receptor modulation are conserved across these models (Gardès et al., 2013). We first evaluated the changes in body weight and food intake following the administration of policosanol or orlistat (positive control) in HFD-induced C57BL/6 mouse model over a period of 12 weeks. As shown in Table 1, body weights in the policosanol-treated groups (Group 4: 36.8± 0.83 g; Group 5: 35.5±0.73 g; Group 6: 31.9±0.43 g) were significantly lower (***p<0.001) than those in the HFD control group (Group 2: 41.3±1.08 g) at the end of the study. Notably, the highest dose of policosanol (20 mg/kg body weight) showed effects comparable to those of the orlistat-treated positive control group (27.4±1.15 g, ***p<0.001). Feed consumption data for all treatment groups were monitored and compared with the pathological control group throughout the experimental period (Table 2). There were no significant differences in feed consumption patterns between treatment groups and the HFD control group (Group 2).
1) Group 1 (G1), normal control group; Group 2 (G2), HFD-induced group; Group 3 (G3), treated orlistat drug (10 mg/kg) in HFD-induced group; Group 4 (G4), treated policosanol (5 mg/kg) in HFD-induced group; Group 5 (G5), treated policosanol (10 mg/kg) in HFD-induced group; Group 6 (G6), treated policosanol (20 mg/kg) in HFD-induced group.
1) Group 1 (G1), normal control group; Group 2 (G2), HFD-induced group; Group 3 (G3), treated orlistat drug (10 mg/kg) in HFD-induced group; Group 4 (G4), treated policosanol (5 mg/kg) in HFD-induced group; Group 5 (G5), treated policosanol (10 mg/kg) in HFD-induced group; Group 6 (G6), treated policosanol (20 mg/kg) in HFD-induced group.
3) Normal control group (G1) showed no significant variation in food intake throughout the 12-week experimental period as compared to Week 1; NS, not significant (p>0.05).
Hypercholesterolemia is characterized by excessive cholesterol accumulation in the bloodstream, primarily resulting from dysregulated hepatic cholesterol metabolism. This condition is a major contributing factor to the development of arterial atherosclerosis and CVD (Song et al., 2021). Hyperlipidemia, driven by an imbalance between LDL and HDL, exacerbates hepatic dysfunction and elevates CVD (Longo et al., 2001). In the present study, we evaluated the effects of an 8-week administration of policosanol and orlistat on lipid profiles across multiple tissues, including plasma, liver, and arteries, with specific focus on total cholesterol, LDL cholesterol, and HDL cholesterol levels (Table 3 and 4).
1) Group 1 (G1), normal control group; Group 2 (G2), HFD-induced group; Group 3 (G3), treated orlistat drug (10 mg/kg) in HFD-induced group; Group 4 (G4), treated policosanol (5 mg/kg) in HFD-induced group; Group 5 (G5), treated policosanol (10 mg/kg) in HFD-induced group; Group 6 (G6), treated policosanol (20 mg/kg) in HFD-induced group.
1) Group 1 (G1), normal control group; Group 2 (G2), HFD-induced group; Group 3 (G3), treated orlistat drug (10 mg/kg) in HFD-induced group; Group 4 (G4), treated policosanol (5 mg/kg) in HFD-induced group; Group 5 (G5), treated policosanol (10 mg/kg) in HFD-induced group; Group 6 (G6), treated policosanol (20 mg/kg) in HFD-induced group.
Our results revealed that both policosanol- and orlistat-treated groups (Groups 3-6) exhibited slight reductions in liver (**p<0.01) and kidney weights, consistent with the observed trends in body weight reduction compared to the HFD-induced control group (Group 2). In addition, HDL-cholesterol levels significantly increased by over 1.5-fold (***p<0.001) in the plasma, liver, and arteries of mice treated with policosanol (Groups 4-6) and orlistat (Group 3), compared to the HFD group. In parallel, LDL-cholesterol levels, which are directly associated with cardiovascular risk, were markedly decreased by ca 1.5-fold (***p<0.001) in all treated groups compared to the HFD-induced controls (Table 3 and 4).
The cholesterol-lowering efficacy of policosanol in serum has been widely studied, though results vary depending on the source and composition of the formulation. In addition, non-Cuban formulations, including those derived from rice bran, have generally demonstrated modest elevations in HDL-cholesterol, with limited or inconsistent efficacy in lowering LDL-cholesterol and total cholesterol. Likewise, beeswax- and insect-derived policosanols have been shown to exert antioxidant effects, particularly through the reduction of oxidized LDL levels, yet current evidence remains insufficient to support a definitive role in modulating serum lipid profiles. (Cho et al., 2024; Uehara et al., 2024).
In contrast, our findings demonstrate that sugarcane-derived policosanol extracted using ethanol from Indian sources significantly and dose-dependently reduced serum total cholesterol and LDL-cholesterol levels. The lipid-lowering efficacy was comparable to orlistat and was accompanied by a beneficial rise in HDL-cholesterol. This enhanced efficacy may be attributed to differences in alcohol composition, extraction methodology, or improved bioavailability inherent to policosanol formulation.
Furthermore, the AI values were calculated, revealing significant improvements (***p<0.001) in the policosanol-treated groups compared with the HFD-induced group. Lower AI values imply a reduced risk of developing atherosclerosis. Previous studies have reported that policosanol supplementation can effectively lower LDL-cholesterol levels in both preclinical and clinical studies (Dulin et al., 2006; Nam et al., 2019; Singh et al., 2006). Thus, the present findings suggest that policosanol, purified from sugarcane ethanol extract, may exhibit similar LDL-cholesterol-lowering effects.
Considering the observed beneficial effects on body weight and cholesterol levels, we further evaluated the hepatic activity of HMGCR, a key enzyme regulating cholesterol biosynthesis. Interestingly, policosanol treatment (Group 4, 14.10±2.04 ng/mL, *p<0.05; Group 5, 13.60±2.51 ng/mL, **p<0.01; Group 6, 10.48±0.95 ng/mL, ***p<0.001) significantly reduced HMGCR activity in a dose-dependent manner, comparable to the effects observed in the orlistat-treated group (Group 3, 10.89±2.75 ng/mL, ***p<0.001) (Table 3). These findings corroborate previously reported mechanisms of policosanol action, such as HMGCR inhibition via AMPK activation (Nam et al., 2019). However, our results extend these findings by evaluating lipid modulation in both hepatic and arterial tissues and identifying significant modulation of HDL and LDL subfractions, which were not explored in earlier studies.
Interestingly, this contrasts with the findings of Singh et al. (2006), who reported that neither sugarcane-derived policosanol nor its major component triacontanol directly inhibited HMGCR in vitro (Singh et al., 2006). This discrepancy may stem from differences in the composition of long-chain aliphatic alcohols such as higher octacosanol content as well as variations in source material (e.g., Cuban vs. Indian sugarcane) and extraction techniques. In our study, Indian sugarcane-derived policosanol, extracted using ethanol, demonstrated potent in vivo suppression of HMGCR activity and downstream cholesterol biosynthesis. It is also plausible that Indian sugarcane-derived policosanol exerts its effects indirectly by modulating upstream regulators such as SREBP2 or AMPK. These findings highlight the possibility that the biological efficacy of policosanol may be influenced by its botanical origin and processing methods, which warrants further comparative studies.
Cholesterol elimination from the body primarily occurs via gastrointestinal and dermal excretion or through its metabolic conversion into various bioactive compounds, including bile acids and steroid hormones (Wang et al., 2017). To determine whether policosanol enhances cholesterol disposal, we measured fecal total cholesterol and bile acid levels in mice following 8 weeks of treatment. As shown in Fig. 1, policosanol-treated groups exhibited significant (***p<0.001) increases in fecal total cholesterol (Group 4, 295.55±3.12 g/kg; Group 5, 316.43±1.25 g/kg; Group 6, 385.76±9.72 g/kg) and bile acid levels (Group 4, 6.97±0.08 g/kg; Group 5, 7.62±0.14 g/kg; Group 6, 12.61±0.14 g/kg) compared to the HFD-induced control group (total cholesterol, 244.31± 7.45 g/kg; bile acid, 6.41±0.10 g/kg). It suggests that policosanol enhances fecal excretion of cholesterol and bile acids, potentially contributing to the reduction of LDL-cholesterol levels in serum and liver.
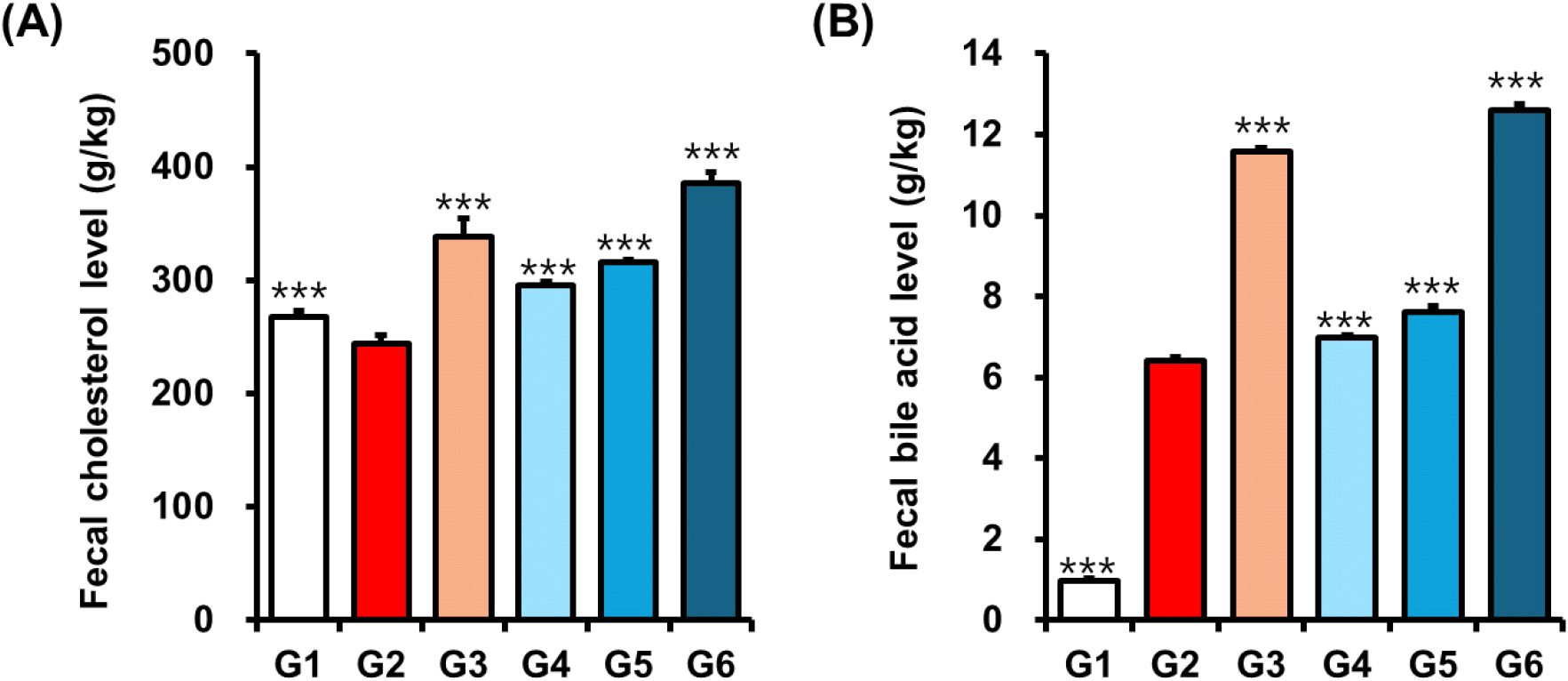
In a study conducted by Ng et al. (2005), policosanol supplementation in hamsters resulted in a significant increase in the excretion of acidic sterols, ranging from 25% to 73% (Ng et al., 2005). This finding indicates that policosanol may contribute to cholesterol reduction by enhancing the elimination of bile acids, thereby potentially decreasing intestinal cholesterol absorption and increasing fecal cholesterol excretion. The study also observed reductions in serum total cholesterol levels by 15% to 25% and elevations in HDL cholesterol by 7% to 16.8% in policosanol-treated hamsters (Ng et al., 2005). These results indicate that the cholesterol-lowering effects of policosanol could be partially mediated through its influence on bile acid metabolism and fecal sterol excretion.
To further elucidate the mechanisms responsible for the cholesterol-lowering effects of policosanol, we investigated its impact on the hepatic cholesterol biosynthetic pathway, with a particular focus on the SREBP2 signaling as a central transcriptional regulator of lipid metabolism. SREBP2 plays a pivotal role in activating the transcription of genes involved in the mevalonate pathway, including HMGCR and FDFT1 (Sharpe and Brown, 2013). These two enzymes are essential for the synthesis of cholesterol, sterols, and other isoprenoid derivatives (Madison, 2016; Sharpe and Brown, 2013). Through the regulation of this pathway, SREBP2 serves as a key homeostatic controller of intracellular cholesterol levels in response to metabolic demands and plays a crucial role in maintaining lipid equilibrium.
Our data showed that the HFD-induced control group (Group 2) exhibited elevated hepatic levels of SREBP2 (8.54 ±2.08 ng/mL), HMGCR (17.04±1.05 ng/mL), and FDFT1 (4.63±0.58 ng/mL), indicating activation of cholesterol biosynthesis. In contrast, mice treated with policosanol (Group 6; 20 mg/kg) showed a significant reduction in the expression of these key regulators: SREBP2 (4.63±0.58 ng/mL, ***p<0.001) and FDFT1 (1.17±0.36 ng/mL, ***p<0.001) (Fig. 2A and 2B), and HMGCR (10.48±0.95 ng/mL, ***p< 0.001) (Table 3) levels comparable to those in the orlistat-treated group. These findings suggest that policosanol may suppress cholesterol biosynthesis through downregulation of the SREBP2-mediated transcriptional cascade. Notably, this in vivo evidence contrasts with Singh et al. (2006), who reported that neither sugarcane-derived policosanol nor its major component triacontanol directly inhibited HMGCR in vitro (Singh et al., 2006). This inconsistency likely reflects differences in experimental models, bioavailability, or the composition of long-chain aliphatic alcohols, as in vivo regulation involves complex upstream modulators such as AMPK and SREBP2 that may not be fully represented or active in in vitro assay.
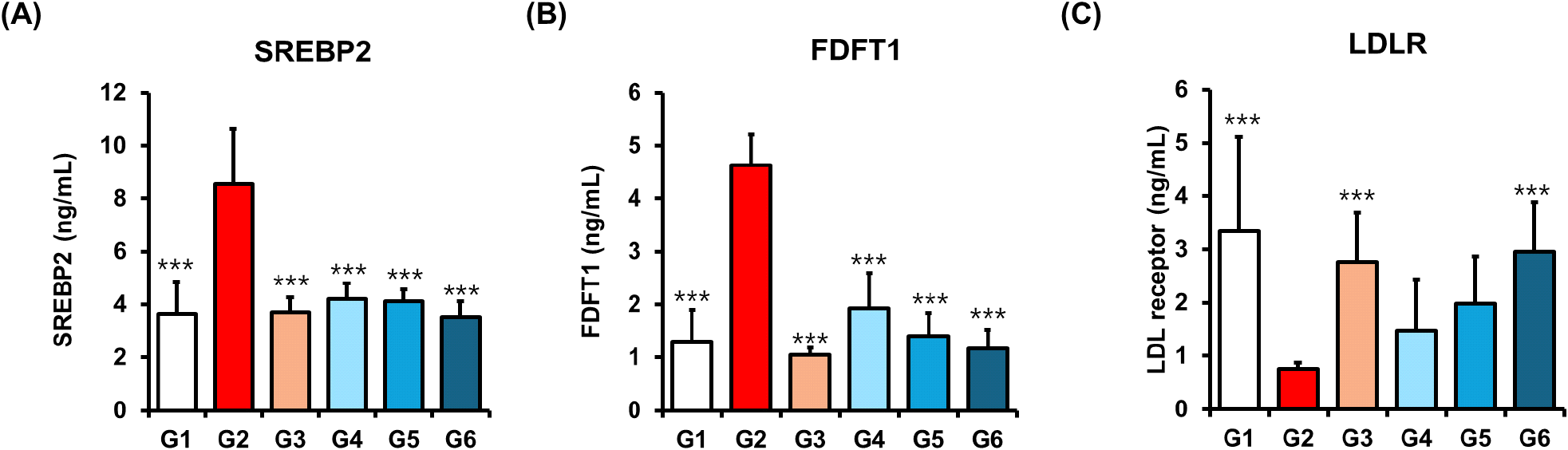
The LDLR is a transmembrane glycoprotein predominantly expressed in hepatocytes, where it plays a central role in cholesterol homeostasis. By binding circulating LDL particles, LDLR mediates their internalization and lysosomal degradation, thereby reducing plasma LDL-cholesterol levels (Goldstein and Brown, 2009; Wang et al., 2017). Its transcription is tightly regulated by SREBP2, which is activated under conditions of intracellular cholesterol depletion. In our study, policosanol significantly enhanced the expression of LDLR in a dose-dependent manner, with levels increasing from 1.48 ±0.96 ng/mL in Group 4 to 2.95±0.93 ng/mL in Group 6 (Fig. 2C). This dose-dependent upregulation of LDLR suggests improved hepatic LDL uptake, consistent with findings by Zhang et al. (Zhang et al., 2012). While previous studies have reported on policosanol-induced LDLR upregulation (Nam et al., 2019), our study provides the first quantitative in vivo confirmation of simultaneous suppression of upstream SREBP2-FDFT1 signaling along with LDLR activation, offering new mechanistic insight.
In comparison with previous studies, Cuban sugarcane-derived policosanol as the most extensively investigated formulation to date has yielded inconsistent outcomes across studies. Initial clinical trials conducted in Cuba reported substantial reductions in total and LDL-cholesterol levels, potentially attributable to mechanisms involving enhanced LDLR activity. However, these early studies lacked clear mechanistic evidence directly linking policosanol to LDLR upregulation (Berthold et al., 2006). Rice bran-derived policosanol has demonstrated modest increases in HDL-C with limited effects on LDL-C and minimal evidence of LDLR involvement. Additionally, beeswax- and insect-derived policosanols exhibit antioxidant activity, particularly against oxidized LDL, but their effects on LDLR expression or cholesterol regulation remain poorly characterized (Cho et al., 2024; Uehara et al., 2024). These alternative sources may act more through preventive antioxidant mechanisms rather than direct modulation of lipid metabolism.
ApoA-1 and ApoB are critical biomarkers associated with the progression of CVD, particularly in the context of obesity and dyslipidemia (Walldius et al., 2021). ApoB is essential for the assembly and transport of LDL particles, facilitating the delivery of cholesterol and triglycerides from the liver to peripheral tissues (Haas et al., 2013), whereas ApoA-1, the major structural protein of HDL, plays an anti-atherogenic role by promoting reverse cholesterol transport (Bhale and Venkataraman, 2022). In our study, policosanol treatment significantly modulated these apolipoproteins. ApoA-1 levels were increased in a dose-dependent manner (Group 4: 49.12 ±8.35; Group 5: 52.57±11.15, *p<0.05; Group 6: 57.46± 11.31 ng/mL, ***p<0.001), while ApoB levels were concurrently reduced (Group 4: 343.94±100.29, **p<0.01; Group 5: 332.06 ±34.15, ***p<0.001; Group 6: 316.91±53.18 ng/mL, ***p<0.001) compared to the HFD-induced control (ApoA-1: 37.63±6.34 ng/mL; ApoB: 566.13±74.13 ng/mL) (Fig. 3A and 3B). These results suggest a shift toward a favorable lipoprotein profile. Notably, earlier clinical studies using rice-derived policosanol reported an increased ApoA-1 without significantly affecting ApoB levels (Reiner et al., 2005), indicating that policosanol may exert more comprehensive lipid-modulating effects.
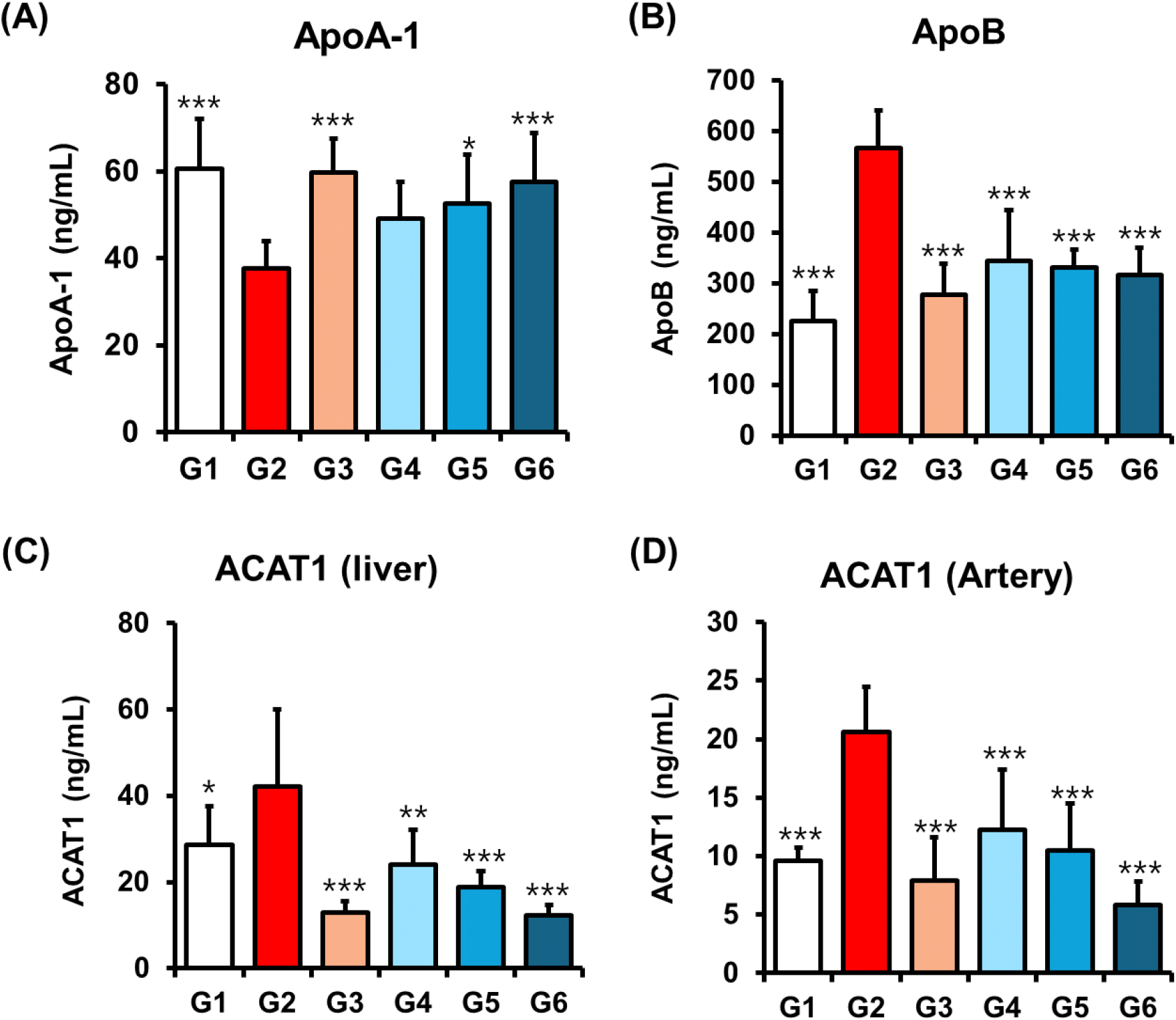
To explore the molecular basis of ApoB regulation, we assessed hepatic expression of ACAT1, an enzyme involved in the esterification and storage of intracellular cholesterol and the formation of ApoB-containing lipoproteins (Dove et al., 2005; Spady et al., 2000). Policosanol treatment significantly reduced hepatic ACAT1 levels in a dose-dependent manner (Group 4: 23.99±8.23, **p<0.01; Group 5: 18.82±3.65, ***p< 0.001; Group 6: 12.28±2.50 ng/mL, ***p<0.001), with reductions comparable to orlistat (12.92±2.64 ng/mL) (Fig. 3C). Additionally, ACAT1 expression was also markedly decreased in arterial tissues (Group 4: 12.26±5.14, ***p<0.001; Group 5: 10.50± 4.00, ***p<0.001; Group 6: 5.86±2.01 ng/mL, ***p<0.001) (Fig. 3D), supporting a potential role in vascular protection. Given that ACAT1 activity in arterial smooth muscle cells contributes to cholesterol accumulation and atherogenesis (Yamazaki et al., 2019), suggesting that policosanol may also offer protective effects against the development of atherosclerotic lesions. Collectively, these findings suggest that policosanol not only improves the apolipoprotein profile by increasing ApoA-1 and decreasing ApoB, but also exerts dual lipid-lowering and atheroprotective effects through the suppression of ACAT1 expression in both hepatic and arterial tissues. Importantly, our study extends previous findings by demonstrating, for the first time to our knowledge, the downregulation of ACAT1 in arterial tissue by policosanol highlighting a previously unrecognized mechanism through which it may confer vascular protection.
While Cuban-derived policosanol has shown variable results in modulating apolipoprotein levels in prior reports (Cho et al., 2023; Cho et al., 2024; Uehara et al., 2024), Indian sugarcane-derived policosanol significantly increased ApoA-1 and decreased ApoB levels, improving the atherogenic profile in HFD-fed mice. Furthermore, the suppression of ACAT1 in both hepatic and arterial tissues suggests that Indian sugarcane-derived policosanol not only impacts circulating lipoproteins but also influences intracellular cholesterol esterification and foam cell formation. This dual effect on apolipoproteins and arterial cholesterol metabolism may reflect compositional or structural differences unique to Indian sugarcane-derived policosanol, warranting further comparative analyses between sources.
In the present study, we observed that policosanol treatment (Groups 4-6) significantly reduced hepatic HMGCR activity compared to the HFD-induced control group (Group 2) (Table 4), indicating its potential to counteract hypercholesterolemia by suppressing key enzymes in cholesterol metabolism. We further investigated the effect of policosanol on two critical regulators of lipoprotein remodeling as CETP and LCAT. CETP facilitates the exchange of cholesteryl esters between HDL and ApoB-containing lipoproteins, thereby influencing HDL composition and function (Zhou et al., 2006). Elevated CETP activity is associated with decreased HDL-C and increased CVD risk. Accordingly, CETP inhibitors have emerged as promising targets in CVD therapy (Schmidt et al., 2021).
Our results demonstrated that policosanol significantly decreased CETP levels in a dose-dependent manner (Group 4: 2.80±0.26 ng/mL, ***p<0.001; Group 5, ***p<0.001: 2.48± 0.33 ng/mL; Group 6: 1.90±0.17 ng/mL, ***p<0.001), with reductions comparable to the orlistat-treated group (1.89± 0.17 ng/mL, ***p<0.001) (Fig. 4A). This suggests that policosanol may help preserve HDL function and reduce the transfer of cholesteryl esters to LDL and VLDL particles. Conversely, LCAT plays a central role in reverse cholesterol transport by esterifying free cholesterol on HDL particles, promoting HDL maturation and facilitating cholesterol clearance from peripheral tissues to the liver (Khovidhunkit et al., 2001). policosanol significantly increased serum LCAT levels across all treatment groups in a dose-dependent manner (Group 4: 1.38±0.65 ng/mL, **p<0.01; Group 5: 1.86±0.52 ng/mL, ***p< 0.001; Group 6: 2.46±0.57 ng/mL, ***p<0.001) compared to the HFD group (0.56±0.30 ng/mL) (Fig. 4B). Taken together, these results indicate that policosanol exerts dual regulatory effects by reducing CETP and enhancing LCAT activity, thereby promoting HDL functionality, inhibiting the redistribution of cholesterol to atherogenic lipoproteins, and potentially lowering CVD risk through improved cholesterol transport dynamics.
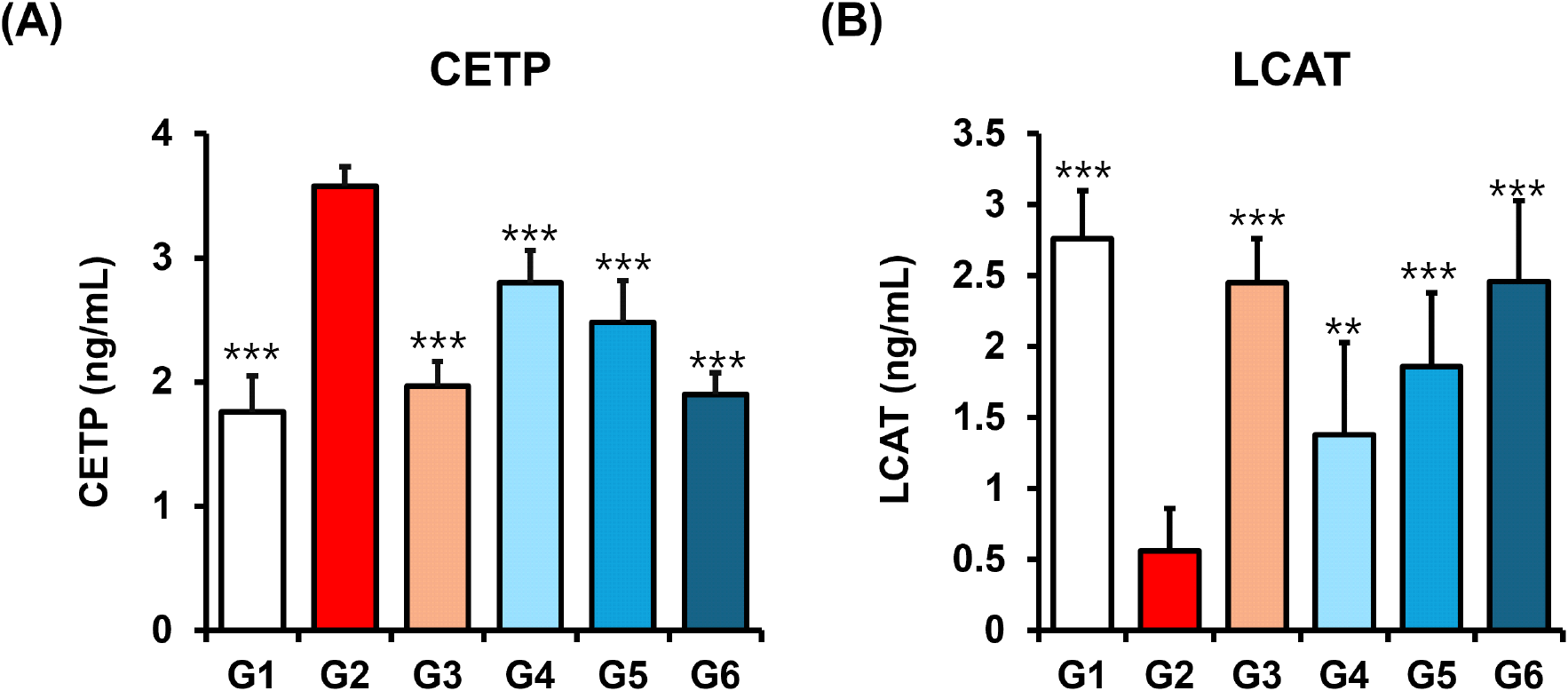
Previous studies indicate that preclinical and clinical evidence regarding the modulation of CETP and LCAT activity by alternative policosanol sources including Cuban sugarcane, rice bran, and beeswax remains limited and inconclusive (Berthold et al., 2006; Cho et al., 2024; Uehara et al., 2024). While Cuban sugarcane-derived policosanol has been reported to elevate HDL-C levels in certain studies, direct mechanistic evidence supporting CETP inhibition is lacking. Similarly, rice bran- and beeswax-derived policosanols have not been consistently shown to modulate CETP activity, as most investigations have focused on general lipid outcomes rather than CETP-specific pathways. Although Cuban policosanol has demonstrated HDL-enhancing effects, LCAT activity has rarely been assessed, leaving its role in reverse cholesterol transport mechanisms unclear. Rice- and insect-derived policosanols have shown modest improvements in HDL-C, yet robust data confirming their ability to upregulate LCAT are still unavailable. As prior studies have not clearly elucidated the underlying mechanisms, we hypothesized that policosanol, owing to its distinct long-chain alcohol composition and ethanol-based extraction process, would exhibit more consistent and potent modulation of CETP and LCAT activity, thereby enhancing HDL functionality and promoting improved lipid homeostasis.
To date, few studies have evaluated the impact of policosanol on HDL remodeling proteins such as CETP and LCAT (Berthold et al., 2006; Cho et al., 2024; Uehara et al., 2024). In this study, Indian sugarcane-derived policosanol significantly decreased CETP expression while increasing LCAT activity, suggesting an improvement in HDL functionality and reverse cholesterol transport. These findings contrast with earlier reports on policosanol from rice or beeswax sources, where such effects were inconsistent or absent. This difference may be attributed to the higher proportion of functionally active long-chain alcohols in Indian sugarcane-derived policosanol or the specificity of its sugarcane origin. These observations highlight the importance of source and formulation in determining the functional spectrum of policosanol and reinforcing the potential of our policosanol as a distinct bioactive agent.
Hypercholesterolemia is strongly associated with elevated levels of oxLDL, a well-established contributor to the initiation and progression of atherosclerosis (Prasad and Mishra, 2022). Previous studies have shown that policosanols derived from rice, oats, insects, and beeswax can inhibit oxLDL formation (Cho et al., 2023). Consistent with these findings, policosanol treatment significantly reduced oxLDL levels in a dose-dependent manner (Group 4: 30.51±10.98 nM/mL, *p<0.05; Group 5: 26.35±5.55 nM/mL, **p<0.01; Group 6: 12.41±2.66 nM/mL, ***p<0.001) compared to the HFD-induced group (Group 2: 43.06±13.06 nM/mL) (Fig. 5A). These results indicate that policosanol exhibits antioxidant and cholesterol-lowering activity comparable to other policosanol sources, effectively attenuating lipid peroxidation in hypercholesterolemic conditions (Ng et al., 2005).
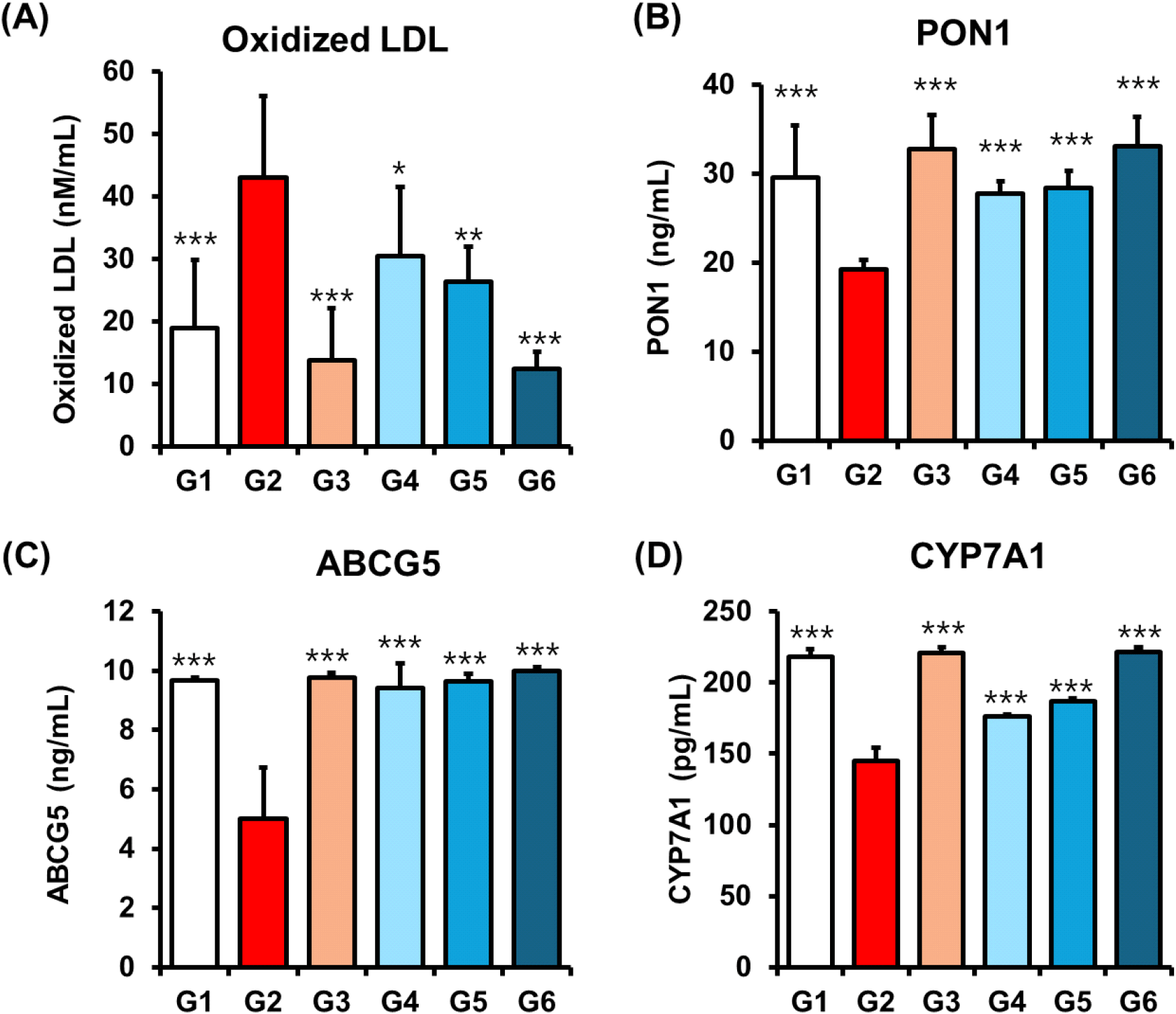
Given the role of oxidized LDL as a direct trigger of atherogenesis, we further investigated the impact of policosanol on PON1 as a key HDL-associated enzyme known to prevent lipid peroxidation (Durrington et al., 2023). Policosanol treatment significantly increased serum PON1 levels in a dose-dependent fashion (Group 4: 27.80±1.31 ng/mL, ***p< 0.001; Group 5: 23.38±1.91 ng/mL, ***p<0.001; Group 6: 33.02±3.33 ng/mL, ***p<0.001), exceeding the effect observed with orlistat (19.18±1.10 ng/mL) (Fig. 5B). These data suggest that policosanol indirectly protects against LDL oxidation by upregulating PON1, thereby contributing to its anti-atherogenic profile.
To further explore cholesterol metabolism, we assessed hepatic expression of ABCG5 and CYP7A1. ABCG5 facilitates cholesterol efflux from hepatocytes into bile, while CYP7A1 catalyzes the rate-limiting step in bile acid synthesis, thus promoting cholesterol elimination (Li et al., 2013; Yu et al., 2002). Policosanol treatment significantly elevated both ABCG5 (Group 4: 9.43±0.82, ***p<0.001; Group 5, ***p<0.001: 9.63±0.26; Group 6, ***p<0.001: 9.99± 0.14 ng/mL) and CYP7A1 (Group 4: 176.10±1.31, ***p< 0.001; Group 5: 186.68±1.91, ***p<0.001; Group 6: 221.32± 3.33 ng/mL, ***p<0.001) expression levels, mirroring the effects seen with orlistat (ABCG5: 9.78±0.16 ng/mL, ***p< 0.001; CYP7A1: 221.02±3.85 ng/mL, ***p<0.001) (Fig. 5C and 5D). Previous reports have shown that SREBP2 suppression promotes ABCG5-mediated biliary cholesterol secretion (Yu et al., 2002). Our findings are consistent with this proposed mechanism, suggesting that policosanol enhances cholesterol elimination through the coordinated upregulation of ABCG5 and CYP7A1, thereby contributing significantly to its overall cholesterol-lowering efficacy.
Interestingly, no other policosanol formulations have clearly demonstrated the coordinated upregulation of ABCG5, CYP7A1, and PON1 as observed with policosanol. While Cuban policosanol has shown lipid-lowering effects in some clinical trials, mechanistic data on bile acid metabolism or cholesterol efflux pathways are lacking (Berthold et al., 2006). Similarly, rice bran- and beeswax-derived policosanols have been associated with modest HDL-C improvements or antioxidant properties, such as reduced oxidized LDL, but robust evidence of their effects on cholesterol excretion markers remains limited (Cho et al., 2024; Uehara et al., 2024). While our study reaffirms well-established mechanisms of policosanol, such as the inhibition of HMGCR and upregulation of LDLR, it uniquely reveals additional regulatory effects on key lipid metabolism and antioxidant pathways including CETP, LCAT, ACAT1, PON1, ABCG5, and CYP7A1 which have not been previously reported in studies of other policosanol sources.
4. Conclusions
In summary, this study demonstrates that policosanol (Reduchole22®), a sugarcane-derived formulation, exerts multi-targeted lipid-lowering effects in a high-fat diet-induced mouse model. Policosanol significantly suppressed cholesterol biosynthesis by inhibiting HMGCR and promoted cholesterol elimination through upregulation of CYP7A1 and ABCG5, enhancing bile acid synthesis and biliary cholesterol excretion. It also improved LDL-cholesterol clearance by upregulating LDLR expression. Importantly, beyond these previously established mechanisms, policosanol uniquely modulated additional lipid and antioxidant regulators including suppression of ACAT1 and CETP, and upregulation of LCAT and PON1 leading to improved HDL functionality and reduced levels of oxidized LDL. These findings distinguish policosanol from previously studied policosanol sources and suggest that it not only reduces cholesterol synthesis and absorption but also enhances reverse cholesterol transport and vascular antioxidant defense. We acknowledge that further mechanistic studies are needed to confirm downstream signaling pathways; however, our findings suggest that policosanol offers broader cardiometabolic benefits than previously studied policosanol formulations. It may serve as a promising nutraceutical for the dietary management of hypercholesterolemia and cardiovascular risk.










