1. Introduction
The rapid growth of the global population has driven an increasing demand for protein, placing immense pressure on the food production industry, particularly meat production. Meeting this demand sustainably has become a significant challenge for the future (Henchion et al., 2017). In this context, insects have emerged as a promising alternative protein source due to their low environmental impact (van Huis, 2016).
Quantifying these environmental benefits, insect farming generally requires significantly less land, water, and feed to produce the same amount of protein as traditional livestock. For instance, studies have shown that insects like crickets and mealworms can convert feed into edible biomass far more efficiently than cattle, pigs, or even chickens, often requiring only 2 kg of feed to produce 1 kg of insect biomass, whereas cattle may need up to 8 kg of feed for the same amount of weight gain (van Huis, 2013). Crucially, insects also produce considerably lower greenhouse gas emissions (e.g., methane and nitrous oxide) compared to ruminant livestock, contributing less to climate change (Oonincx and de Boer, 2012). While specific data for Lethocerus indicus on these metrics are still emerging, its aquatic nature and small-scale farming potential inherently suggest that it has similar, if not superior, efficiency compared to land-based livestock.
The practice of consuming insects has existed for centuries in many countries, especially in developing regions. In Southeast Asia, where 40% of the population faces chronic malnutrition, insect consumption is an integral part of the culinary culture (Tao and Lee, 2018). It is estimated that approximately 2 billion people throughout the world incorporate insects into their traditional diets (van Huis, 2013).
Insects are considered to be a rich and diverse source of nutrition, offering fats, proteins, vitamins, fiber, and minerals. Amino acids such as phenylalanine and tyrosine, found in insects, are regarded as particularly nutritionally valuable. Furthermore, certain insect species contain significant amounts of lysine, tryptophan, and threonine-amino acids that are often deficient in cereal-based proteins (Kouřimská and Adámková, 2011). In addition to their high nutritional value, edible insects can also be considered a viable and practical business opportunity, providing a means for income generation, particularly in rural areas where insects are raised as micro-livestock for human consumption and animal feed (Williams and Williams, 2017).
According to research, the protein digestibility of insects, excluding their exoskeleton (a hard outer covering composed of indigestible chitin), ranges from 77% to 98% (Afanassieva, 2024; Defoliart, 1992). The nutritional value of edible insects varies greatly even within the same species group and is influenced by factors such as developmental stage, habitat, diet, and gender (Kulma et al., 2017; Rumpold and Schluter, 2013b). One major challenge related to insect protein digestion is the structure of their exoskeleton. Composed primarily of chitin (a rigid, fibrous carbohydrate found in insect shells), the exoskeleton is difficult to digest (Polhemus, 2008; Yeul and Rayalu, 2013). Processing methods can remove the exoskeleton and thus improve protein digestibility (Rumpold and Schluter, 2013b).
In terms of the protein content, edible insects provide 35-60 g of protein per 100 g of dry weight or 10-25 g of protein per 100 g of fresh weight (Melo et al., 2011; Schluter et al., 2017). These figures surpass the protein content of cereals (17.90±0.03 g/100 g), soybeans (16.73±0.21 g/100 g), and red beans (21.32±1.50 g/100 g) (Fan and Beta, 2016; Oluwajuyitan et al., 2021; Tufa et al., 2016). Remarkably, certain insect species with the highest protein content also exceed the protein levels of meat and chicken eggs (Mlcek et al., 2014). Insects from the order Orthoptera, such as crickets and grasshoppers, are particularly protein-rich (Rumpold and Schluter, 2013a). Specifically, Lethocerus indicus stands out with a protein content of up to 60.12±0.50 g/100 g (Melo-Ruíz et al., 2016). In addition to its remarkable protein profile, L. indicus contains essential oils (EOs) with promising applications in food preservation, cosmetic, and health features rarely found in other edible insect species (Fig. 1). Unlike terrestrial insects that often require feed and controlled substrates, L. indicus thrives in freshwater environments, which aligns well with Vietnam’s geography of dense river systems, ponds, and rice fields. This allows low-input, small-scale farming at the household level using minimal infrastructure. Furthermore, the commercial value of L. indicus is significantly higher: both the insect itself and its EO command premium prices in domestic markets due to their rarity and culinary demand. These advantages-economic profitability, farming feasibility in rural areas, and bioactive uniqueness underscores its strategic importance in Vietnam’s efforts to diversify protein sources and develop sustainable insect-based industries. Therefore, greater research attention and policy support toward L. indicus would directly benefit national food security and rural livelihoods. To further understand the potential of edible insects in meeting sustainable protein demands, this article delves into the biological characteristics, nutritional value, and roles of L. indicus in the culinary and economic landscapes of Southeast Asia.
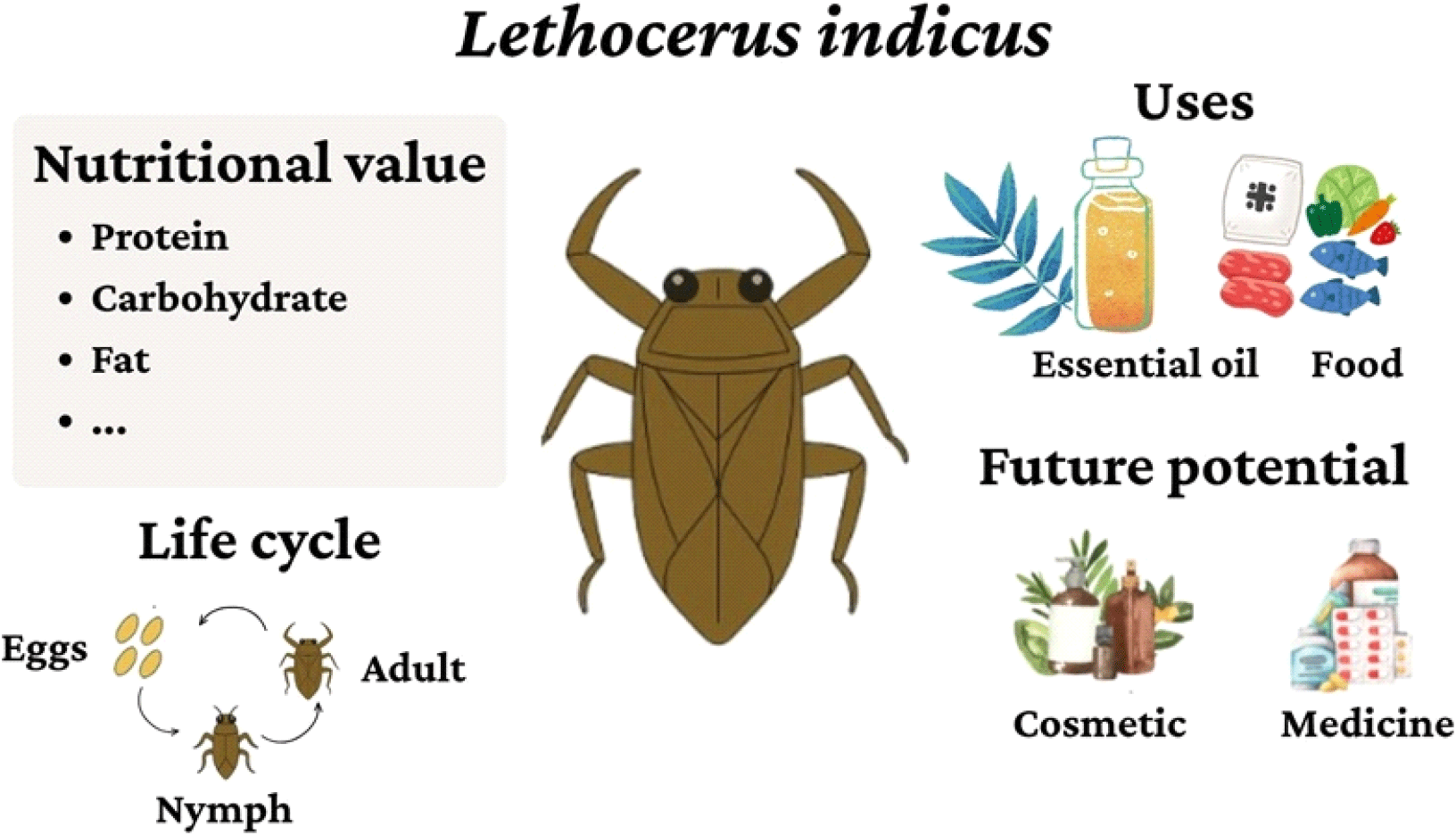
2. A comprehensive overview of L. indicus
Lethocerus indicus belongs to the insect group Hemiptera = Heteroptera: Belostomatidae: Lethocerinae. It is a large aquatic insect (measuring 3-5 cm in length) commonly found in ponds (Fig. 2), rice fields, and slow-moving freshwater streams. This species is one of the many edible insects popular in the northern and northeastern regions of Thailand, as well as in other Southeast Asian countries, such as Laos, Vietnam, and Cambodia. It is also favored by insect consumers in certain regions outside Southeast Asia (Lokeshwari and Shantibala, 2010; Srivastava et al., 2009). The species is characterized by its broad, flattened hind legs resembling paddles, which enable swift and agile movement underwater, aiding in its predatory behavior. Aquatic insects, including L. indicus, possess two types of scent glands: one type located in the thoracic region during the adult stage and another in the abdominal region during the larval stage (Dettner, 2019). These insects utilize the chemicals they secrete for defense against predators and to inhibit bacterial growth within their bodies (Kovac and Maschwitz, 1989).
Lethocerus indicus has long been an important flavoring ingredient in the cuisines of Manipur and Thailand. Unlike females, male L. indicus are noted for their distinctive aroma and flavor, primarily due to the presence of a scent gland. (Melo-Ruíz et al., 2016; Shantibala et al., 2014). Edible insects, including L. indicus, have gained attention as a future food source due to their favorable nutritional profile and low environmental impact, such as reduced greenhouse gas emissions, lower land use, and minimal water requirements, when compared to traditional livestock (Kapesa et al., 2020; van Huis et al., 2013).
Beyond its culinary significance, L. indicus has been studied for its biological and ecological roles. A study in India by Bali et al. (1984) revealed that L. indicus primarily preys on mollusks, which are known to negatively impact aquatic ecosystems (Bali et al., 1984). Notably, in the United States, L. indicus is consumed and sold as a popular item within Asian American communities in California, where it is often featured as a delicacy during parties and festivals (Pemberton, 1988). Lethocerus indicus plays a multifaceted role in maintaining ecological balance, contributing to human health, and supporting food security (Zhao et al., 2021). Thus, L. indicus is an ecologically valuable insect and an essential contributor to the culinary and economic landscapes of many countries throughout the world.
3. Biological characteristics and life cycle of L. indicus
During summer, L. indicus adults are often attracted to light sources, especially lamps, and their activity peaks on rainy days. In winter, they tend to enter a state of dormancy, burrowing into mud to conserve energy and avoid cold conditions. During this time, their metabolic activity decreases significantly, and predation nearly ceases (Stoianova et al., 2020). When spring arrives, L. indicus enters its reproductive season, typically coinciding with rainy days. Adult individuals migrate to freshwater habitats such as ponds, lakes, or rice fields to lay eggs. They often select water bodies with dense aquatic vegetation and abundant tadpoles or aquatic insects, which support the development and survival of their offspring (Barbosa et al., 2017).
As a distinctive freshwater insect native to Vietnam, L. indicus inhabits calm water bodies with abundant aquatic plants. These environments provide ideal conditions for hiding, hunting, and reproduction. A natural predator, L. indicus uses its piercing-sucking mouthparts to inject venom into prey such as small insects, juvenile fish, shrimp, and other crustaceans, and subsequently extracts the internal fluids. With a predominantly nocturnal lifestyle, L. indicus actively hunts and seeks mates at night; during the day, it hides to rest and to avoid predators.
The growth of L. indicus involves multiple molting stages, during which the insect increases in size and progresses toward maturity. However, immediately after molting, their bodies are soft and vulnerable, making them susceptible to damage. The life cycle of L. indicus is a journey of physical development as well as a process of adaptation to its surroundings. Upon reaching maturity, during the breeding season, males and females mate, after which females lay their eggs on aquatic plants or hard surfaces near the water’s edge. The eggs of L. indicus typically hatch after 6-8 days (Fig. 3), depending on temperature and humidity conditions.
The farming of L. indicus is not overly complex, but it requires meticulous care. They can be raised in Styrofoam boxes or tarpaulin-lined ponds with clean and stable water. Their primary diet consists of live prey such as small fish, tadpoles, frogs, and toads. It takes approximately 35 days from hatching to the final molting stage. After this final molt, these insects do not grow in size; rather, they transition into the stages of maturity and aging. Around 70 days after hatching, L. indicus enters the reproductive phase or can be harvested for processing into regional edible insect products and delicacies. Each individual weighs about 2-5 g, depending on nutritional conditions, including diet and the environment.
Currently, the L. indicus farming model remains unpopular, mainly on a small scale within households. A 1-2 m2 plastic tank can support 20-50 adults; however, low productivity restricts their survival and reproduction rates. Scaling up is difficult because this insect is sensitive to environmental factors such as temperature, water quality, and live food (tadpoles, juvenile fish). Additionally, water pollution and climate change make natural farming challenging to sustain, which requires a controlled, closed system, but high initial investment costs remain a major obstacle.
4. Nutritional profile of L. indicus and comparison with other insects
The nutritional value of L. indicus has garnered significant attention due to its rich nutrient profile and broad potential applications in the food industry. Table 1 provides an overview of the key components of L. indicus based on studies conducted in Mexico, India, and Thailand. Table 2 provides the nutritional profile of some other insects, namely Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. This information serves as a basis for evaluating the dietary value and substitution potential of L. indicus in daily diets.
1) Data from Akhtar and Das (2025), Melo-Ruíz et al. (2016).
2) Data from Sarmah et al. (2022).
3) Data from Melo-Ruíz et al. (2016).
1) Data from Hong et al. (2020).
Protein is the most abundant macronutrient in L. indicus, with values ranging from 30.97 g/100 g (India) to 60.12 g/100 g (Mexico) (Akhtar and Das, 2025; Sarmah et al., 2022). These figures are comparable to or even higher than the protein content of other commonly consumed insects, such as T. molitor (45.10-67.60%), A. diaperinus (58.0-65.0%), and H. illucens (41.1-47.6%) (Hong et al., 2020; Rumbos et al., 2018). Given the recommended daily protein intake of 65 g/day/kg body weight (BW) for women and 78 g/day/kg BW for men (Tieland et al., 2015), consuming L. indicus in sufficient quantities could meet or exceed daily protein requirements, especially in protein-deficient regions. Currently, L. indicus does not have specific protein intake recommendations, as do many other edible insects. This is an important gap that requires further research, particularly regarding its absorption, safety, and tolerability in humans.
Regarding fiber, L. indicus consistently contains high levels across all samples: 10.95 g/100 g (Mexico), 11.66 g/100 g (India), and 12.23 g/100 g (Thailand) (Melo-Ruíz et al., 2016; Sarmah et al., 2022). These values are substantially higher than the fiber range in T. molitor (4.58-22.35%, often closer to the lower end) and are particularly beneficial given the recommended fiber intake of 28 g/day/kg BW for women and 36 g/day/kg BW for men (Anderson et al., 2009; Hong et al., 2020). The high fiber content is largely attributed to the chitinous exoskeleton of L. indicus, offering benefits for gut health and digestion, and positioning it as a natural source of dietary fiber.
In terms of lipid content, L. indicus exhibits variable fat levels depending on geographic origin: 5.72% (Mexico), 8.15% (Thailand), and a notably higher 26.63% (India) (Melo-Ruíz et al., 2016; Sarmah et al., 2022). This wide variation is consistent with trends seen in other insects, where lipid ranges fluctuate based on developmental stage, diet, and environment. For comparison, T. molitor contains 14.8-43.1% lipids, A. diaperinus 13.4-29.0%, and H. illucens 11.8-36.1% (Hong et al., 2020; Rumbos et al., 2018). Lipids are the second-largest macronutrient in L. indicus after protein, and they play a key role in energy storage, flavor, and industrial applications.
The carbohydrate content in L. indicus is also notable, especially in the samples from Mexico (17.75%) and Thailand (19.74%), compared to a lower value in India (0.61-2.92%) (Akhtar and Das, 2025; Melo-Ruíz et al., 2016; Sarmah et al., 2022). Although carbohydrates are not the primary nutrient in insects, their presence contributes to total caloric content and may support gluconeogenesis when protein intake exceeds metabolic needs.
Moisture levels also vary, with high values in Mexico (47.75%) and Thailand (44.91%), but markedly low in India (3.17-3.38%), likely due to differences in drying methods or measurement basis (Akhtar and Das, 2025; Melo-Ruíz et al., 2016; Sarmah et al., 2022). The energy values reflect these compositions: L. indicus from India yields 874-1,986 kJ/100 g DW, which is within the range of T. molitor (1,600-2,730 kJ) and H. illucens (2,210 kJ) (Akhtar and Das, 2025; Hong et al., 2020; Rumbos et al., 2018).
In addition to these macronutrients, L. indicus has been reported to contain bioactive compounds such as (E)-2-hexenal, contributing to its distinctive aroma and potential functional uses in food preservation (Kiatbenjakul et al., 2015). These compounds, along with their essential oil (EO) content, open possibilities for pharmaceutical and cosmetic applications, similar to the oil of T. molitor, which has demonstrated benefits in wound healing (Kim et al., 2021), or H. illucens, which has been incorporated in baked products (Delicato et al., 2020).
Taken together, the nutrient density, functional components, and industrial potential of L. indicus clearly position it as a multifunctional insect species. Its high protein and fiber content, moderate fat, and market value per unit insect make it a strong candidate for food security strategies, particularly in developing nations seeking sustainable, low-cost, and environmentally friendly alternatives to traditional animal proteins.
5. Chemical composition of L. indicus and other insects
Gas chromatography-mass spectrometry (GC-MS) analysis has been employed to identify the characteristic chemical composition of L. indicus and several other insects, aiming to compare and evaluate their potential applications in fields such as pharmaceuticals, cosmetics, and biotechnology (Krone et al., 2010). Table 3 summarizes the major compounds identified, highlighting the ecological roles and application values of each species.
Table 3 shows that L. indicus possesses a chemically rich and unique volatile profile, with at least 37 identified compounds, dominated by 2-Hexan-1-ol (54.86%). This alcohol functions as a pheromone (a signaling chemical that influences behavior within the same species), playing a significant role in insect behavior regulation, defense mechanisms, and antibacterial activity, making it valuable in food preservation, cosmetics, and fragrance industries (Fleischer and Krieger, 2018; Kim et al., 2005). Additionally, cholesterol (7.83%) and 10-Pentadecen-5-yn-1-ol (6.39%) are other notable compounds associated with maintaining cellular membrane structure and enhancing metabolic activity in L. indicus (Ding et al., 2019).
In contrast, D. gigas (Table 3) expresses an even more diverse array of 30 volatile compounds, including ethyl oleate (29.78%) and n-Hexadecanoic acid (17.54%). These fatty acid esters are commonly involved in chemical communication among social insects and have anti-inflammatory or emollient properties, with widespread use in pharmaceutical and cosmetic products (Castillo et al., 2012). S. sacer, though taxonomically and ecologically distinct, is no less chemically complex, with over 40 compounds identified, notably neophytadiene (6.43%) and 3,7,11,15-tetramethyl-2-hexadecen-1-ol (6.43%). These molecules possess antioxidant and antibacterial effects, which are consistent with the dung beetle’s ecological role in organic waste degradation (Bhardwaj et al., 2020).
A notable observation is the apparent lack of overlap in dominant volatile compounds among the three species, suggesting that each may possess a distinct chemical signature. These differences could be linked to species-specific ecological roles or chemical defense mechanisms. In edible insect research, strong or pungent odors are sometimes considered undesirable, potentially limiting consumption unless processed appropriately. However, further studies are needed to confirm these assumptions and clarify the relationship between volatile composition and edibility.
Furthermore, the chemical richness of L. indicus-with a high proportion of alcohols, sterols, and defensive volatiles-positions it as a strong candidate for industrial applications (Jing and Behmer, 2020). Its compounds are especially promising for bioactive product development, including natural antimicrobials, perfumery ingredients, and cosmeceuticals.
In summary, the species-specific and functionally diverse volatile compounds detected across these three insects not only illustrate their ecological specialization but also underscore their potential in multidisciplinary industries, ranging from food science and biotechnology to pharmaceuticals and cosmetics.
6. Lethocerus indicus and unique flavor: The quintessence of Vietnamese cuisine
Lethocerus indicus, commonly known as “cà cuống,” is a distinctive insect species in Vietnam, widely distributed across the country’s freshwater regions. Beyond being a part of the ecosystem, it is a valuable resource, raised for its essential oil and used in preparing traditional dishes. The essential oil extracted from the male’s scent gland is highly regarded for its rare and unique aroma (Dettner, 2019), often referred to as the “truffle of Asia” for its ability to enhance the flavor of dishes. Regarded as a traditional food item with notable economic value, L. indicus commands a price ranging from 100 to 200 USD per kilogram, making it not only a culinary specialty but also a lucrative source of income for those who farm and harvest this exceptional insect.
With an extraction yield of only about 5-15%, 100 grams of L. indicus produce merely 5-15 mL of essential oil (Fig. 4). This scarce yield not only increases its value but also reinforces the distinctive status of this special seasoning, as a 5 mL bottle can sell for up to 24 USD. The oil tends to permeate the insect’s body; therefore, chopping the insect into smaller portions helps distribute the aroma evenly when used in sauces.
L. indicus essential oil is considered an important aromatic component in many Vietnamese dishes. A small quantity can enhance the flavor of regional specialties such as “bún thang” and “bún chả”. Its strong and distinctive aroma leaves a lasting impression and contributes to the depth and identity of Vietnamese cuisine. In addition to oil extraction, L. indicus is also processed in various forms, such as fried, salted, infused in wine or fish sauce, etc. (Fig. 5).
One particularly beloved dish is L. indicus sauce, where the bugs are soaked in fish sauce fermented for 9-12 months. The essential oil from the abdomen slowly blends with the sauce, creating a rich, unforgettable flavor. This method reflects traditional culinary knowledge and emphasizes the integration of natural ingredients into local food systems.
Salted L. indicus is another traditional preservation and preparation method that retains the unique flavor of this insect. The process involves removing inedible parts such as wings and legs, soaking the cleaned insects in wine to neutralize strong odors, then salting and layering them in jars with lime leaves. After being pressed and sealed, the product is ready for use after 3-5 days, though a more intense flavor develops over 1-2 weeks. Salted L. indicus can be consumed directly or used as a seasoning in dipping sauces, noodle soups, or broths. Refrigeration is recommended for longer shelf life.
In recent years, in addition to traditional preparations, modern processing techniques such as boiling, drying, and grinding have been increasingly applied to optimize the safety, digestibility, and versatility of L. indicus in food systems. For instance, blanching or boiling the insects at 95-100°C for 2-3 min helps in reducing the number of the microorganisms and deactivating certain antinutritional factors, while preserving most volatile compounds responsible for the aroma (Ribeiro et al., 2024). Drying-especially oven-drying at 60-70°C, effectively lowers moisture content to below 10%, improving shelf life and making the texture more suitable for grinding (Lampová et al., 2024). These processing steps are particularly important when considering broader commercialization or export of L. indicus-based products. By combining traditional Vietnamese techniques with systematic modern processing, the value chain of this edible insect can be expanded significantly, allowing L. indicus to be not only a cultural delicacy but also a scalable functional ingredient in global gastronomy and food innovation.
While L. indicus offers valuable nutritional and economic benefits, consumer acceptance remains a significant barrier. Like many edible insects, it faces cultural resistance, often due to the “disgust factor” or fear of allergies. However, studies have shown that consumer education, effective processing, and appealing packaging can improve acceptance (Hartmann and Siegrist, 2017). Cooking methods such as marinating in fish sauce, deep-frying, or integrating into traditional dishes like bún thang help mask the insect’s appearance and aroma, making it more palatable. Clear labeling, allergen information, and developing familiar formats (e.g., oils, powders, or sauces) will be critical in fostering broader acceptance and safe consumption.
However, from a long-term perspective, L. indicus remains a promising new ingredient that can contribute to food diversification and sustainable nutrition security. With proper communication, combined with improvements in processing, packaging, and safety, L. indicus could become an acceptable food option in the wider market.
7. Challenges and prospects for the future
While L. indicus demonstrates significant potential as a nutrient-rich food source and a provider of high-value essential oil, several challenges remain that limit its widespread use and industrial scalability. Environmental pollution, particularly water contamination and pesticide use, makes natural farming increasingly unfeasible. As a result, closed-system rearing models must be developed and optimized to ensure safe and consistent production; however, these systems involve technical and financial barriers that are not yet fully addressed in Vietnam or other Southeast Asian countries. Its large size, relatively low population density, and oil yield restricted to males present difficulties in turning it into powder or bulk feed ingredients. Furthermore, there is no established recommended daily intake (RDI) or official nutritional guidelines for L. indicus protein or essential oil, creating a research gap that must be filled through clinical or nutritional studies. Although L. indicus essential oil is prized in Vietnamese cuisine, its bioactivity and pharmacological properties remain underexplored. Currently, there are no published studies on the full chemical composition, pharmacokinetics or potential toxicity of this oil, despite its aromatic similarity to known bioactive plant compounds. Only local products exist, often without standardization. To harness the full potential of L. indicus, future research should focus on developing standardized farming systems for safe and efficient cultivation; exploring formulation into value-added products such as oil-based seasonings, capsules, or topical cosmetics; conducting toxicological and allergenic risk assessments, and allergenic risk assessments; and clarifying nutritional and pharmacological properties, especially of the essential oil, through scientific trials. Given the growing interest in sustainable protein and natural ingredients, L. indicus has strong potential to emerge not only as a culinary specialty but also as a functional food or natural bioresource with cultural, nutritional, and economic value.
8. Conclusions
Lethocerus indicus represents a sustainable and nutrient-rich protein source, holding the promise of addressing future food security challenges. With its high protein content, excellent digestibility, and economic value from essential oil production, this insect not only meets nutritional needs but also reduces environmental impact compared to traditional protein sources such as meat or fish. In Vietnam and Southeast Asia, L. indicus plays a significant role in local cuisine and is also being researched for its potential as a high-value commercial product. The responsible exploitation and cultivation of L. indicus could contribute to global sustainable nutrition solutions, while preserving the cultural and ecological values this species offers. Despite its culinary and nutritional relevance, research on L. indicus - particularly its essential oil-remains limited. Future studies should focus on the chemical characterization of its volatile compounds, their mechanisms of bioactivity, and potential applications in food preservation, pharmaceuticals, and cosmetics. Additionally, standardizing farming techniques, assessing allergenicity, and developing scalable processing methods would be critical to unlocking its full commercial potential. Interdisciplinary research integrating food science, chemistry, and entomology is essential to advance the understanding and sustainable use of L. indicus as a functional food and bioresource.














