1. Introduction
Recently, as national income has increased, consumer interest has shifted from survival-oriented consumption to a focus on improving quality of life, leading to heightened awareness of health. Accordingly, the market for probiotics, known for their health benefits, is rapidly growing. According to the U.S. market research firm Markets and Markets, the global probiotics market is projected to grow from $61.4 billion in 2021 to $91.5 billion in 2026, with an average annual growth rate of 8.3% (Son, 2022). The domestic (Korean) market is also expected to expand from 842 billion KRW in 2021 to 2.5143 trillion KRW in 2026, representing a high annual growth rate of 16% (Shim et al., 2024).
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Al-Shawi et al., 2020). This definition implies that probiotics must remain viable at the time of consumption.
Therefore, the selection of an appropriate food matrix and the processing and storage conditions for delivering probiotics are of great importance (Wang et al., 2024). In addition, for probiotics to confer health benefits, the minimum viable cell count in probiotic products should meet the threshold of 1 to 10 billion CFU (Ministry of Food and Drug Safety, 2025). These benefits are generally strain- or species-specific and include maintenance of a healthy gut microbiota, potential anti-cancer effects observed in experimental studies, and alleviation of lactose intolerance symptoms. Furthermore, probiotics are also used for therapeutic purposes such as the management of constipation, diarrhea, hypercholesterolemia, and inflammatory bowel disease (Lee et al., 2021; Rastogi and Singh, 2022; Sugimura et al., 2020).
However, despite the recent rapid growth of the probiotic market, the quality of many commercial probiotic products remains substandard, with viable cell counts often falling short of the levels claimed on product labels (Sarkar, 2018). The survival of probiotics is influenced by various factors during food processing, storage, and gastrointestinal transit, including the presence of oxygen, pH, water activity (aw), and temperature (Albadran et al., 2015). In particular, during export and distribution, factors such as temperature, humidity, and storage conditions can lead to a rapid decline in viable counts, with many cases failing to meet the minimum required levels (Kang, 2021). To address these issues, the Ministry of Food and Drug Safety (MFDS) in Korea has implemented mandatory inspection orders, and several imported and exported products have been subject to regulatory action due to non-compliance with viable cell standards. Furthermore, ensuring quality reliability is a key factor for competitiveness in global markets, highlighting the urgent need for research on probiotic viability and functionality under export-related environmental conditions.
Therefore, various technologies have been proposed to maintain the viability and functionality of probiotics during storage. Fredua-Agyeman (2024) applied protective agents such as glycerol, skim milk, and trehalose, along with enteric coating techniques, to effectively minimize viability loss of Lactobacillus acidophilus during storage at 4°C and 25°C. Nag et al. (2013) reported that Lactobacillus casei CRL 431, stabilized through fluidized bed drying and osmotic stress adaptation, showed significantly higher survival rates after 20 weeks of storage at 25°C when treated with vitamin E, compared to the control. Additionally, Afzaal et al. (2022) confirmed that alginate-inulin-based encapsulation of L. acidophilus maintained the probiotic viability within the recommended levels (107−108 CFU/mL) for food carriers during storage. However, most of these studies have been limited to laboratory-scale conditions, and research reflecting real distribution environments particularly high-temperature and high-humidity conditions encountered during long-distance sea transportation is lacking. Simulated experiments and the development of preservation technologies that reflect transport conditions remain limited, highlighting the need for more empirical studies to ensure product stability and enhance consumer trust in global markets.
Therefore, this study aims to identify the key factors contributing to the reduction in viable cell counts and functionality of lactic acid bacteria (LAB) under various maritime distribution conditions and to provide foundational data for enhancing the global competitiveness of LAB-based health functional foods. Through this, it is expected to establish a scientific basis for the development of LAB products suitable for export and distribution environments and to contribute to securing quality reliability in international markets.
2. Materials and methods
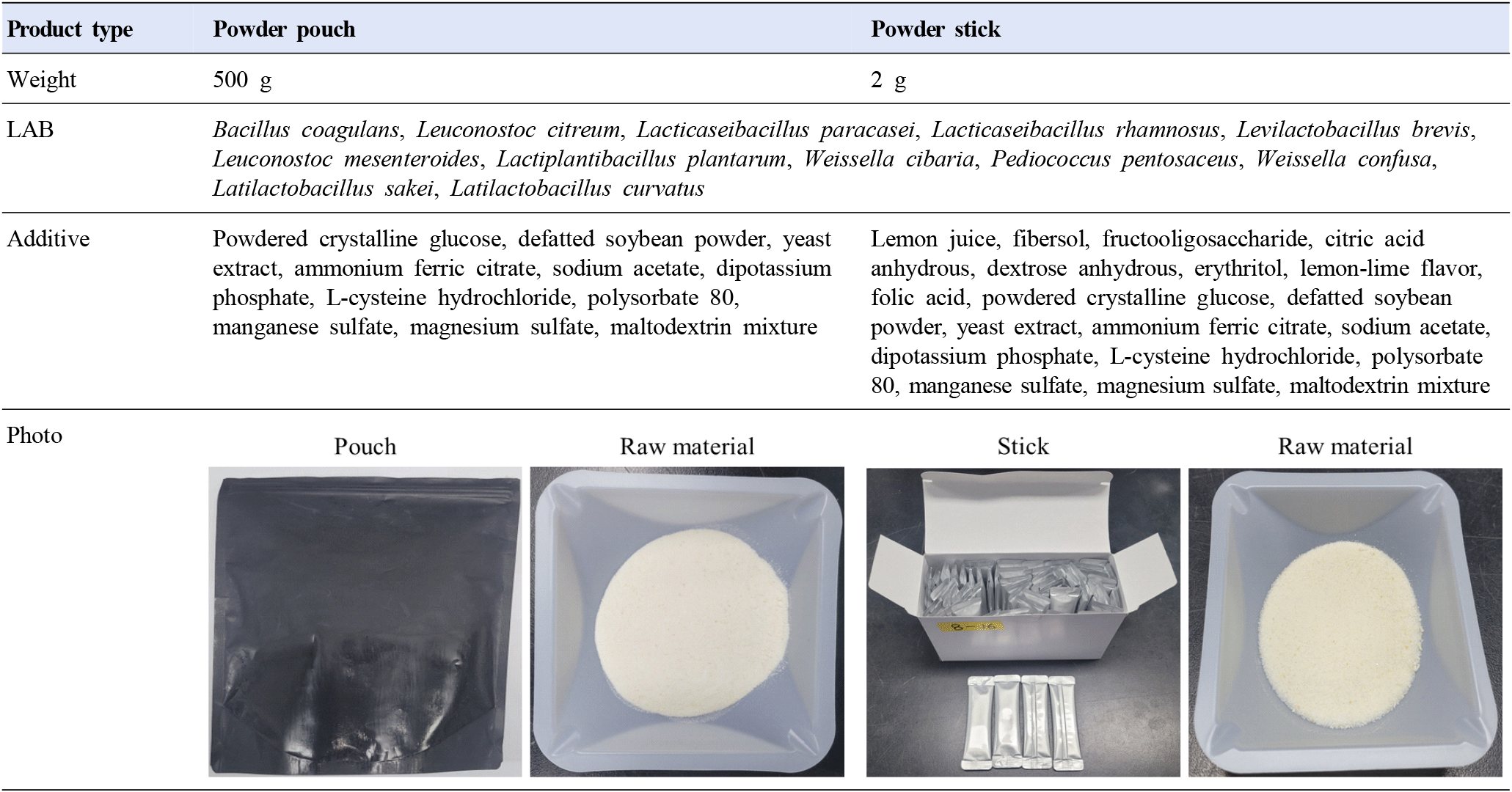
This experiment used two types of LAB products manufactured by InoB Science, and the specifications of these products are shown in Fig. 1. De Man, Rogosa, and Sharpe (MRS) medium was purchased from BD Difco (Sparks, MD, USA). Lipopolysaccharide (LPS) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin (PS) were purchased from HyClone (Pittsburgh, PA, USA). The Mouse TNF ELISA set was obtained from BD Biosciences (San Diego, CA, USA).
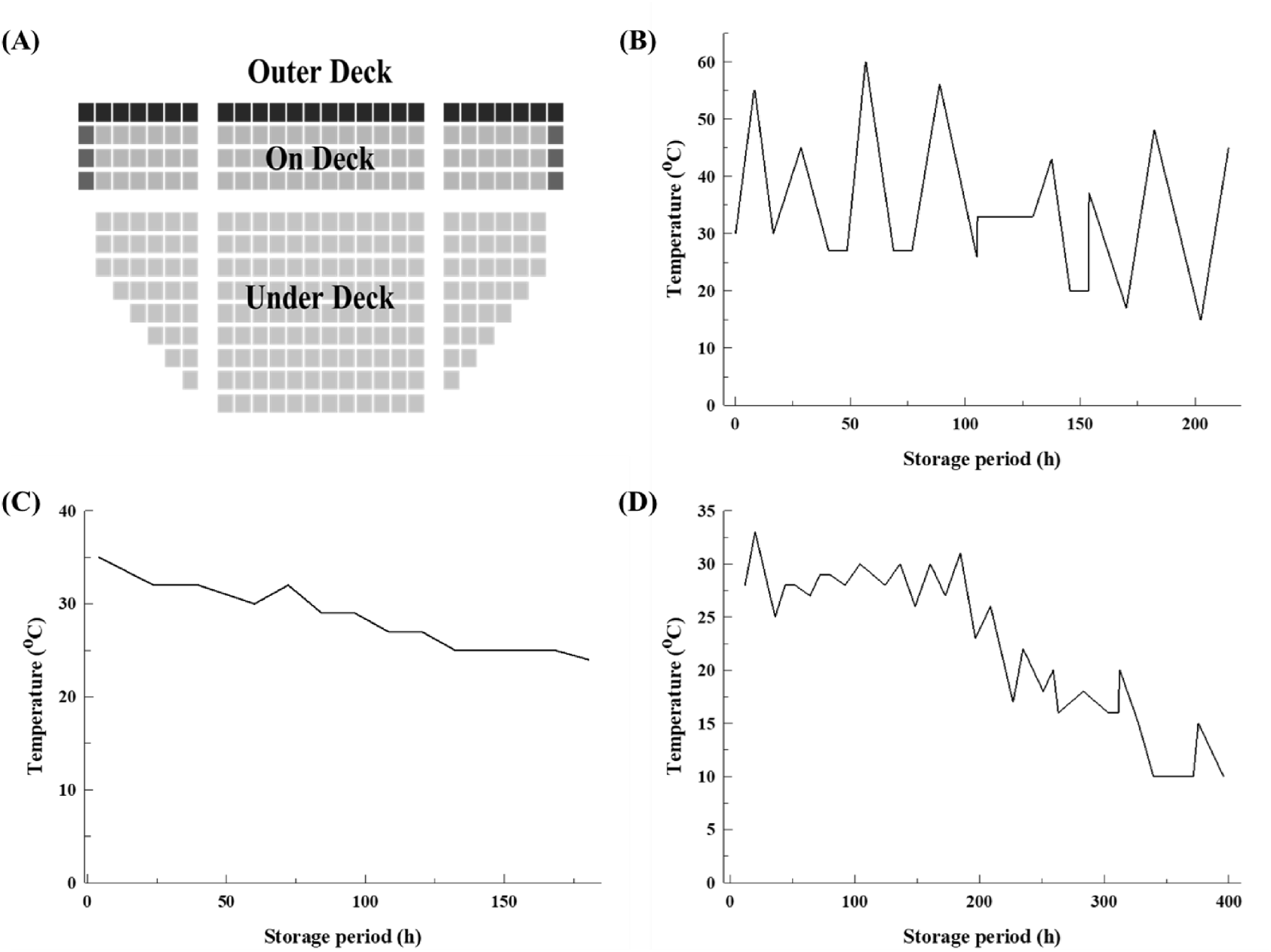
For the export of LAB products, transportation temperature and period were based on typical shipping conditions to South America. As shown in Fig. 2, export distribution temperatures were set in a walk-in chamber (THC3000, JinsungPLT, Incheon, Korea) to 15-60°C for the outermost on-deck, 10-33°C for the on-deck, and 24-35°C for the under-deck conditions. These temperature settings were established with reference to the marine container thermal conditions reported by Kondo (2011), and the experiment was conducted over 21 days.
To measure the viable cell count of LAB in the products used for evaluating simulated export distribution conditions, 1 g of the sample was mixed with 9 mL of PBS buffer and homogenized at 180 rpm for 10 min. Then, the homogenate was diluted with PBS buffer to a concentration of 101 to 109 and plated in triplicate on MRS agar. The Petri dishes inoculated with the samples were incubated at 37°C for 24 h, after which the colonies were counted, and the viable cell count per gram of the sample was calculated.
The macrophage cell line RAW 264.7 was obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea) and cultured in DMEM medium supplemented with 10% FBS and 100 units/mL of penicillin-streptomycin (PS) in a 37°C incubator with 5% CO2.
RAW 264.7 cells were seeded in 24-well plates at a density of 5×105 cells/well and incubated for 24 h in a 5% CO2 incubator. Pouch products (log CFU/g) stored for 1, 11, and 21 days, and stick products stored for 1, 11, and 21 days were added and incubated for 24 h. After incubation, 0.5 mg/mL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution was added, and the cells were further incubated for 4 h. Then, the supernatant was removed, and the resulting formazan crystals were dissolved by adding 100 μL of DMSO. The absorbance of the dissolved solution was measured at 570 nm using a microplate reader.
To evaluate the influence of LAB on NO production under used simulated export distribution conditions, measurements were performed using the method adapted from Green et al. (1982). RAW 264.7 cells were seeded in a 24-well plate at a density of 5×105 cells/well and incubated for 24 h. The LAB products with storage periods of 1, 11, and 21 days were pre-treated with the cells for 2 h, followed by the addition of 1 μg/mL LPS, and cultured for 24 h. Subsequently, 100 μL of the cell culture supernatant was mixed with 100 μL of Griess reagent [0.1% N-(1-naphthyl) ethylenediamine and 1% sulfanilamide in a 1:1 ratio] in a new 96-well plate and allowed to react for 10 min. The absorbance was measured at 570 nm using a microplate reader.
RAW 264.7 cells were seeded in a 24-well plate at a density of 5×105 cells/well and cultured in a 37°C incubator with 5% CO2. After pre-treating the cells with lactic acid bacteria products at 1, 11, and 21 days of storage for 2 h, 1 μg/mL of LPS was added to induce cytokine production for 24 h. The culture supernatant was then collected, and the secretion levels of TNF-α, IL-1β, and IL-6 were measured using the ELISA MAX™ Deluxe Set (BioLegend, San Diego, CA, USA) ELISA kit. The experimental procedure was conducted according to the manufacturer’s instructions (Jo et al., 2019).
Statistical analysis was performed using the statistical package GraphPad Prism 8.0 (Graph Pad Software Inc., San Diego, CA, United States). Results were analyzed using one-way analysis of variance (ANOVA), and statistical significance was evaluated using Tukey’s multiple comparison test at p<0.05.
3. Results and discussion
In this experiment, LAB products (pouch and stick) were stored for 21 days under temperature conditions representing the outer-deck, on-deck, and under-deck areas of a ship, and changes in the viable counts of lactic acid bacteria detected on MRS agar were investigated.
The results of the viable cell count of LAB in pouch products, according to storage period and loading position under a simulated ship transport environment, are shown in Fig. 3. Under the outer-deck condition, the viable cell counts on days 0, 1, 7, 14, and 21 were 13.5, 13.5, 11.1, 7.5, and 8.0 log CFU/g, respectively. For the on-deck condition, the counts were 13.5, 13.5, 11.0, 8.9, and 9.2 log CFU/g, and for the under-deck condition, they were 13.5, 13.4, 11.3, 8.7, and 9.0 log CFU/g (Fig. 3A).
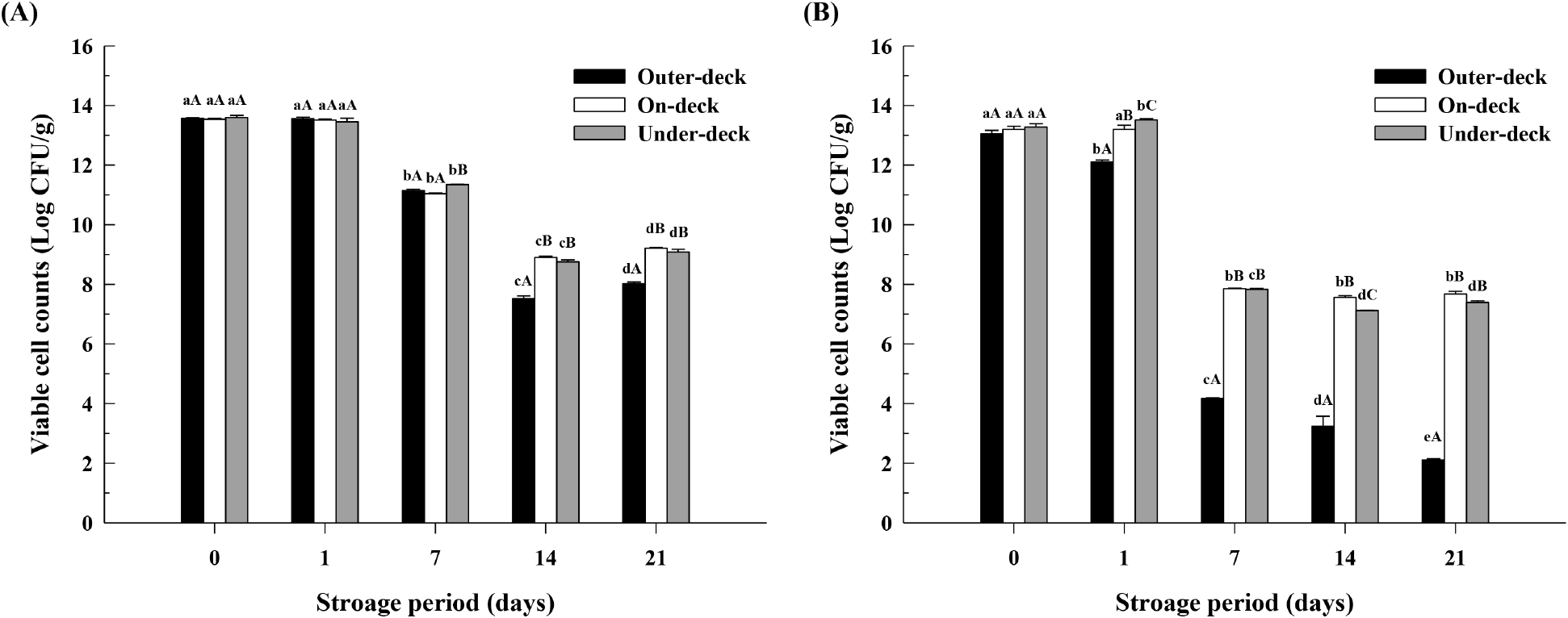
The results of the viable cell count of LAB in stick products, according to storage period and loading position under a simulated ship transport environment, are shown in Fig. 3B. Under the outer-deck condition, the viable cell counts on days 0, 1, 7, 14, and 21 were 13.1, 12.0, 4.1, 3.2, and 2.1 log CFU/g, respectively. For the on-deck condition, the counts were 13.0, 13.1, 7.8, 7.5, and 7.6 log CFU/g, and for the under-deck condition, they were 13.0, 13.5, 7.8, 7.1, and 7.3 log CFU/g (Fig. 3B).
The observation of differences in viable counts of LAB revealed a decreasing trend over the storage period on MRS agar. Notably, the highest reduction in viable counts occurred under the outer-deck conditions, indicating that the rapidly fluctuating temperatures (15-60°C) during the storage process in the shipping environment significantly affect the survival rate of LAB. Furthermore, it was confirmed that the product type also influenced the viable counts. The pouch product maintained counts above 10 log CFU/g from days 1 to 8, whereas the stick product dropped below 10 log CFU/g starting from day 6, with the LAB in the outer-deck condition decreasing to 6.47 log CFU/g by day 3. This is
attributed to the greater surface area of the stick product, which allows external temperatures to be transmitted more easily to the interior, thereby reducing the viable counts. This phenomenon can be attributed to the greater surface area-to-volume ratio of the stick product, which facilitates faster heat transfer from the external environment to the interior. As a result, the stick product is more susceptible to rapid temperature fluctuations, leading to greater thermal stress and reduced LAB viability. This aligns with previous findings that products with higher surface area-to-volume ratios tend to exhibit more pronounced temperature-induced degradation due to enhanced heat transfer efficiency (Raval et al., 2013; Singh et al., 2017; Yin et al., 2024). According to Kim et al. (2018), a study measuring changes in viable counts of LAB at various preservation temperatures (-20, 4, 20, and 40°C) over preservation periods (1, 3, and 6 months) showed an average reduction rate of 59% over 1 to 6 months. The reduction rates due to temperature changes were 41% at 4°C, 54% at 20°C, 55% at −20°C, and 84% at 40°C. Thus, the lowest reduction rate was observed at 4°C, while the highest occurred at 40°C.
Prior to evaluating the NO production of the LAB products, the cell viability of RAW 264.7 cells was assessed according to the stacking position and storage period (Fig. 4). For the pouch products stored for 1, 11, and 21 days, cytotoxicity was evaluated at the outer-deck (13.5, 8.4, 7.9 log CFU/g), on-deck (13.5, 8.9, 9.1 log CFU/g), and under-deck (13.4, 8.9, 9.0 log CFU/g), and over 95% cell viability was observed in all cases (Fig. 4A). Likewise, for the stick products stored for 1, 11, and 21 days, cytotoxicity was evaluated at the outer-deck (12.0, 4.3, 2.0 log CFU/g), on-deck (13.2, 7.5, 7.5 log CFU/g), and under-deck (13.5, 7.4, 7.3 log CFU/g), all of which also exhibited over 95% cell viability (Fig. 4B).
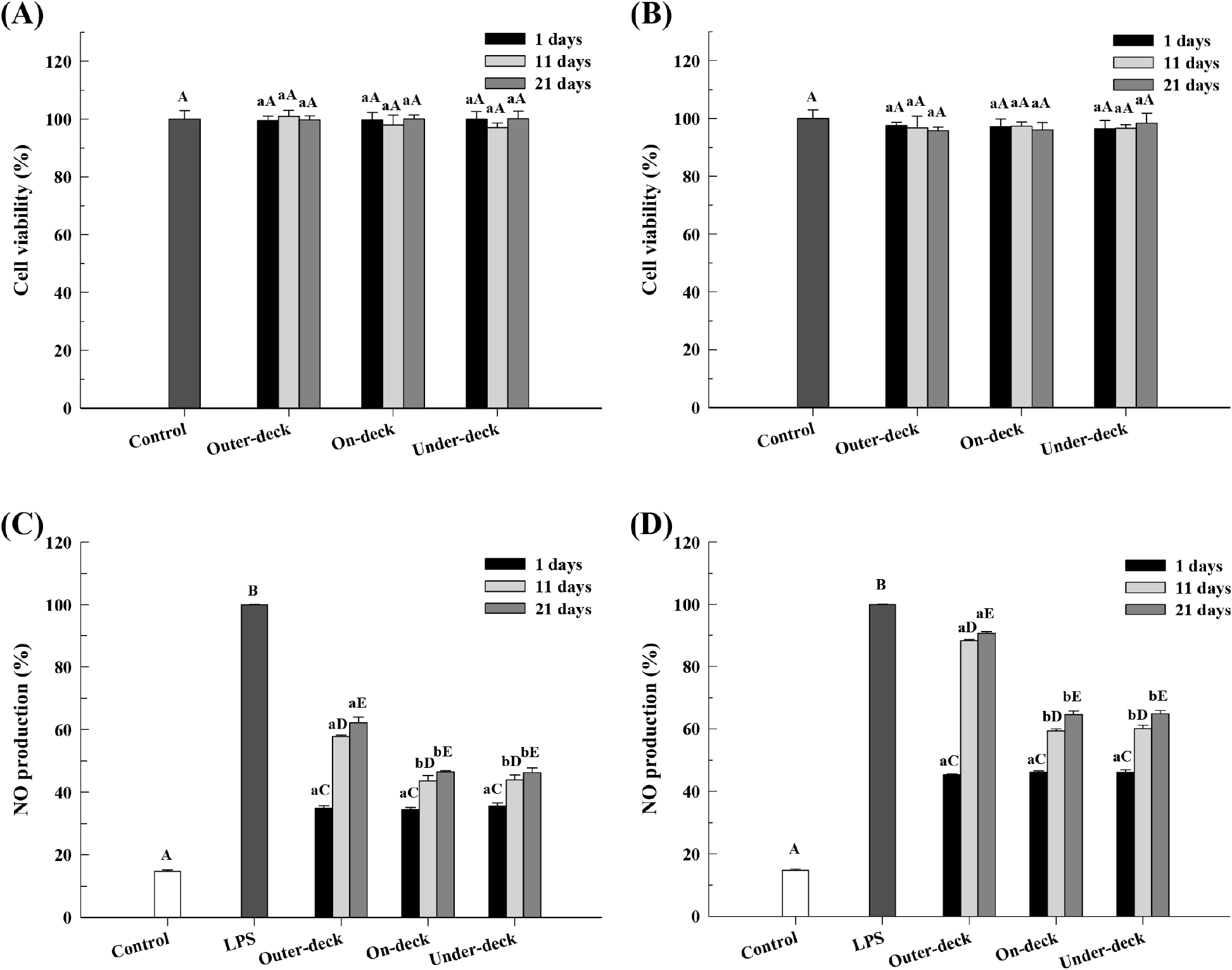
LAB have been reported to exhibit anti-inflammatory effects by modulating immune responses and suppressing the production of NO, a pro-inflammatory mediator. Among these, NO plays a critical role in inflammatory conditions such as sepsis and LPS-induced inflammation and is widely used as a key indicator for evaluating the anti-inflammatory activity of LAB (Lee et al., 2015). In this study, we aimed to investigate how changes in storage conditions, including loading position on a ship and storage period, affect the anti-inflammatory activity of LAB products by evaluating their NO production.
The NO production of pouch-type products according to the storage period and loading location on the ship is shown in Fig. 4C. The NO production reduction effect of products stored under-deck for 1-21 days was 35%, 43%, and 46%, on-deck was 34%, 43%, and 46%, and outer-deck was 34%, 57%, and 62%, showing the greatest reduction in NO production.
The NO production of stick-type products according to the storage period and loading location on the simulated ship is shown in Fig. 4D. The NO production reduction effect of products stored under-deck for 1-21 days was 46%, 60%, and 64%, on-deck was 46%, 59%, and 64%, and outer-deck was 45%, 88%, and 90%, showing the greatest reduction in NO production.
Evaluation of the NO inhibition capacity of LAB products showed a trend of decreasing activity with longer storage periods. For pouch products, the NO inhibition capacity decreased to similar levels on days 11 and 21 under both under-deck and on-deck conditions, while it continued to decline through day 21 under outer-deck conditions. The stick products displayed a similar trend to the pouch products but exhibited a greater decrease in NO inhibition capacity. This significant reduction is likely to be due to a greater decline in the viable cell count of LAB in the stick products. The survival of LAB strains during storage is influenced by factors such as water activity (aw), glass transition temperature (Tg), types of coating materials, and drying methods. In particular, elevated storage temperature and water activity have been reported to induce structural changes and reduce physiological activity of the strains, thereby leading to a decline in their functional properties (Dianawati et al., 2013; Higl et al., 2007; Kurtmann et al., 2009).
Macrophages play a crucial role in maintaining homeostasis and mediating inflammatory responses by eliminating invading bacteria and relaying this information to T-cells, thus contributing to immune defense. When stimulated by external factors, macrophages produce inflammatory substances, such as TNF-α, via signal transduction (Lee et al., 2022). Cytokines secreted by immune cells regulate the proliferation, activation, and differentiation of immune cells, thereby promoting inflammation (Namkoong et al., 2015). When the inflammatory inducer LPS is applied to macrophages, it stimulates cytokine production, and TNF-α, IL-1β, and IL-6 are known as key pro-inflammatory cytokines involved in regulating the inflammatory response (Chung et al., 2001). Accordingly, this study compared the anti-inflammatory effects of LAB products (pouch and stick) based on their loading positions on the ship by evaluating the production levels of pro-inflammatory cytokines (Fig. 5).
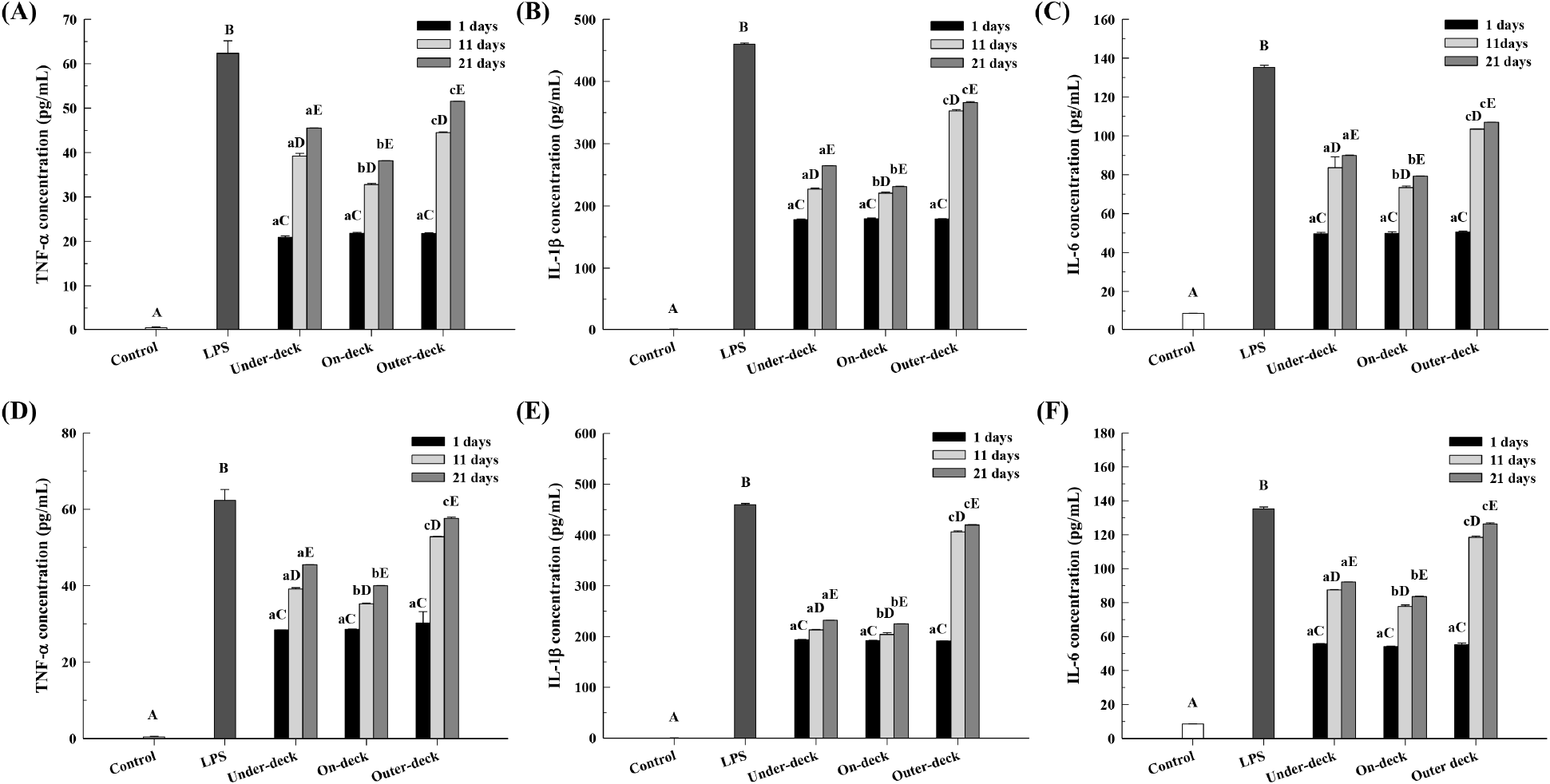
When RAW 264.7 cells were treated with LPS, production of TNF-α, IL-1β, and IL-6 increased; however, cytokine levels decreased with LAB product treatment. Nevertheless, the cytokine inhibition efficacy declined with extended storage period, with the largest decrease observed under outer-deck conditions, where temperature fluctuations were greatest. Similar to the NO activity results, it is inferred that the reduction in functional efficacy, such as anti-inflammatory activity, is due to the decline in viable LAB cell count within the products over time.
4. Conclusions
LAB provide health benefits, including immune enhancement, anticancer effects, and lowering blood cholesterol levels. As a result, they have been incorporated into various products. While the LAB market is continually expanding with increasing exports, concerns have emerged regarding the guaranteed viable cell counts due to external factors such as distribution conditions. Therefore, this study evaluated the viable cell counts and strain-based functional efficacy (anti-inflammatory effect) of LAB products (pouch and stick forms) under simulated marine shipping environments (outer-deck, on-deck, under-deck). The change in the viable cell count compared to the initial concentration for 21 days in the pouch product was approximately 4.5 log CFU/g in the under-deck, 4.3 log CFU/g in the on-deck, and 5.5 log CFU/g in the outer-deck. In the case of the stick product, the decrease was 5.7 log CFU/g in the under-deck, 5.4 log CFU/g in the on-deck, and 11.0 log CFU/g in the outer-deck. As a result of evaluating the NO production ability of the products according to the loading location and storage period (1 day, 11 days, and 21 days), the NO production ability increased by 35.6%, 43.9%, and 46.2% for the under-deck of the pouch product; 34.4%, 43.5%, and 46.4% for the on-deck; and 34.8%, 57.7%, and 62.1% for the outer-deck, thereby decreasing the anti-inflammatory effect. In the case of the stick product, the anti-inflammatory effect decreased over time by 46.1%, 60.1%, and 64.8% for the under-deck; 46.1%, 59.3%, and 64.5% for the on-deck; and 45.3%, 88.2%, and 90.5% for the outer-deck. The levels of cytokine production (TNF-α, IL-1β, IL-6) followed a similar pattern to the NO inhibition results, with a noticeable decrease in efficacy over time, especially under outer-deck conditions. This study confirmed that the viable bacterial count of LAB products decreases during transportation in the following order: outer-deck ˃ on-deck ˃ under-deck. Therefore, it is essential to devise a strategy to maintain the initial quality and prevent loss of functional efficacy until the product is delivered to the consumer. This will ultimately contribute to increasing the reliability of LAB products and improving consumer satisfaction.










