1. Introduction
In the modern world, consumer preference is more towards healthy and quality of the food because of their awareness about calories, fat, and cholesterol levels of the product, more than those of consumers who lived twenty years ago. There is an increasing preference among global consumers for ready-to-eat food products (Ramos-Diaz et al., 2022; Souza et al., 2019; Yadav et al., 2016) based on the diversification of taste, flavor, and the sensory properties, which creates a remarkable challenge in the meat industry. As a solution for that, the meat products are incorporated with healthy ingredients as well as some ingredients which used within appropriate limits of phosphate and nitrite (Jimènez-Colmenero et al., 2001). Meat has a superior nutritive value with excellent biological value of dietary protein, fatty acids, and required micronutrients, including vitamins (B12) and minerals (Fe, Zn, Se, and P) for the growth and development of human beings. (Grasso et al., 2024; Larsson and Wolk, 2006; Yadav et al., 2016). The loss of fluids from the meat product (drip loss) can be considered a major problem in the meat processing industry due to its huge influence on both consumer acceptance and economic losses (Troy and Kerry, 2010). A significant drip loss resulted in declined sensory attributes (appearance, texture, mouthfeel, and overall acceptability) of the meat products (Otto et al., 2004) as well as negatively impacts high protein denaturation because water holding capacity (WHC) of meat is affected by the muscle protein structure (Torres Filho et al., 2017).
Among the available meat products, one of the earliest and the greatest comminuted processed meat products is sausage (Abdolghafour and Saghir, 2014), which is mainly made with the combination of meat (finely ground, minced, chopped) and non-meat ingredients (salt, spices) by curing, smoking, and cooking (Sindelar, 2006). There is a broad spectrum of non-meat substances/ingredients/compounds such as binders, extenders, fillers, emulsifiers, and stabilizers that are incorporated into the sausage mixture according to the standard levels for the purpose of improved formulation, maximizing the final product’s quality and quantity (Essien, 2003). In general, meat lacks fiber (Yang et al., 2007), whereas dietary fiber represents one of the important functional food components that can be added into meat products with the aim of improving WHC, fat binding capacity, lubrication, expanded shelf life, viscosity, gel forming ability, less formulation costs, and freeze/thaw stability (Cofrades et al., 2000; Gelroth and Ranhotra, 2001; Kehlet et al., 2017; Souza et al., 2019). In addition to that, the dietary fiber is extremely important for the human body regulation by preventing heart diseases, diabetes, colon cancers, and improving short/long-term memory functions (Anderson et al., 2009). Furthermore, the dietary fiber can promote the growth and proliferation of gut flora and probiotics as an energy source (Sterna et al., 2016). According to the recommendations of the American Dietetic Association, the adult individual to consume dietary fiber, which ranges from 25 to 30 g/day with a 3:1 ratio of insoluble to soluble fiber ratio (Sofi et al., 2017). However, considerable number of people do not have the chance to consume at least half of the required daily dietary fiber due to less availability and poor awareness about the importance of dietary fiber (Anderson et al., 2009). At present, the industries are increasingly practicing the incorporation of dietary fiber into many foods; dairy products, flour products, and meat products, including sausages, surimi, meatballs, meat emulsion, and other meat-based functional food items (Dhingra et al., 2012; Talukder, 2015).
Among the Poaceae family, oat (Avena sativa L.) is one of the vital cereals, which is a perennial crop cultivated in temperate countries (Biel et al., 2009). It can be considered as a health-promoting food based on its superior qualities as high content of soluble dietary fiber, β-glucans, fat-soluble vitamin E, and polyunsaturated fatty acids (Biel et al., 2009; Jandyal et al., 2022; Sterna et al., 2016). With the findings of Alemayehu et al. (2023) and Tanwar and Goyal (2021) typical soluble fiber content (average dietary fiber) in husked oat grain, naked oat grain and the entire fiber content of oat grain were reported as 14.32, 17.63 and 13.66-30.17 g/100 g, respectively. Retaining more water and reduction of fat content in frankfurter has been observed with the highest incorporation level of oat bran (Elleuch et al., 2010; Hughes et al., 1997). Modified food starch is used to enhance thickening properties, stability while changing the texture in various situations as high temperature, acidic environment, increased shear forces and the storage period to tolerate changes in cooling and freezing (Abbas et al., 2010). Rusk powder is a popular baked, ground product made from a combination of wheat flour, corn flour, soy, egg, and rice flour, which is used in various food industries to entrap moisture and fat within the product (Giacco et al., 2002). Thus, incorporation of oat fiber with modified starch and rusk powder into the sausages plays a special role as a binder, and inclusion of the aforementioned three ingredients as a combination could be a solution for the reduction of drip loss while improving the healthiness of the product, which was the study’s uniqueness over other studies. Therefore, the present study was focused on reducing drip loss, assessing and comparing the physiochemical and microbial properties between the different levels of oat fiber incorporated chicken bockwurst sausages, which was the main objective. Another special objective was to determine the most appropriate incorporation level of oat fiber in the chicken bockwurst sausages.
2. Materials and methods
Preliminary trials, product development, and microbial analysis of sausage samples were conducted at the Quality Assurance Laboratory of Cargill’s Quality Foods (Pvt.) Ltd., Ja-Ela, Sri Lanka and physiochemical analysis of sausage samples was done at Nutrition and Meat Technology Laboratories, Department of Animal Science, Faculty of Agriculture, University of Peradeniya, Sri Lanka.
Mechanically separated boneless chicken meat (MSM), boneless meat, chicken skin, fat and skin emulsion, rusk powder, modified starch, spices, food grade colorants, nitrite, salt, phosphate, ascorbic acid and chicken flavor were obtained from Cargill’s Quality Foods (Pvt) Ltd., and Oat (Avena sativa L.) fiber samples were gifted from J.L. Morison Son & Jones PLC, Biyagama Road, Pethiyagoda, Sri Lanka.
A series of preliminary trails were done in order to investigate the optimum level of oat fiber that could be incorporated into chicken bockwurst sausages manufacturing. The oat fiber was used as a binder as well as a replacement for the modified starch and rusk powder. Therefore, eleven types of binders, including ten treatments and a control, were prepared using different levels of oat fiber, modified starch and rusk powder in this study. The incorporation ratios of the aforementioned three ingredients to the sausage mixture were selected according to the previous studies (Smith, 2017), and the formulations were developed by Cargill’s Quality Foods (Pvt) Ltd, Sri Lanka. In this study, the different levels of oat fiber were used in sausage formulation to select the best level of oat fiber, which should be incorporated into sausages with favorable sensory properties, as matched for Sri Lankan consumers. In the preliminary experiments, the amount of oat fiber inclusion in bockwurst sausages made from chicken were illustrated in Table 1 and other ingredients required for manufacturing of the sausages remained in all the treatments.
The chicken bockwurst sausages were produced as their traditional formula: with chicken, salt, sugar, spices, binders, color and flavor enhancers in line with the techniques outlined by Ham et al. (2017) and Yang et al. (2010). Mechanically separated meat (MSM), boneless meat, and chicken skin were minced using a mincer (AU-C-114 200 S, Fritz Reimers GmbH and Co., Baujahr, Germany) to reduce the meat particle size into small particles. Meat and non-meat ingredients were measured (RADWAG®, AS 220/C/2, RADWAG Wagi Electrroonicizne, Poland) according to the composition as shown in Table 2.
Meat components with oil and fat emulsions were loaded into the bowl chopper (MADO Super 130, Maschinenfabrik, Dornhan, Germany) and salt, nitrite salt, spices mixture, ice and water were added gradually and mixed by the bowl chopper. The sausage batter was loaded into a vacuum filling machine (HATDMON vacuum filler, Germany) and filling was done into collagen casings with a 26 mm diameter. After filling, the stuffed sausages were arranged and positioned in the smoking chamber (VERINOX, Italy) to complete the drying, cooking and smoking process until the internal temperature of the sausages reached 72°C. Subsequently, the cooked sausages were showered and transferred to a packing chiller at 4°C. Finally, the sausages were vacuum packed using a multi-vacuum machine (R140, Sepp Hannenmuller, Germany) in nylon-mixed polyethylene packaging materials and kept in the blast freezer at −18°C.
The sensory evaluation at this stage was conducted to select the most preferred oat fiber incorporation level in the chicken bockwurst sausages out of ten treatments in this study using ten trained panelists at Cargill’s Quality Foods (Pvt) Ltd. Frozen sausages were subjected to evaluate texture, taste, color, aroma, appearance and overall acceptability according to five-point hedonic scale (Fernández-Ginés et al., 2003). Among all the treatments, two were selected as the best treatments according to the sensory evaluation in the preliminary study, and these two products were developed for further analysis in this study with the control sample.
Drip loss, WHC, cooking loss, TBARS, pH, and color of the chosen two sausages and control sausage samples were analyzed at weekly intervals during one month of the storage period at −18°C with three replicates per treatment (n=36). Nine samples were analyzed per timepoint during the four weeks of the storage period.
Weights of the sausage samples were measured initially, and the sausage samples with the container were allowed to stand for 24 h at 4°C for thawing. The containers were removed, and the liquid accumulated inside the containers was collected. The sausage sample was wiped using tissue paper and weighed. Formula (1) was used to determine the sausage drip loss (Torres Filho et al., 2017).
Where; W1 = initial weight (g) of the sample, W2 = weight (g) of the sample before thawed, and W3 = weight (g) of the sample after thawed.
A sausage sample (2 g) was weighed and wrapped in Whatman No. 1 filter paper. Then, the wrapped sample was positioned in centrifuge tubes and subjected to centrifugation (Himac CF 15D2, Hitachi, Japan) at 2,600 rpm for 4 min. Then, the weighed samples were transferred to the oven (DX 600, Yamota, Japan) and dried at 70°C temperature overnight. Finally, the oven-dried samples were weighed and determined WHC as follows (2) as according to the protocols of the Association of Analytical Chemistry (AOAC, 2005).
Where; W1 = initial weight of the sample, W2 = weight of the sample after centrifuged, and W3 = weight of the sample after drying (all the weights are taken as grams)
Before cooking, the thawed sausage samples were weighed and the weights of the sausages after cooking were taken after internal temperature reached to 72°C for 5 min as described by Syuhairah et al. (2016).
A maximum of two grams of the ground sausage samples was weighed and transferred into the centrifuge tube. Then, 5 mL of 10%(w/v) solution of trichloroacetic (TCA) was added and the mixture was vortexed for 2 min (VM-96B, Jeio Tech, Korea). After that, 5 mL of 0.02 M aqueous solution of 2-Thiobarbituric acid (TBA) was added and further vortexed for an additional 30 sec. Then, the samples were centrifuged at 3,000 ×g for 10 min using (CUR-1A, Hitachi koki Co Ltd., Japan) and supernatants were filtered. The filtrate was placed in a boiling (100°C) water bath (GmbH + Co. KG 8540 Schwabach, Memmert, Germany) for 45 min. After chilling the test tubes to room temperature, a spectrophotometer (G10S UV-Vis, Thermo Fisher Scientific, USA) was used to determine the absorbance of resulting supernatants at 532 nm. The absorbance reading from a standard line generated 1,1,3,3-tetramethoxy methane as a precursor of malonaldehyde was used to determine the TBARS values of the sausages as described by AOAC, 1995).
Five grams of the ground sausage sample were measured and transferred into a beaker (or 100 mL container). The mixture was then homogenized (BM-4, Nissei, Nihonseki Kaisha Ltd., Japan) for 1 min until the formation of suspension formed after 50 mL of distilled water was added to the beaker. Finally, the reading of the samples was recorded using a pH meter (pH 211, Hannah Instruments, Mauritius) (AOAC, 2005; Özünlü and Ergezer, 2022).
External/outer color of the sausage samples was recorded at one-week intervals using a colorimeter (CR-10, Konica Minolta Sensing, Japan) and the values were presented in three forms as L* (Lightness), a* (redness) and b* (yellowness). When taking the color measurements, tissue papers were used to avoid extra moisture on the outer surface. Measurements of the outer color of the sausages were taken from three places of each replicate (Fernández-Ginés et al., 2003; Zhang et al., 2020).
The proximate composition of the oat fiber incorporated two sausage samples and the control samples was analyzed for moisture, crude protein, crude fat, ash content, crude fiber and dry matter after one week of manufacturing with three replicates per each treatment as described by AOAC (2005) and Acateca-Hernández et al. (2024) standard procedures.
At the fourth week of the storage period, the microbiological properties of all the treatments were assessed for Total Aerobic Plate Count (TAPC), detection of Staphylococcus aureus and Escherichia coli. In order to prepare a dilution series for the specific dilution series of each sausage sample, 1 mL of the particular sample was inserted into a test tube containing 9 mL of peptone water (Wijayawardana et al., 2022). The pour plate method was used to preparation of culture plates, followed the standard protocol outlined by the AOAC (2005). Determination of E coli and Staphylococcus was tested according to the SLS 516 part III: 1982 and SLS part VI: 1992, respectively. Colony forming unit per gram was used to express the microbial count of each plate (CFU/g).
Data collected from proximate composition (n=9) was analyzed by Completely Randomized Design (CRD) using one-way ANOVA and data of the keeping quality parameters evaluation (repeatable data) collected during one month of the storage period (n=36) were analyzed using repeated measures ANOVA by SAS program (SAS Institute Inc., 2004) version 9.0 with a 95% confidence interval. The Least Significant Difference (LSD) test was used to determine mean separation of all treatments.
3. Results and discussion
In this study, oat fiber was used as binding component for sausage formulation compared to other binders used in meat industry as corn flour, soy proteins and wheat flour because of its unique favorable qualities such as nutritional value (dietary fiber, β glycan, minerals, proteins and phytochemicals), functional properties (greater WHC, fat binding capacity and texture), healthiness (non-allergenic), sensory (neutral taste and odor) and economical advantage for the industries (Chauhan et al., 2018). Preliminary studies were directed to find out the amount of oat fiber to be incorporated in the chicken sausages in order to obtain the correct level of the binder composition with modified starch and rusk powder in the chicken sausage mixture. Among the preliminary trials, 25.00% and 16.50% oat fiber levels out of 3% of binder composition in the sausages were selected for further studies (data not shown). According to the studies of Talukder and Sharma (2010), consumers decided that oat bran could be included to a maximum of 10% in chicken meat patties, out of 5% and 10%. The meat batter was altered with wheat and oat fibers at 5% and 10% levels of fibers; 7.50% of fiber was selected as the best fat replacement level (Baranowska et al., 2007). In order to produce low-fat sausages, 10% oatmeal could potentially be added to the sausages for substitution of fat (Yang et al., 2010). Further, Santhi and Kalaikannan (2014) stated that, incorporation of 10% oat flour can be acceptable when producing dietary fiber-enriched low-fat chicken nuggets. Furthermore, 9% oat flour can be incorporated into chicken sausages as a binder out of three levels (3%, 6% and 9%), which can improve the yield, higher WHC, and organoleptic traits (Reddy et al., 2017). In addition to that, black quinoa co-products were used as binders for Bologna-type sausages in order to improve sensory attributes, nutritional and technological properties as described by Fernández-López et al. (2020); Mongi and Gomezulu (2022).
As illustrated in Fig. 1, the drip loss percentage of oat fiber added and control treatments showed a significant difference up to three weeks of their storage period. When compared to the other two treatments, T1 showed the lowest (p<0.05) drip loss at the fourth week of the storage period. It might have happened due to the high incorporation ratios of oat fiber (25.00% out of 3% of binder) into the sausages because dietary fiber can hold and bind more water during thawing (Kim et al., 2014). However, the T1 sausage contained the highest (p<0.05) crude fiber content than the T2 and the control (as described in Table 3), which may be the possible reason for the findings of drip loss in this study. The drip loss of three sausages in the first week showed a significantly lower value compared to the drip loss of three sausages in other weeks of the storage period. As well as the drip loss percentage of all four treatments was raised (p<0.05) throughout the storage period, which described by Manzoor et al. (2023). According to the results of Shashin et al. (2016), the drip loss percentage of chicken burger treatments gradually climbed with the storage period because of the development process of protein denaturation and aggregation in sausage samples. The drip loss of the sausages can be varied according to the thawing method, such as thawing at chilling temperature, thawing at room temperature, cold water thawing, and microwave thawing (Berry, 1994). The findings of Elleuch et al. (2010) stated that oat fiber, as well as wheat fiber, can strengthen the water and fat absorption capacity while reducing the drip loss during cooking operations in food products.
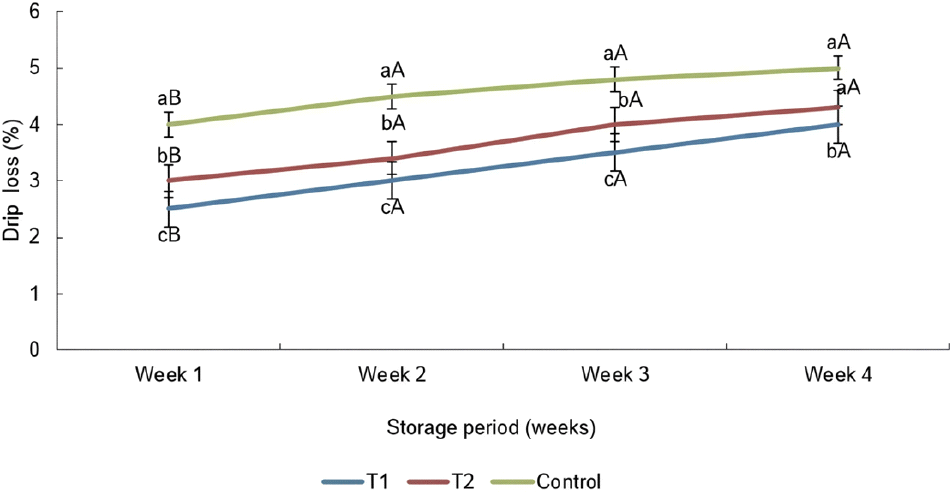
According to Fig. 2, oat fiber enriched two sausages (T1 and T2) exhibited higher (p<0.05) initial WHC than the sausages without oat fiber. It might have occurred due to the incorporation of oat fiber into the chicken sausages (Park et al., 2013). Cofrades et al. (2000) also proved that the fiber is appropriate for inclusion in cooked meat products with the objective of enhancing WHC. The findings of the current study are matched with respect to the outcomes obtained by Reddy et al. (2012) and Isnaeni et al. (2023), who found that an increase in WHC of both oat flour fortified raw and cooked low-fat chicken sausages was due to the significant moisture absorbance ability of oat flour in meat emulsion. Even though there was a significant difference in crude fiber and moisture percentages of the oats containing in two sausages (T1 and T2), they did not show any significant difference with respect to WHC. A similar WHC was observed in both oat fiber-incorporated sausages due to β-glucan content in oat fiber act as a binder by absorbing water and forming a gel, which influences water retention than the quantity of fiber content (Malki et al., 2022). During the three weeks following the completion of the storage period in this study, oats incorporated two sausages exhibited higher (p<0.05) WHC than the control.
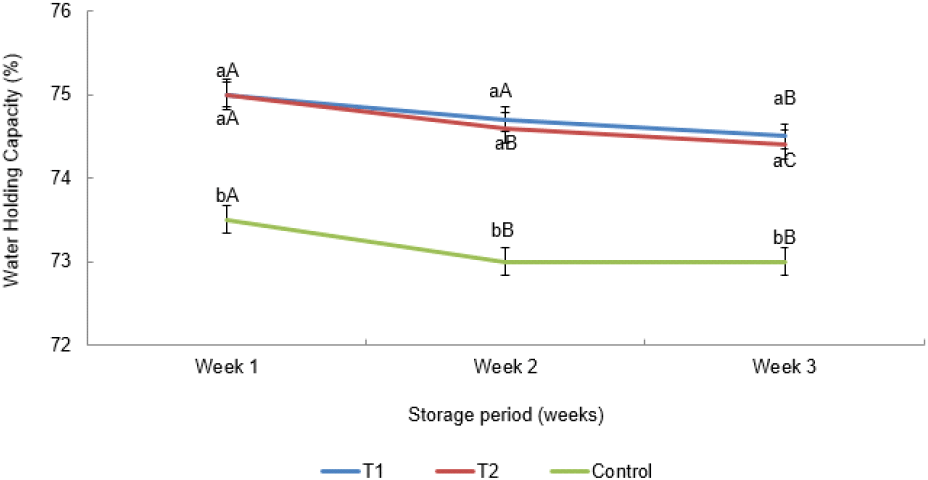
The capability of meat to hold water throughout the processing, which is added or naturally present in the meat, is referred to as WHC and it positively impacts several important qualities, including eating qualities; tenderness, juiciness, thawing drip, and cooking loss of meat (Cheng and Sun, 2008). In this study, WHC had declined continuously by numerical values with the progression of the storage period; however, it was not significantly different. Protein denaturation and declines in protein solubility might influence the losses of WHC during freezing storage (Qiao et al., 2001). The existing study did not measure the degree of protein denaturation of the chicken bockwurst sausages, and we recommend to perform a test to analyze the degree of denaturation of proteins in the sausages during the storage period.
In general, the holistic fiber structure during fiber processing creates a porous fiber network with a high interior surface area, which helps to create a variety of attachments in dietary fiber, including hydrogen ties among soluble and insoluble fiber to water as well as interactions between protein to fat. As a result of that, water binds and holds in the fiber incorporated products through baking, freezing, thawing, and storage, than the normal products (Talukder et al., 2017). In the studies of Hughes et al. (1997) found that, combining oat fiber and carrageenan on frankfurters that had various fat percentages resulted to increment in WHC by b 2% compared to the control. Further, water is retained in the frankfurters made with 29% dietary fiber suspension due to their good characteristics such as their absorption capacity (Fernandez-Gines et al., 2005).
As illustrated in Fig. 3, the sausage in all treatments showed a difference (p<0.05) until the second week of storage. The sausages without oat fiber showed higher (p<0.05) shrinkage over to the oat fiber incorporated into two sausages at the third and fourth week of the storage period. The incorporation of the oat fiber in the sausage mixture could be the reason for the reduction of shrinkage in both sausages at the completion of the storage period. Results of the present study suggested that, incorporation of oat fiber positively influenced to increase in cooking yield of the sausages as described by Trindade et al (2017). Chil et al. (2010) and Zhao et al. (2021) showed that the inclusion of dietary fiber into meat emulsion could be able to reduce shrinkage, improve emulsion stability and viscosity. Yang et al. (2010) reported that the sausages made with oatmeal had different cooking yield, hardness and a low percentage of shrinkage, which may be occurred due to the water holding properties of oat fiber. The chicken sausages prepared from low-fat with 9% of oat flour showed the highest cooking yield, followed by three different extension levels of oat flour: 6%, 3% and 0% due to the function of oat hydrocolloidal fibers which creates tridimensional matrix to hold water and fat in the sausage mixture (Reddy et al., 2012). According to the outcomes of this study, there was an influence of the storage time (week) on the shrinkage of the T1 and T2 sausages in the first and second week of the storage period.
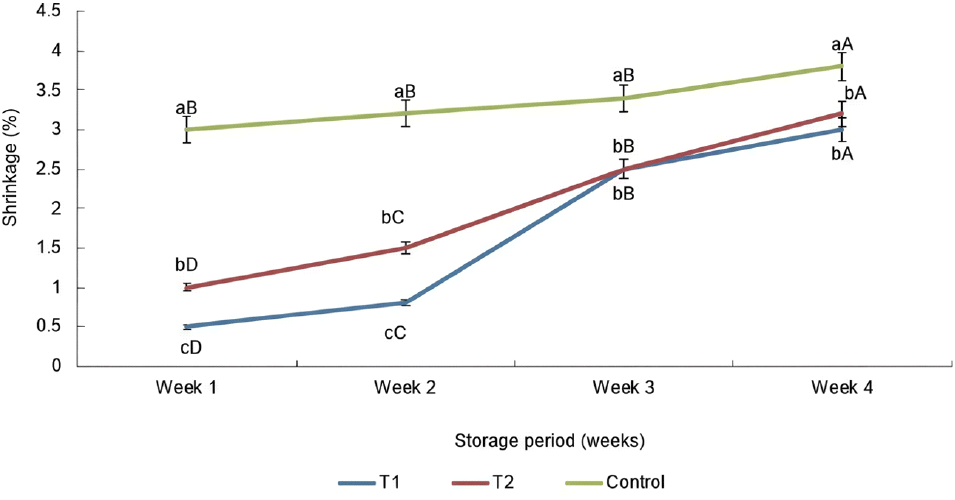
In the present study, the percentage of shrinkage of the T1 sausage sample increased (p<0.05) from the second week to the third week during the entire storage period. Comparatable to this study, the shrinkage of every sausage progressively increased as the entire storage period increased (Darwish et al., 2014; Naveen et al., 2016). These studies further mentioned that the rise of shrinkage during freezing occurred due to several causes as extreme fat separation and the discharge of water when cooking. According to Darwish et al. (2014), protein denaturation can occur, leading to deterioration of WHC that directly impacts shrinkage with the extending storage time. However, according to these investigations, the highest shrinkage was identified in the control, while the oat incorporated two sausages corresponded with the lowest (p<0.05) shrinkage of the sausages. Results highlighted that, lower shrinkage in T1 and T2 sausages can occur due to the incorporation of oat fiber in the sausages. In contrast, T1 and T2 treatments had no significant difference in shrinkage, which cannot be explained by the perfect reason. However, this study is further described that the water is likely lost as a result of heat-induced meat cooking techniques, which reduce the amount of water entrapped in protein structures by capillary forces that may not by denaturation of proteins in the sausages during storage. With the Sri Lankan cooking culture, raw and processed meat products were subjected to different cooking methods, which may have caused the shrinkage. El-sayed (2013) also reported that protein denaturation, expulsion of fat and water in beef burger patties, caused cooking loss. As well as, several factors as raw meat quality, meat composition, pre-cooking treatment, cooking technique, heating rate, cooking temperature and endpoint center temperature, impact on the cooking loss of the meat (Serrano et al., 2007). As well as Hui et al. (2001) noted that the moisture can be lost during prolonged frozen storage due to the vapor pressure gradient within the product and between the products. A similar outcome was found by Cofrades et al. (2000) and Verma et al. (2015), that retention/preservation of water and fat in the fiber incorporated sausages enhanced the cooking yield and emulsion stability.
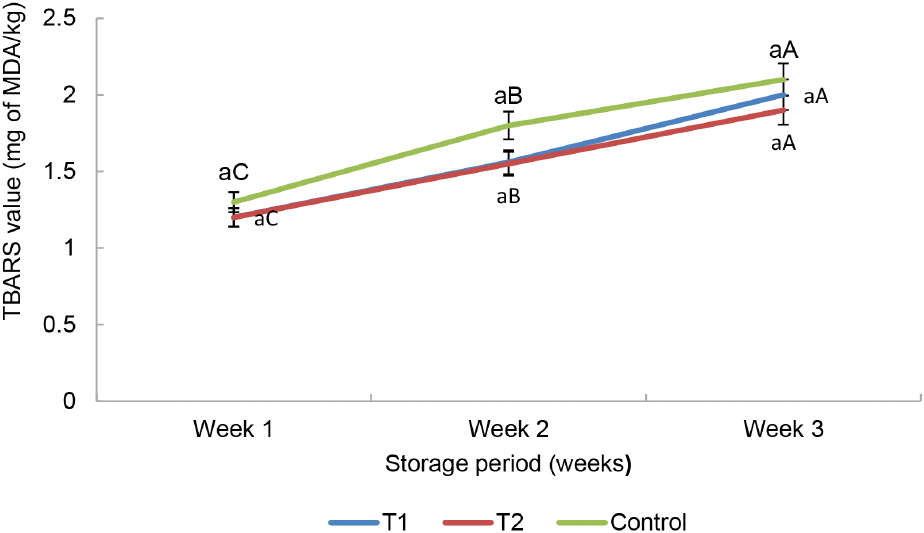
No difference (p>0.05) of TBARS was identified among all the treatment samples and during the storage period at −18°C, as shown in Fig. 4. Therefore, the results revealed that the incorporation of oat fiber in sausages didn’t impact on the TBARS value of all the samples. The conclusions drawn from the study of Souza et al. (2019) are aligned with the findings of this study. However, TBARS of all the treatments increased numerically during the storage. Hui et al. (2001) conveyed that the formation of a lower quantity of TBA acid in beef burger after processing could be primarily attributed to the auto-oxidation of lipids. The optimum limit of the TBARS value of the sausages is 2 mg MDA/kg (Connell, 1990; Wenjiao et al., 2014). Thus, the TBARS value of all the sausages in this experiment was at an acceptable level. In general, TBARS value of any sausages can be raised during the entire storage period, and it could be a reason to increase oxidation of unsaturated fatty acids and increase partial dehydration of the sausages (Wenjiao et al., 2014). Furthermore, the influence of storage time on TBARS of the sausages increased when increasing the storage period.
As illustrated in Fig. 5, pH values of all treatments were not different (p>0.05) during the four weeks of the storage period. The pH values of the control sausage at the second week showed a significant difference compared to other treatments, with the time, which was expressed as the time (week), affecting the pH values of the sausage. Results of pH values of chicken bockwurst sausages concluded that, inclusion of fiber in sausages did not influenced for pH value of the samples. The outcomes generated in this investigation are matched with the conclusions of García et al. (2002) and Mendoza et al. (2001) and who stated that incorporation of dietary fiber did not impact on pH of dry fermented sausages. However, Talukder (2015) found that, inclusion of fibers could be a reason for the change of pH values of the meat products. Similar results were revealed by Yilmaz and Daglioglu (2003) that oat bran incorporation at different levels, such as 5%, 10%, 15%, and 20% exhibited a difference in pH values of the meatballs. Moreover, Yilmaz (2004) found that the dietary fiber causes changes in the pH of wheat bran incorporated meatballs with enhancing the incorporation level of wheat bran. Steenblock et al. (2001) found that light bologna produced from oat fiber and frankfurters (fat-free) did not show any significant difference in pH between treatments and control. Therefore, the outcomes of the present study agree with the results of the study conducted by Steenblock et al. (2001).
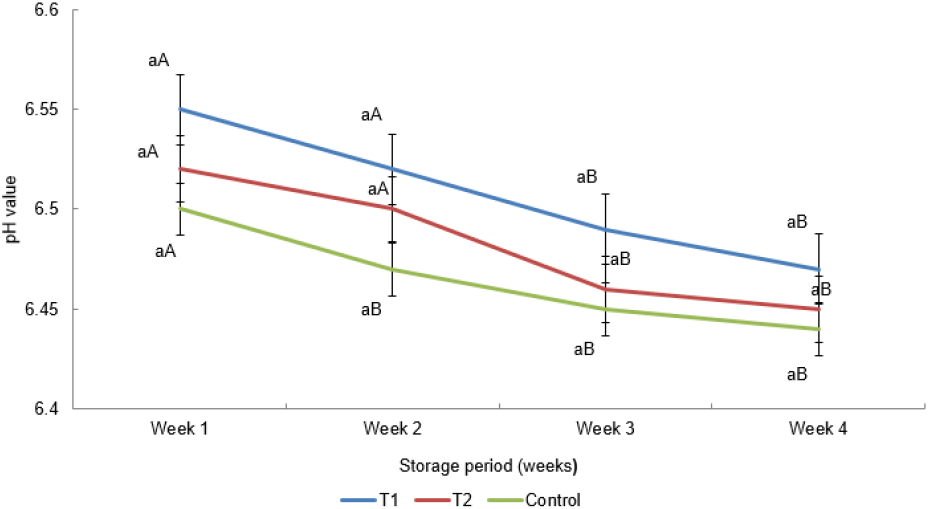
In this study, a reduction of pH of the treatments can be observed due to the conversion of sugars into lactic acid by lactic acid bacteria during the fermentation process. As a result of that, it might be caused to slow down the fat oxidation by inhibiting spoilage bacteria and oxidative enzymes because of an acidic environment (Wang et al., 2021). However, the TBARS value of all the treatments was increased during the storage period. It might have happened due to other factors that contribute to increasing the oxidation during storage, rather than pH. Huff-Lonergan and Lonergan (2005) reported that unacceptable high drip loss in the meat and the onset of low retaining capacity were linked to a drop in rapid pH and low ultimate pH. According to Choi et al. (2009; 2010), rice bran has an alkaline pH, which influenced on pH of frankfurters produced with rice bran. Khan et al. (2011) reported that the escape of imidazolium (a base active group) and the occurrence of amino acids during cooking caused attain higher pH in cooked frankfurters containing chicken skin and wheat fiber than in uncooked frankfurters.
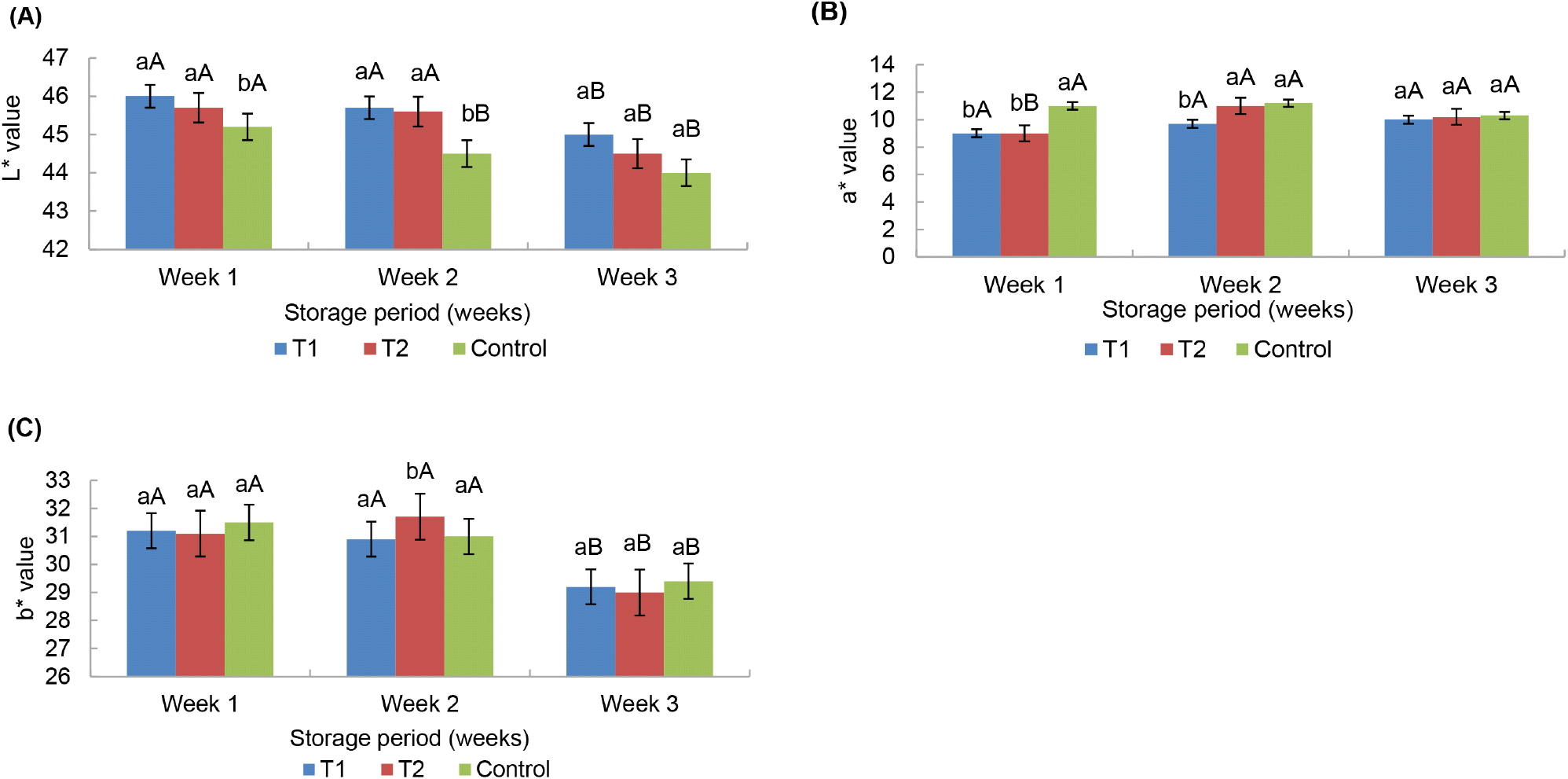
Fig. 6 shows the variation in outer color values of three chicken bockwurst sausage samples for outer lightness (A), outer redness (B), and outer yellowness (C), respectively, during three weeks of storage at −18°C. According to the L* value of the sausages in this study, the oat fiber incorporated into two sausages had higher (p<0.05) L* values related to the untreated/control sample up to the second week of the storage period. The possible reason for that could be the addition of oat fiber into sausage mixtures. When considering the storage period, there was an effect of time for the L* value of the sausages in the middle of the storage period (at the second week). According to Yilmaz and Daglioglu (2003), lightness value was increased with the inclusion of oat bran in meatballs. The studies of Yilmaz (2004) showed that the highest lightness value was given by the meatballs prepared with 20% oat bran among of different levels of oat bran incorporation: 5%, 10%, 15% and 20%. Even though the oat incorporated two sausage groups had lower (p<0.05) a* value at the first week of the entire storage period, no difference (p>0.05) was recorded between the three treatments at the end of the four weeks. Yilmaz and Daglioglu (2003) showed a simultaneously low a* value in oat bran incorporated meatballs. In the studies of Horita et al. (2011) and Steenblock et al. (2001) the oat fiber inclusion improved the inner L* value of frankfurters and simultaneously decreased the a* value. As well as, no effect of the time (week) on the a* value of the sausages was recorded at the second and third week of the storage. In the second week of the storage time framework, the sausages made from 16.50% oat fiber showed a difference in b* value. However, Steenblock et al. (2001) stated that including a higher level of oat fiber in frankfurter mixtures resulted in higher yellowness in the product compared to the products made from lower fiber levels. The added fiber content and retained extra moisture in the sausages could be the reason for this occurrence. Uneven smoking in the smoking chamber might be the cause for unusual results related to the color of sausages. Therefore, this condition should be controlled throughout the production process by providing unique smoke penetration, maintaining proper temperature and time as described by Duma-Kocan et al. (2020). Moreover, the outer color of the sausages largely varies according to the poisoning of the sausages inside the chamber used in this study.
As shown in Table 3, the highest (p<0.05) crude fiber and moisture content was observed in T1 sausage samples over to the other two samples. This could have possibly happened due to the highest incorporation level of oat fiber in the T1 sausage. The increment of moisture percentage in the sausages may positively stimulus sensory properties of the product as texture and juiciness, by creating a more porous structure of the product, which affects on improvement of WHC (Bekhit, 2017). Similar endings were obtained by Choi et al. (2009; 2012) who found that higher moisture content in meat batter samples produced with vegetable oil and rice bran fiber over to the control sample. Mansour and Khalil (1999) revealed that wheat fiber added to beef burgers had higher moisture content than the control sample due to the retention of more moisture during cooking because of the ability to bind water in fibers. Souza et al. (2019) highlighted that the oat fiber added (0.85%) to cooked Paio sausage had an increment of moisture content due to its higher water-binding capacity. According to the results in this study, no difference (p>0.05) in crude fat, crude protein and ash content was identified among all three samples. Therefore, this study stated that, inclusion of oat fiber did not impact several proximate components: crude fat, crude protein, and ash content, while the inclusion of oat fiber influenced on moisture and crude fiber content of the chicken bockwurst sausages. Reddy et al. (2012) noted that the oat incorporation did not affect on crude protein and ash content of low-fat chicken sausages. In the studies of Huang et al. (2010), the crude protein and crude fat content of the control and experimental Chinese-style sausages made from different percentages of wheat, oat and inulin did not exhibited a significant difference. Yang et al. (2010) stated that the chicken sausage batter with the incorporation of 10% hydrated oatmeal showed the moisture, protein and fat composition as 65.95±0.63%, 20.55±0.31% and 2.94±0.60%, respectively.
The total viable plate count of all the sausage samples was less than 1×105 log CFU/g, with no significant difference between the samples. The inclusion of oat fiber did not influence on microbial composition of chicken bockwurst sausages. The microbiological findings of this study are matched with the findings of Huang et al. (2010). The studies of García et al. (2002) noted that dietary fiber incorporation did not impact on microbial growth of the dry fermented sausages. Escherichia coli was not detected in any of the sausages during the storage at −18°C after one month of the storage period. The microbiological results were at an acceptable level for human consumption according to Sri Lanka Standards Specifications; SLS 1218:2001 (Sri Lanka Standard Specification for commented Meat Products). The oat fiber incorporated chicken bockwurst sausages were microbiologically safe and a quality product for the consumers within one month of the storage period.
4. Conclusions
The oat fiber incorporated into two sausages (25.00% and 16.50%) had the highest WHC and the lowest cooking loss. Incorporation of oat fiber did not influence to change in TBARS and pH of the treatments. The oat fiber (25.00%) added to the binder mixture (out of 3%) with modified starch and rusk powder could be identified as the best incorporation level because of the lowest drip loss. The 25.00% oat fiber incorporated sausage had the highest crude fiber and moisture at the end of the period, at 3.91±0.14% and 70.95±0.21%, respectively. The oat (Avena sativa L.) fiber can be introduced as an excellent binding compound in order to reduce the drip loss in the chicken bockwurst sausage by replacing modified starch in the sausage mixture. The oat fiber incorporated chicken bockwurst sausages could be introduced into the market as a nutritious, microbiological sound, good quality product, and consumer-acceptable product within one month of the storage period. Further studies should be conducted to analyze the changes of sensory properties: flavor, texture, mouth-feel, and taste, and to identify how different thawing methods affect the drip loss of meat products, such as chilling temperature, thawing at room temperature, cold water thawing, and microwave thawing.










