1. Introduction
Oyster mushrooms (Pleurotus ostreatus), which belong to the family Pleurotaceae and class Agaricomycetes, are among the most cultivated species in many countries in the world due to their taste, flavor, high nutritional values, and medicinal properties (Deepalakshmi and Mirunalini, 2014). Their protein content is higher than in other foods, and they contain all nine essential amino acids required by humans, enabling their use as a substitute for a meat diet (Kakon et al., 2012). Due to its ease in cultivation and short growth time, this agricultural commodity is the most preferable option as a strategy of economic empowerment for poor farmers in many rural communities in Indonesia (Istikhoroh et al., 2018).
Reports indicate that cultivated oyster mushrooms in various regions were affected by microbial contamination coming from the surroundings and the growing media (Suada et al., 2015). As reported by BPS Statistics Indonesia, the national production of oyster mushrooms in 2022 reached 63.15 tonnes, reflecting a decrease of approximately 30.15% compared to the 90.42 tonnes produced in 2021. The decrease can be attributed to the presence of some bacteria strains such as Pseudomonas fluorescens, Erwinia carotovora, Bacillus sp., and Clostridium (Yuniarta et al., 2022). This matches what Mu et al. (2015) found and was also supported by later research from Schill et al. (2021), which showed that the most common type in all mushroom samples was the Pseudomonas fluorescens species complex, making up 58.4%.
Post-harvest oyster mushrooms only stand in fresh condition for 3-5 days before turning yellow (Lo Cantore and Iacobellis, 2014) and slimy, making them unsellable and causing financial losses for farmers. This finding is consistent with the research of Wang et al. (2017), who reported that oyster mushrooms can maintain their commercial quality for 1-3 days at room temperature or 4-7 days at 4°C. It is therefore necessary to treat the harvested oyster mushroom to prolong its shelf life so the product can still be in a fresh condition in consumers’ hands.
Post-harvest management has a tremendous role in assuring the quality and safety of agricultural products (Aier et al, 2024; Kader and Rolle, 2004), particularly for the perishable ones, like fruit and vegetables, that have a short shelf life (Elhadi, 2019). Disinfection of products is one of the crucial steps in handling agricultural products (Siddiqui, 2018). There are many disinfection techniques applied in handling agricultural products, ranging from chemical to physical ones (Deng et al., 2020).
Among the hurdle technologies developed (Singh and Shalini, 2016), the one using UV light to inactivate pathogenic bacteria in fresh agricultural products showed increasing potential as an alternative nonthermal technology for food processing (Guerrero et al., 2021). Because the UV-C light overlaps with the peak of maximum DNA absorption, exposing products to this light causes absorbed photons to stimulate several photochemical reactions that lead to microbial inactivation. The pyrimidine dimers are then formed in the DNA and RNA of the pathogenic microorganisms, which will impede their cell replication process (Gayán et al., 2014).
It was reported in previous works that many treatment strategies have been implemented to extend the shelf life of oyster mushrooms; among others are modified atmosphere packaging (MAP) and active packaging (Han Lyn et al., 2020), edible coating (Samadpour and Behesti, 2020), vacuum cooling (Tao et al., 2007), chemical treatments (Olotu et al., 2015), and ozone treatment (Anjaly et al., 2020). Even if all the above-mentioned can prolong the shelf life, they also provide some drawbacks. So a more powerful pre-treatment system which is low-cost (in either equipment or its maintenance as well as its energy consumption), fast and environmentally friendly, is needed. A UV-C radiation-based pre-treatment is then considered to be the potential candidate due to the above-mentioned favorable advantages, in addition to the benefits of minimal processing efforts, enhanced commercial and nutritional values, undegraded sensory quality, and the removal of microbial contamination and spoilage (Islam et al., 2016).
An innovative technology involving post-harvest disinfection of oyster mushrooms to extend their shelf life and prevent rapid spoilage represents an ideal solution. According to Wang et al. (2017), ultraviolet C (UV-C) radiation at a dose of 4 kJ/m2 can effectively extend the storage life of oyster mushrooms. This radiation dose reduces the growth of bacteria that cause spoilage, lowers the number of microbes, and enhances the activity of the catalase enzyme, which breaks down hydrogen peroxide into oxygen and water. It also raises the levels of phenylalanine ammonia-lyase (PAL), which helps to protect against harmful pathogens. Consequently, the mushrooms remain fresh longer and are less prone to discoloration from spoilage, since UV-C radiation induces mutations in nitrogenous base chains of microbial RNA and DNA, leading to microbial mortality (Sánchez-Navarrete et al., 2020).
Therefore, it is necessary to develop a disinfection system for farmers to extend the post-harvest shelf life of oyster mushrooms. This paper describes efforts to design, calibrate, and test a low-cost automated UV-C radiation-based disinfection system for decontaminating harvested oyster mushrooms from rural area farmers.
2. Materials and methods
A low-cost and easy-to-operate UV-C radiation-based device for sterilizing oyster mushrooms with fuzzy logic is proposed in this study. The fuzzy logic method automates the activation of the UV-C lamps based on inputs such as temperature, weight, and humidity. This system works by automating the lamp switches to provide radiation tailored to the sterilizing needs of the mushrooms. UV-C radiation sterilizes bacteria and enhances enzymatic activity in the mushrooms, resulting in prolonged shelf life. The primary objectives of this study are to analyze the effectiveness of fuzzy logic in a UV-C-based sterilization device for reducing bacteria that cause spoilage in oyster mushrooms and also to evaluate the differences in morphology and texture of oyster mushrooms after exposure to UV-C radiation to improve their shelf life.
The design for the proposed UV-C disinfection system for post-harvest oyster mushrooms were based on the National Indonesian Standard (SNI 7388:2009, 2009). This standard specifies that the maximum microbial contamination for dried vegetables, including oyster mushrooms, is 105 colony-forming units per gram (CFU/g) or 2 log reduction, as determined using the total plate count (TPC) method. The device was calibrated to deliver suitable doses to achieve a certain log of bacterial inactivation (Sun et al., 2023). The device ensures optimal radiation exposure with a minimum duration of 20 sec to effectively reduce bacterial contamination, particularly Pseudomonas fluorescens (Zekanović et al., 2022).
The proposed UV-C disinfection system was constructed in a rectangular box form, which was constructed from 2×2 hollow steel with an overall dimension of 1×1×2.378, with the walls made from thin aluminum sheets (1.5-2 mm thickness). The interior of the box is divided equally into two rooms, which are separated by a wire mesh located 1.188 meters above the bottom. On this wire mesh, the oyster mushroom samples will be placed for disinfection. Two UV-C TL lamps (30 watts) were installed on the bottom, and the other two lamps on the top, and they were arranged in such a way as to expose the oyster mushroom samples from below. On top of the box, an exhaust fan is installed to flow out the evaporated moisture from the samples during the exposure.
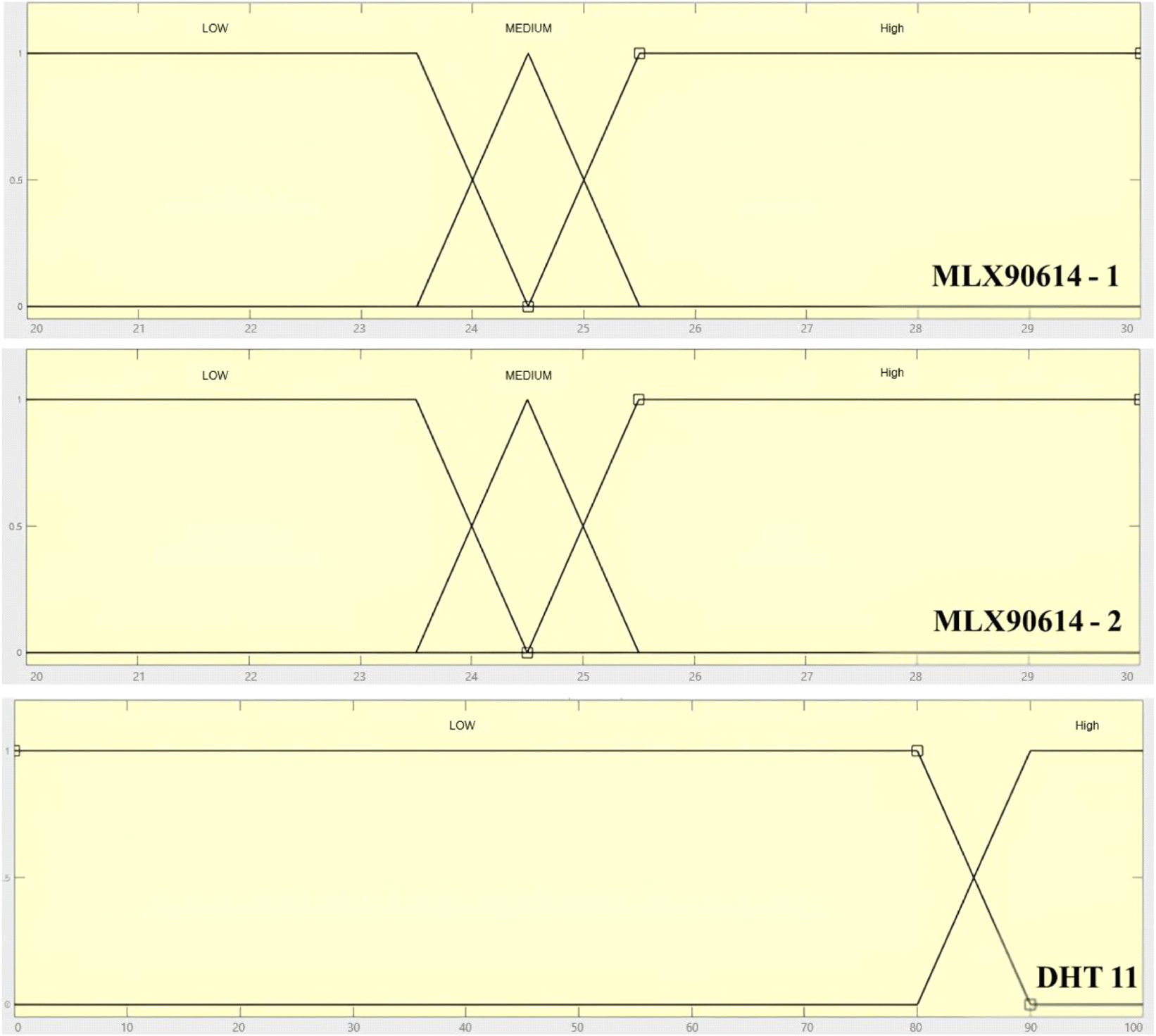
To automate the exposure time, two temperature sensors, MLX90614, and one humidity sensor, DHT11, were installed at the locations above the wire mesh plane in the box, which will feed their readings to a Raspberry Pi 3B+ microcontroller. An embedded Mamdani fuzzy logic (Iancu, 2012) algorithm will process these temperature and humidity inputs to provide an automated exposure time to the UV-C TL lamps, which acts as an intelligent disinfection system. In order to ensure the accuracy and precision level of the sensors, they were carefully calibrated using an IR thermogun and hygrometer for temperature and humidity sensors, respectively. Each of the measurements was made in three repetitions to ensure highly accurate and precise measurements. Fig. 1 shows a sketch of the proposed disinfection room along with the system’s wiring diagram.
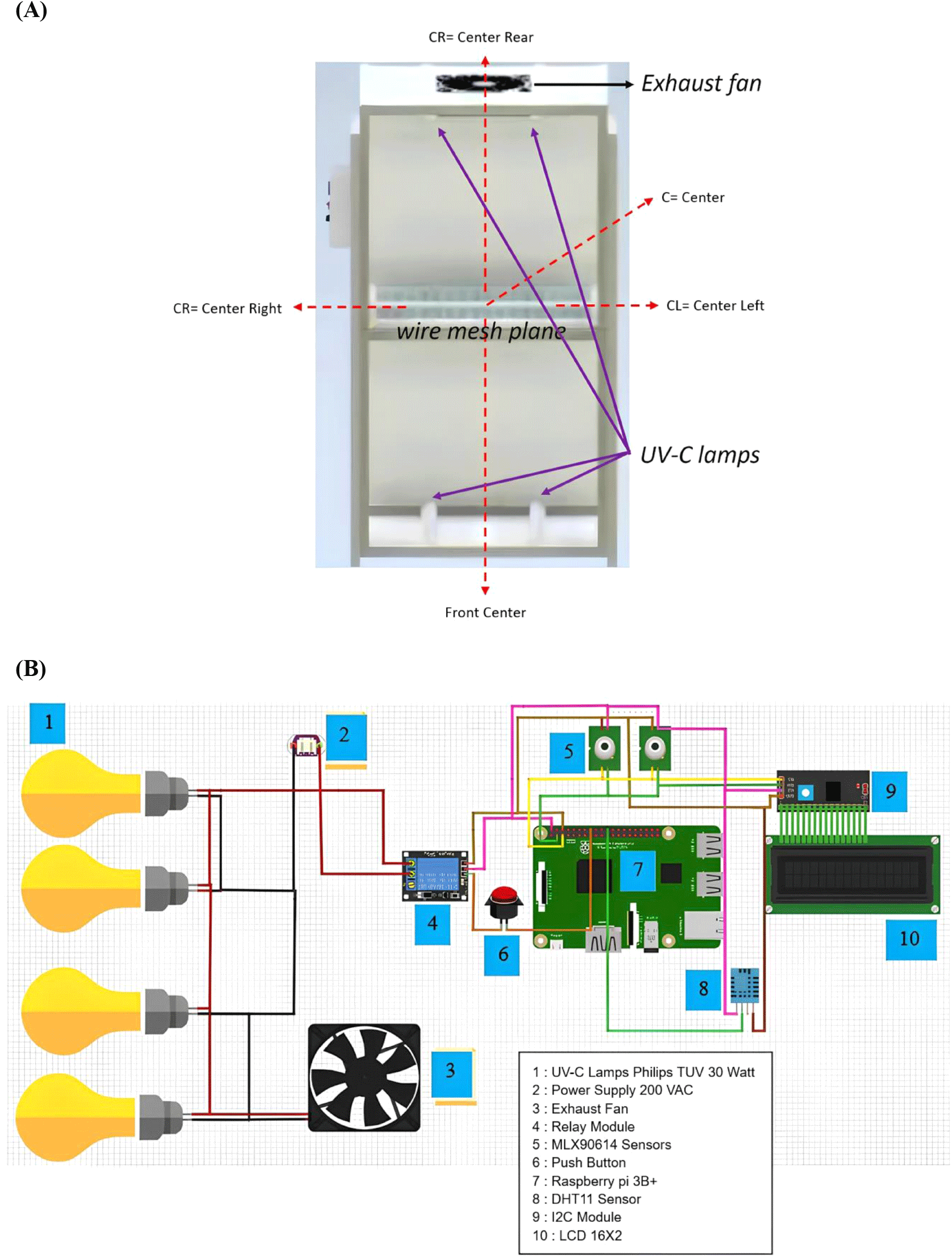
The uniformity of the UV-C exposure through the wire-mesh plane (the samples’ location) was evaluated using the YK-37UVSD Lutron UV Light Meter with its UV-C sensor’s head. The intensity (in W/cm2) at five locations on the wire-mesh plane was measured, each with three repetitions, to ensure precise exposure across the plane. These measurements were important to determine a precise exposure time to provide a consistent dose of about 70 J/m2 or an effective microbial reduction.
The data obtained from TPC analysis demonstrated a significant reduction in the Pseudomonas fluorescens species complex following UV-C exposure. These colonies were isolated using nutrient agar supplemented with chloramphenicol, which selectively inhibits Gram-positive bacteria, allowing for the enrichment of Gram-negative organisms such as Pseudomonas spp. Colonies exhibiting mucoid texture, irregular edges, and greenish pigmentation were tentatively identified as members of the P. fluorescens species complex, consistent with previous studies on mushroom spoilage flora.
The bacterial reduction from the irradiated samples with several disinfection times was calculated using the total plate count (TCP) method and will be compared to the control samples, i.e., the unirradiated oyster mushroom samples. Nutrient agar (NA) for bacterial growing media were prepared following controlled standard procedures as normally used in microbiology laboratory of the university. The prepared growing media were then being poured into petri dishes and kept to solidify.
Moist filter papers, i.e., after dipping them into aquadest, were affixed to the fresh samples and kept for 1 min before being attached to the growing media in 30 petri dishes for further cultivation. The cultivated bacterial samples were then divided into 5 groups, i.e., one group for the samples that were unexposed to UV-C radiation. Meanwhile, the other 4 groups were exposed to the UV-C radiation at four different location, i.e., labeled with position TR (=TopRight), TL (=TopLeft), BR (=BottomRight), BL (=BottomLeft), all under different exposure times of 20, 30, 40, and 50 sec. The forming of bacterial colonies in these growing media was then observed under a microscope and counted using a counting chamber every 24 h for three consecutive days. For the calculation of bacterial colonies cultivated, the area of petri dishes was divided into four quadrants by marking two perpendicular diameter lines on the bottom surface of petri dishes, and calculations were repeated for every three petri dishes for each cluster of samples. The average reduction log for each treatment was calculated by dividing the average number of colonies for each treatment by the one calculated from the control unexposed samples.
For testing the designed system, 5 kg samples of oyster mushrooms were collected from the local farmer. For each of the exposure sessions, thirty pieces of mushrooms were evenly arranged on the wire-mesh plane for irradiation. Overexposure to UV-C light was avoided to prevent structural damage to the mushrooms, as excessive radiation can harm mushroom cells (Feng et al., 2023).
The number of bacterial reduction was assessed using the TPC method and was benchmarked to the SNI 7388:2009, 2009, which specifies a maximum contamination level of 105 CFU/g. Shelf-life tests involved storing irradiated mushrooms under two conditions: refrigerated storage at a temperature of 3°C and ambient storage at temperature of 25°C using oxygen-permeable plastic packaging. These packaging methods were selected for their cost-effectiveness and suitability to the farmers’ practices. Meanwhile, vacuum packaging necessitates specialized equipment and may cause fragility in the mushrooms (Anderson et al., 2021). The morphological quality of mushrooms, including color, wilting, and slime formation, was then being evaluated to determine the extension of shelf life for the irradiated samples in comparison to the control (i.e., unirradiated samples).
Disinfection will start after all samples are evenly arranged and the door is closed. Turning on the switch button will initiate the measurement preparation, and the LCD will light up. Pressing the push button will start the disinfection process, turn ON the two UV-C lamps, and activate the temperature and humidity sensors to sense the temperature on the surface of samples and the humidity inside the disinfection chamber, respectively, and feed these measurements to the Raspberry Pi 3B+ microcontroller. The fuzzification (based on the Mamdani method) was then started, converting sensors’ inputs into a fuzzy set and executing the inference process with the designed rule base. Output results will change the ON or OFF commands and their duration for the exhaust fan and the four UV-C lamps. A disinfection time in the range of 20-40 sec is generally achieved for bacterial reduction of 2 logs, and for the test, the disinfection times of 20, 30, 40, and 50 sec were set for irradiating samples. Completing the disinfection process, the door can be opened, and the samples can be taken out for further handling, i.e., packaging and storage. The rule-based fuzzy inference used is tabulated in Table 1 and schematically depicted in Fig. 2.
Color is one of the object’s properties that will be transformed during the imaging process. In digital images, the color of an object can be quantified into color spaces. In color image processing, several color spaces can be used to quantify the color properties of an imaged object (Velastegui et al., 2021), among others are RGB and CIE L*, a*, b*. These two color spaces will be used to quantify the color changes in oyster mushrooms during the different preservation modes. It can be represented by the parameter of total color difference, which can be calculated from the CIE L*, a*, b* color spaces, as given in the following equation (Engin, 2020).
where ΔL*, Δa*, and Δb* are the difference in respective CIE L*, a*, b* color spaces between two sample’s images being compared.
To observe the samples’ shelf life, the UV-C irradiated oyster mushroom samples were stored after disinfection in either A. the products’ shelf under room temperature or B. in a refrigerator. Both of the clusters of irradiated samples were then compared to the similar cluster of unirradiated samples. These samples were observed day by day, and the shelf life will be determined with reference to the observation days where the onset of changes in appearance and smell of the samples was recognized. The effect of UV-C disinfection on the extension of oyster mushroom samples’ shelf life will be determined.
All tabulated data, as well as the plotted data on graphs, are represented in calculated mean±standard deviation (SD) from repeatable measurements. To analyze the dependency of location and UV-C exposure time and their effect on the number of bacteria (c.f. Section 2.3), a two-way ANOVA was implemented. All statistical analysis of the measurement data and their graphical plots were performed using OriginPro 8.5 from OriginLab Corporation, Northampton, MA, USA (https://www.originlab.com).
3. Results and discussion
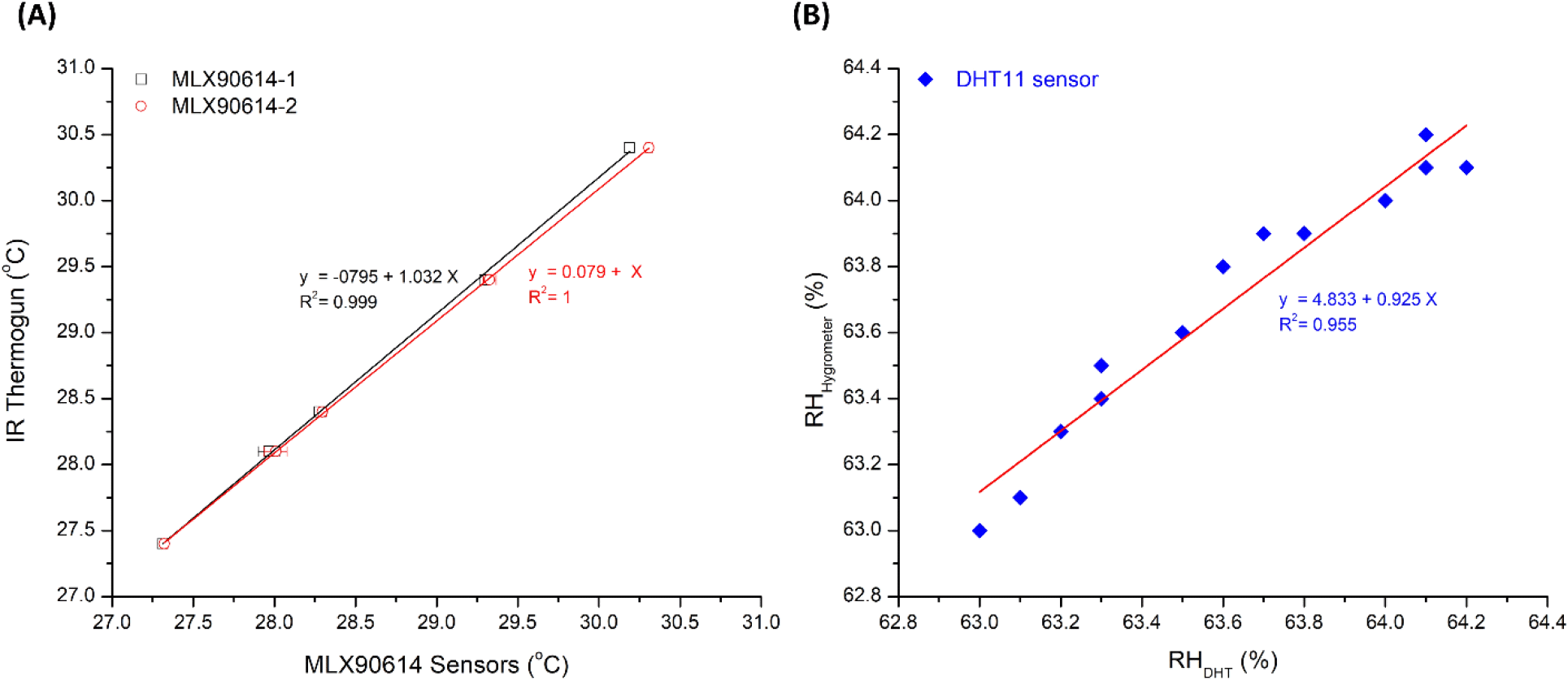
Results of calibration can be given in Fig. 3 for both MLX90614 temperature and DHT11 humidity sensors. After 6 measurements, the MLX90614 temperature sensors showed an excellent precision level, i.e., with an average relative standard deviation of 0.112±0.080 (%) and 0.122± 0.076 (%) for MLX90614-1 and MLX90614-2, respectively. The fitting slope (and coefficient of correlation − R2) to the IR thermometer for both sensors was 1.032 (0.999) and 1.000 (1.000), respectively. Meanwhile, the fitting slope (and coefficient of correlation − R2) for DHT11 humidity sensors was 0.925 (0.955). These results showed that both the MLX90614 temperature sensors and the DHT11 humidity sensors had a very good accuracy and precision level, as well as linearity responses in the range of calibrated values.
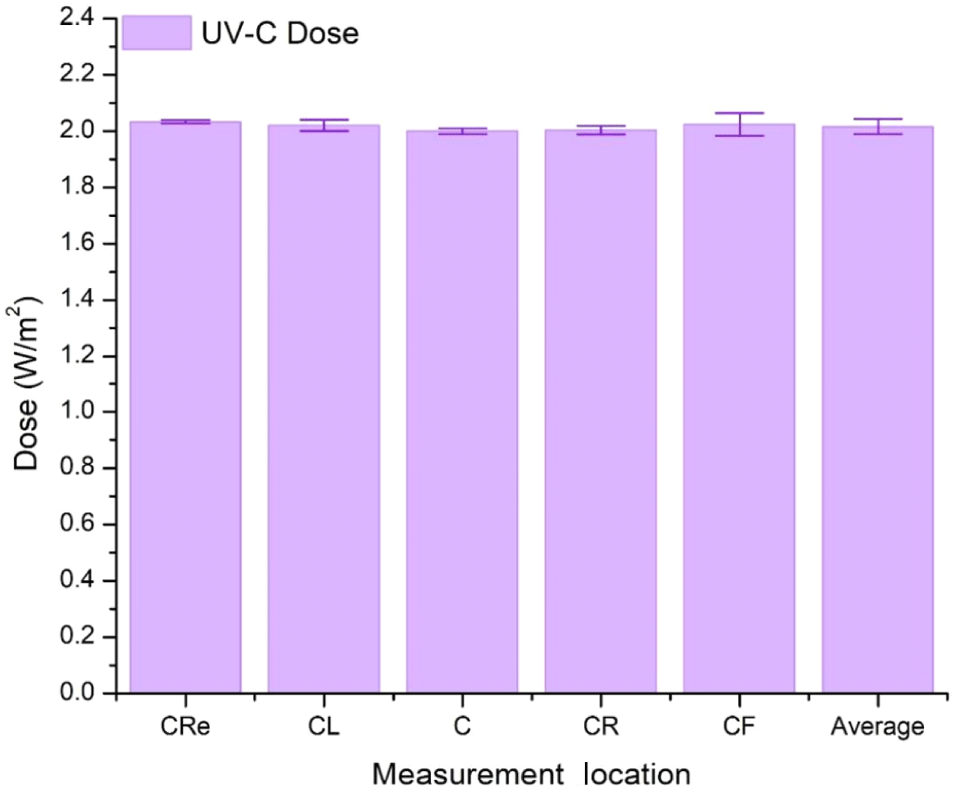
UV-C doses were measured at 5 different point locations on the wire-mesh plane (as all of the lamps were ON), i.e., at points C=centre, CL=centre-left, CR=centre-right, CF= centre-front, and CRe=centre-rear. Measurements were acquired with 3 repetitions for each point. Results were as follow (in Watts/m2), i.e., C=2.000±0.010, CL=2.020±0.020, CR=2.003 ±0.015, CF=2.023±0.040 and CRe=2.033±0.006. The average dose was 2.016±0.027. The dose of UV-C exposure seems to be almost uniform, with excellent precision level, i.e., spread between 0.284-1.997%. These measurements are given in Fig. 4.
The calculated number of bacterial colonies formed in the growing media, for every exposure time, is given in Table 2, with respective reduction either in logs or percentage. Its trend is also visualized in Fig. 5. Results showed that UV-C disinfection can significantly reduce the infected bacteria Pseudomonas fluorescens in the oyster mushroom samples, i.e., circa 50% under UV-C exposure of 20 sec and even higher for longer duration after exposure. Such a trend might be attributed to phenomena of photoreactivation following UV irradiation (Kashimada et al., 1996). Photoreactivation is the process by which inactivated microorganisms recover their capability by repair pyrimidine dimers in their DNA utilizing the enzyme photolyase, without excising the affected area. Several investigations have discovered that photoreactivation may promote the regrowth of microbial populations under certain conditions, thereby decreasing the efficacy of UV-C inactivation (Sanz et al., 2007).
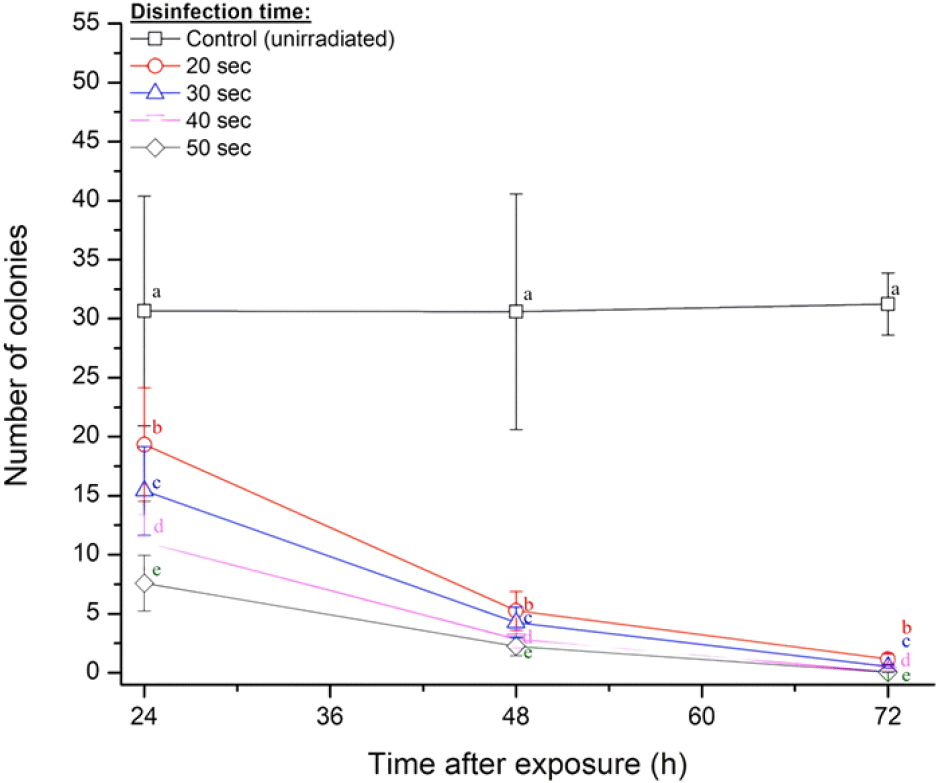
The observed bacterial reductions at the first 24 h after UV-C exposure were around 50-75%, for all treatments’ time, and showed a significant increase on the next days, i.e., 83-93% and 96-99.9% for 48 h and 72 h, respectively. These results proved the advantage of UV-C radiation exposure in decontaminating bacteria on the oyster mushroom products.
The Indonesian National Standard (SNI), number 01-6945-2003, guides the quality standards for fresh mushrooms in Indonesia. This standard specifies that the quality of fresh mushrooms must meet both physical and chemical requirements. The physical ones, among others, are of homogeneous size, have undamaged form, have a strong but lithe outer texture, have no mushy and slimy appearance, and have a bright color without pale color spots, a.k.a. the blotch. Bacterial blotch is regarded as the most significant mushroom disease with global occurrence and economic impact (Osdaghi et al., 2019). Meanwhile, the chemical ones are related to the levels of water, protein, and fat contents, as well as requirements for being free of any pesticides and other chemical residues. The acceptable duration of shelf life should follow the above requirements to guarantee that the products are still acceptable for consumption. The physical properties of the product are the easiest way to determine their quality, and color changes are one of the easier to perceive.
After disinfecting the samples of oyster mushrooms at several exposure times using the proposed system and storing the samples, observations on the shelf life were carried out to see the effect of disinfected samples on the unexposed control samples, as well as the effect of storing conditions after disinfection. Two storing conditions were compared, i.e., storing at room temperature and storing in the refrigerator. Fig. 6 displays the visual appearance of the oyster mushroom samples under various treatment conditions. The color spaces from each image can be tabulated in Table 3, i.e., given in both RGB and CIE L*, a*, b* color spaces. Color changing in oyster mushroom samples for each treatment scheme, as described in Section 2.6, is quantified based on the changes in total color difference ΔE. It was shown that values ΔE= 2.323 is the lowest for combined UV-C irradiation and stored in refrigeration afterward. It does growingly increase ΔE= 11.604 for the treatment of UV-C and is stored at room temperature, and ΔE=31.054 for treatment samples by chilling in refrigeration only. The highest ΔE=42.502 is for untreated control samples.
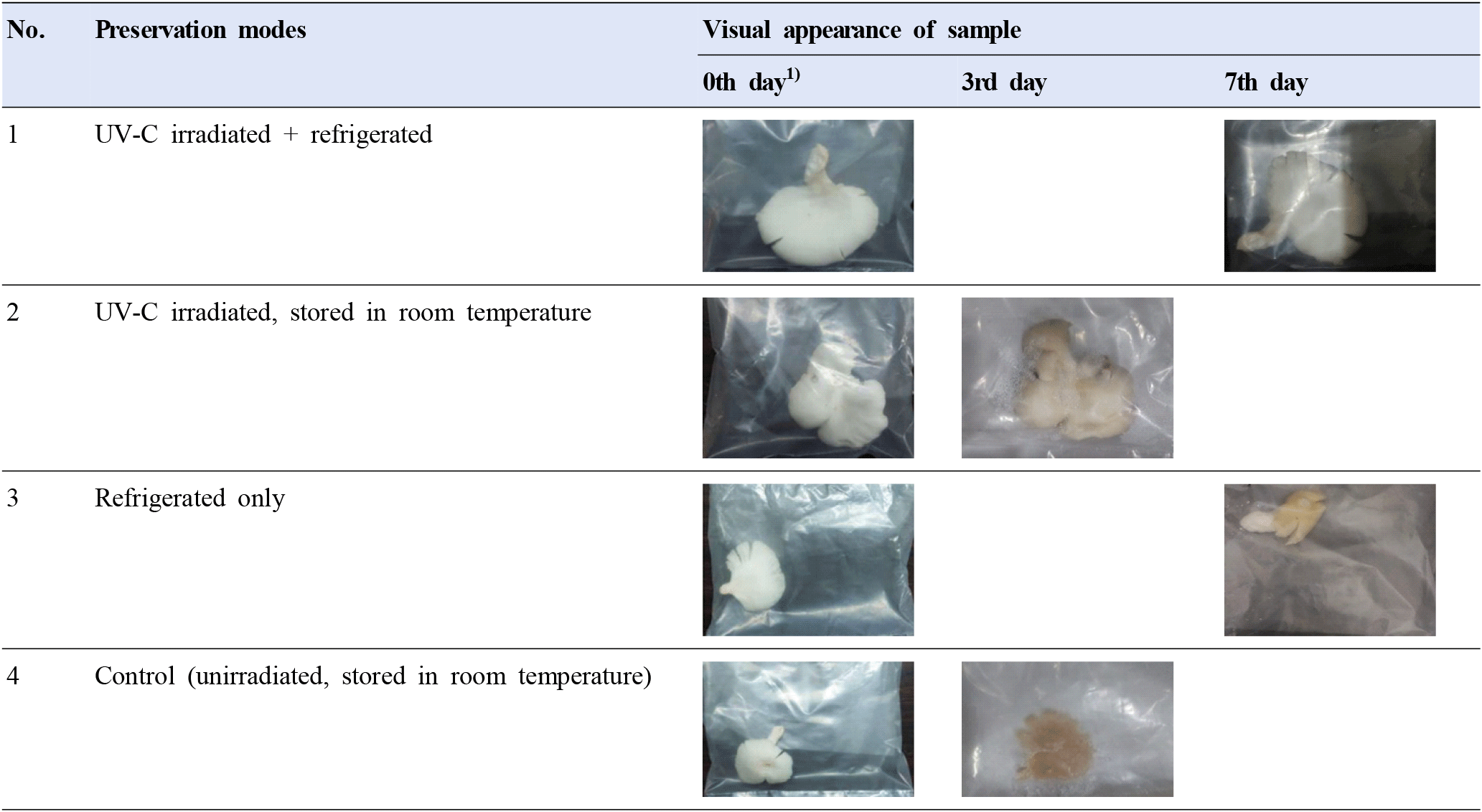
The higher the exposure time of UV-C radiation, the more bacterial killing in the oyster mushroom samples, but exceeding a certain exposure time limit, the disinfection will change the sensory attributes of the samples. In the test carried on, disinfecting the samples for 50 sec caused the samples to look burnt, and a burnt aroma was smelt. So it is not recommended to disinfect the oyster mushroom samples for more than 40 sec.
Meanwhile, the proper storing of the disinfected oyster mushroom will add to its shelf life. Storing the samples at room temperature after disinfection will only double the shelf life of the samples, i.e., in comparison to the untreated ones. Storing in a refrigerator will make the shelf life even longer, i.e., up to 3 to 4 times longer. Storing oyster mushrooms in a refrigerator after UV-C treatment will extend their shelf life by an additional 1.5-2 times compared to storing them at room temperature, regardless of the investigated exposure durations (Table 4). The possible cause of this additional improvement could be due to the additional suppression of the regrowth of dormant bacteria that survived after UV-C irradiation, which did not happen as it was stored at room temperature.
The costs for the proposed UV-C disinfection system reported here can be split into the fixed and variable parts. The fixed part was the cost for required components, as well as construction and testing costs, which was about USD 400. Meanwhile, the variable cost is the operational cost, which covers electricity and labor costs, which depend on the quantities of disinfected products.
During the test, 5 kg of oyster mushroom samples (medium-large size) can be disinfected in two cycles, i.e., 2.5 kg each. The required power for electricity, i.e., for energizing lamps, an exhaust fan, Raspberry cards, and sensors, was circa 84.5 W. As the optimum disinfection time was 40 sec, each cycle consumed 0.001 kWh. The local electricity price for the Micro, Small, and Medium Enterprises is 8.35 cents per kWh; hence, the disinfection cost per cycle per kg is 0.003 cents. On average, a rural farmer can harvest ca. 100 kg daily, which means that the disinfection cost will be only 0.3 cents. Since the average daily labor cost in local rural areas is USD 5, this means the total daily operational cost will be USD 5.03.
Currently, the cost of oyster mushrooms in the local market is ca. USD 2.16 per kg, so a rural farmer can earn a net income of USD 210.97 daily. If 5% of this daily net income is paid for the daily installments for the above disinfection system, i.e., USD 10.55 daily, then a rural farmer can pay off the purchased UV-C disinfection system only in 38 days. These calculations reflect the cost-effectiveness of the proposed UV-C disinfection system, as also confirmed by the published studies (Shahi et al., 2021; Zhang et al., 2018).
4. Conclusions
Several important conclusions can be drawn as follows: The calibrated and tested sensors and lamps utilized in the construction of the proposed UV-C disinfection system showed outstanding accuracy and precision, in addition to excellent linearity within the applied dosage range. The UV-C radiation doses were uniformly distributed at the wire-mesh plane, as measured by the calibrated UV-C light meter. Exposing the cultivated oyster mushroom samples caused a significant reduction in the bacterial colonies formed for different exposure duration treatments. These results proved the usability of the UV-C disinfection system to reduce the contaminated bacteria on the oyster mushroom products. For a daily harvest of 100 kg, the operational cost for the proposed UV-C disinfection system is quite cheap, i.e., only 0.3 USD cents. When the oyster mushroom samples were stored at room temperature after being irradiated, the developed disinfection system proved to prolong the shelf life by two times. Storing the disinfected samples in the refrigerator made the shelf life 3-4 times longer.