1. Introduction
Seaweed is one of the major aquatic resources utilized across different industries, including food, pharmaceuticals, and cosmetics, due to its diverse functional properties. According to FAO (2021), global algae production has increased more than 60-fold from 1950 to 2019, with Asia contributing most of this growth. Countries such as China, Indonesia, Korea, and the Philippines rank among the world’s leading seaweed producers. Additionally, it was reported that seaweed aquaculture constitutes 51.3% of global mariculture, having an annual growth rate of 6.2% (Duarte et al., 2021).
Based on pigments and chloroplasts, seaweeds are divided into three major groups: brown (Phaeophyceae), green (Chlorophyta), and red (Rhodophyta). Among the brown seaweeds, Undaria pinnatifida, Saccharina japonica, and Sargassum fusiforme are the most widely cultivated species (Sugumaran et al., 2022). U. pinnatifida, locally known as miyeok, is one of the primary seaweed species produced in Korea, with a production volume of 585,955 metric tons in 2022 (Marine Fisheries Statistics System, 2022). It has been cultivated for centuries, primarily for food consumption. Traditionally, it is prepared as a soup and served to mothers after childbirth, as it is believed to have detoxifying properties and support recovery.
U. pinnatifida is popular among consumers not only for its rich flavor but also for its health-promoting properties. It is a valuable reservoir of dietary fiber, minerals, vitamins, and bioactive compounds including polyphenols, flavonoids, and polysaccharides, having several health benefits, such as anti-inflammatory, anti-diabetic, anti-hypertensive, anti-cancer, antimicrobial, antioxidant, and photoprotective activities (Jiang et al., 2021). Moreover, aside from phenolic compounds, the functional pigments in U. pinnatifida act as potent antioxidants, inhibiting enzymes associated with skin wrinkling and aging (Martinez and Becherucci, 2022). With the increasing risks and adverse effects of synthetic chemicals, natural product-derived ingredients, such as seaweeds, are gaining popularity as efficient, cost-effective, and safe alternatives in both the pharmaceutical and cosmetic industries (Liu, 2022).
Since seaweeds naturally have high moisture content and are highly perishable, drying is an essential pre-treatment step before industrial processing. It also plays a key role in material preparation for compound extraction, such as pigments (Santhoshkumar et al., 2023). In many coastal areas, seaweeds are commonly dried using solar heat. However, sun-drying often results in significant quality changes. Higher drying temperatures can induce biochemical reactions such as Maillard reactions, vitamin degradation, fat oxidation, protein denaturation, and changes in sensory properties (Bonazzi and Dumoulin, 2011). For the production of high-value products, freeze-drying is often preferred, although its high cost remains a major drawback. Oven drying at 40°C, on the other hand, showed a favorable yield of fucoxanthin and pheophytin a from the brown seaweed Fucus vesiculosus, while negatively impacting the extraction of phenolic compounds (Silva et al., 2019). Microwave drying, which offers the advantage of shorter drying times compared to other techniques, showed higher antioxidant capacities in some brown seaweed species compared to oven drying (Badmus et al., 2019).
Given the growing demand for stable, high-quality seaweed products, alternative drying methods are being explored to retain bioactive compounds while minimizing costs. In this study, the influence of various drying conditions (freeze, hot-air, and microwave drying) on the functional pigments and antioxidant properties of U. pinnatifida were investigated.
2. Materials and methods
The standard chemicals utilized in this research, including DPPH reagent, gallic acid, ABTS reagent, ascorbic acid, Folin reagent, quercetin, sodium carbonate, phloroglucinol, and standards for phylloquinone and menaquinone, were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium cyanide and sodium acetate trihydrate were purchased from Wako Pure Chemical Industries (Osaka, Japan), while acetone and potassium carbonate anhydrous were sourced from Daejung Chemicals & Metals Co., Ltd. (Siheung, Korea).
Fresh U. pinnatifida samples were obtained from Bada & Haecho Fishery Corp. and harvested from the coastal waters of Goheung, Jeonnam, Korea, between March and May 2023. The fresh samples were thoroughly washed with running water, drained, and dried before used in the analysis. The drying process was carried out using three distinct methods: freeze-drying at −70°C with a freeze dryer (Il-Shin Freeze Dryer Series, Il-Shinbiobase Co., Ltd., Yangju, Korea), hot-air drying at 40°C (HB-502M, HanBaek Scientific Co., Ltd., Bucheon, Korea), and microwave drying at 260 W (ER-4320B, LG Electronics Tianjin Appliances Co., Ltd., Seoul, Korea) (Templonuevo et al., 2014). The dried U. pinnatifida was pulverized with a blender (HMF-3250S, Hanil Science Industrial Co., Ltd., Gwangju, Korea) and passed through a 300 μm mesh sieve (Laboratory Test Sieve No. 50). The processed samples were then used for further analyses.
The color properties of the dried U. pinnatifida samples were evaluated using a Chroma Meter (Minolta CR-200, Minolta Co., Osaka, Japan), calibrated with standard white plates (L=97.06, a=−0.15, b=+1.94). Chromaticity values for each sample were recorded five times, and the average was then computed. The results were presented as follows: L* (lightness), a* (redness), and b* (yellowness).
The total carotenoid and fucoxanthin contents as well as the chlorophyll content were determined using the modified methods of Osório et al. (2020). All measurements were performed in triplicate (n=3).
The total carotenoid content was determined by adding 10 mL of 100% methanol to 100 mg of dried samples and vortexing for 1 min. The mixture was then centrifuged at 700 ×g for 10 min using a centrifuge (MF-550, Hanil Science Co., Gwangju, Korea). The resulting supernatant was subjected to a second centrifugation at 1,413.2 ×g for 5 min. Absorbance of the final supernatant was measured at 480 nm and 750 nm using a microplate reader (Eon, BioTek Instruments, Winooski, VT, USA), with 100% methanol as the blank. The total carotenoid content was calculated according to the following equation (1).
The total fucoxanthin content was analyzed by mixing 100 mg of dried samples with 10 mL of a DMSO-water mixture (4:1, v/v) and vortexing for one minute. The mixture was then centrifuged at 700 ×g for 10 min (MF-550, Hanil Science Co.), and the supernatant underwent further centrifugation at 1,413.2 ×g for 5 min. Absorbance of the supernatant was measured at 480, 582, 631, 665, and 750 nm using a microplate reader (Eon, BioTek Instruments), with a DMSO-water blank as the reference. The total fucoxanthin level was calculated according to the following equation (2).
The chlorophyll contents were measured by mixing 100 mg of dried samples in 10 mL of 90% acetone, followed by 1 min vortexing. Then, mixture was then centrifuged at 700 ×g for 10 min (MF-550, Hanil Science Co.), and the supernatant was further centrifuged for 5 min at 1,413.2 ×g. Absorbance readings were taken at 630, 647, 664, 691, and 750 nm using a microplate reader (Eon, BioTek Instruments), with 90% acetone serving as the blank. The chlorophyll contents were calculated according to the following equation (3)-(7).
The total polyphenol content was quantified following a modified version of the method described by Singleton et al. (1999). Dried samples were extracted in 70% ethanol at a ratio of 1:20 (w/v) using an ultrasonic extractor (8893-DHT, Cole-Parmer) at 40°C for 1 h. Briefly, 40 μL of the U. pinnatifida extract was mixed with 200 μL of distilled water and 20 μL of Folin–Ciocalteu reagent, followed by a 3-min incubation. Next, 40 μL of sodium nitrate (Na2NO3) was added, and the reaction mixture was kept in the dark for 1 h. Absorbance was recorded at 750 nm using a microplate reader (Eon, BioTek Instruments). Gallic acid served as the reference standard, and results were expressed as gallic acid equivalents (μg/g). A standard curve was constructed using gallic acid concentrations of 0, 20, 40, 60, 80, and 100 μg/mL. All measurements were conducted in triplicate.
The total phlorotannin content was quantified using a modified method described by Li et al. (2017). Briefly, 20 μL of U. pinnatifida extract was mixed with 130 μL of double-distilled water, 50 μL of Folin–Ciocalteu reagent, and 100 μL of sodium carbonate solution. The mixture was then incubated in the dark for 1 h to allow the reaction to proceed. Absorbance was recorded at 770 nm using a microplate reader. Phloroglucinol was used as the calibration standard, and results were reported as phloroglucinol equivalents (μg/g). A standard curve was generated using phloroglucinol concentrations of 0, 20, 40, 60, 80, and 100 μg/mL. All analyses were performed in triplicate.
The total flavonoid content was determined using a modified method from Zhishen et al. (1999). Briefly, 100 μL of U. pinnatifida extract was mixed with 400 μL of 80% ethanol and 30 μL of 5% sodium nitrite (NaNO2). The mixture was vortexed for 30 sec and reaction was allowed within 5 min. Following this, 30 μL of 10% aluminum chloride (AlCl3) was added, and the reaction was continued for another 5 min. Afterwards, 200 μL of double-distilled water was added, followed by an additional 30-sec vortexing. Absorbance was measured at 420 nm using a microplate reader (Eon, BioTek Instruments), with quercetin used as the standard. The results were expressed as quercetin equivalents (μg/g). A calibration curve was constructed using standard concentrations of 0, 100, 200, 400, 600, 800, and 1,000 μg/mL, and analysis was performed in triplicate.
The modified version of the method by Blois (1958) was used to determine the DPPH radical scavenging activity. First, 60 μL of U. pinnatifida extract was mixed with 240 μL of a 0.02 mM DPPH solution and incubated for 30 min in the dark to allow reaction. The DPPH solution was made by mixing 0.00789 g of DPPH reagent with 100 mL of 70% ethanol. A microplate reader (Eon, BioTek Instruments) was used to measure the absorbance at 517 nm. The results were reported as gallic acid equivalents (μg/g), with gallic acid serving as the standard. A calibration curve was constructed using concentrations of 0, 2, 4, 8, 12, 16, and 20 μg/mL, and measurements were performed in triplicate.
The ABTS radical scavenging activity was evaluated based on the modified method from Arts et al. (2004). Briefly, 15 μL of U. pinnatifida extract was combined with 285 μL of ABTS solution, prepared by dissolving ABTS reagent in a 2.45 mM potassium persulfate buffer. The reaction mixture was incubated in the dark for 7 min, after which the absorbance was measured at 734 nm using a microplate reader (Eon, BioTek Instruments). Ascorbic acid was used as the standard, and the antioxidant activity was expressed as ascorbic acid equivalents (mg/g). A calibration curve was constructed using standard concentrations of 0, 20, 40, 60, 80, and 100 μg/mL, analysis was performed in triplicate.
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA followed by Duncan’s multiple range test was used to determine the significant differences among the treatments at p<0.05. Correlation analysis was conducted using Pearson’s correlation coefficient.
3. Results and discussion
The color properties of dried U. pinnatifida is presented in Table 1. Among the drying methods, freeze-dried sample exhibited the highest L* value (p<0.05), showing the lightest color, followed by both hot-air dried sample and microwave-dried samples. The lower L* values observed in heat drying suggests color deterioration due to high temperature exposure. Comparable results were observed by Zhao et al. (2022), wherein lower L* and b* values were attained by hot-air, microwave, and sun-dried Sargassum fusiforme brown alga compared to freeze-dried samples. This phenomenon may be attributed to the Maillard and enzymatic browning reactions which usually happen during the drying process (Bejar et al., 2011).
| Drying conditions | L*1) | a*2) | b*3) |
|---|---|---|---|
| Freeze-dried | 42.03±0.064)a | −4.22±0.04b | 9.96±0.01a |
| Hot-air dried | 38.85±0.02b | −3.69±0.03b | 9.51±0.02b |
| Microwave-dried | 38.87±0.05b | −3.21±0.02a | 7.11±0.00c |
All a* values were negative, indicating the green coloration of the samples, while b* values were positive, reflecting a slightly yellowish hue. Brown seaweeds contain various pigments, including fucoxanthin, violaxanthin, β-carotene, and chlorophyll, which contribute to their characteristic coloration (Zhao et al., 2022). During the drying process, these pigments may degrade or undergo chemical reactions, leading to the changes in color properties.
Carotenoids are terpenoid pigments commonly present in vegetables, fruits, and algae that produce yellow, orange, and red colors. It can be divided into two major groups: carotenes, consisting of hydrocarbons, and xanthophylls, comprising oxygenated hydrocarbons. (Bhat et al., 2021). These pigments are necessary in light harvesting during photosynthesis, as well as providing photoprotection. More than 600 carotenoids have been identified to date (Hammond and Renzi, 2013). In seaweeds, notable carotenoids include fucoxanthin, β-carotene, astaxanthin, and zeaxanthin, which contribute to their diverse biological and ecological functions (Poojari et al., 2016).
Fig. 1 shows the total carotenoid content of U. pinnatifida wherein highest was obtained when freeze-dried, reaching 155.37±0.58 μg/g. According to Atencio et al. (2022), carotenoid stability may be influenced by factors such as heat, light, air exposure, and the presence of acids or metals. While heat drying significantly reduced the total carotenoid content, microwave drying (63.56±0.78 μg/g) preserved carotenoids better than hot-air drying (55.44±0.97 μg/g). These findings align in the report of Maurya et al. (2018), where five pepper cultivars exhibited higher β-carotene content when microwave-dried compared to hot-air and sun drying. Furthermore, microwave-assisted extraction has recently been demonstrated as an efficient method for isolating carotenoids from seaweeds (Poojari et al., 2016). This efficiency may be attributed to the significant pressure generated during the process, which causes cell membrane rupture and facilitates the release of intracellular compounds. Additionally, the shorter drying periods associated with microwave drying may reduce the occurrence of oxidation due to exposure to light and oxygen.
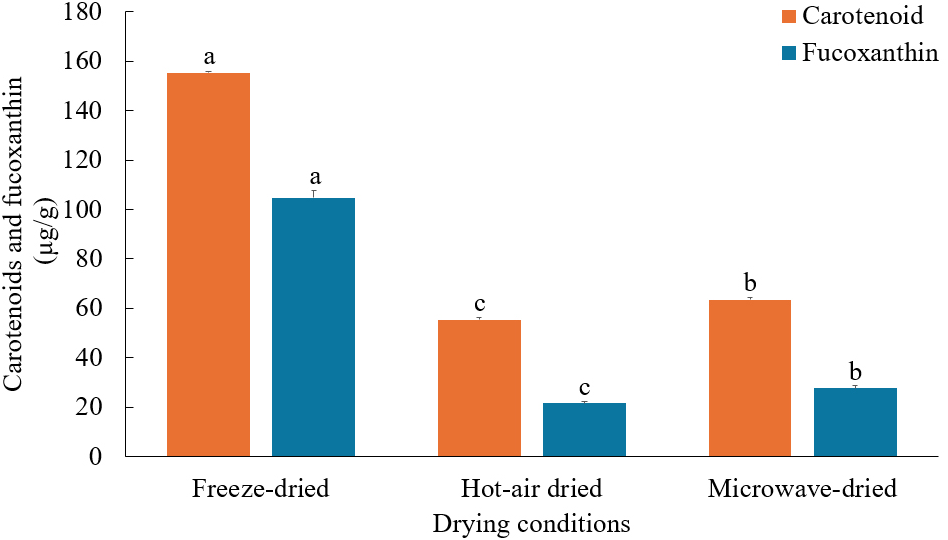
Carotenoids derived from algae are increasingly utilized in the food, medicine, and cosmetics industries owing to its diverse bioactive properties, such as anti-inflammatory, antioxidant, and anti-tumor effects. The demand for natural products like carotenoids has grown steadily over the years, reaching a market size of US$ 123 million in 2019, driven by their safety, wide availability, and cost-effective production (Lourenço-Lopes et al., 2022).
Fucoxanthin, a xanthophyll carotenoid uniquely found in brown and golden-brown macro- and microalgae, represents roughly 10% of the overall carotenoid production in ecosystems. It absorbs blue-green light, which chlorophylls cannot, thereby enhancing photosynthetic efficiency (Chini Zittelli et al., 2023).
As presented in Fig. 1, the highest fucoxanthin content was attained by freeze-dried samples (104.77±2.87 μg/g), approximately four times higher than in microwave-dried samples and five times greater than in hot-air dried samples. The same trend was recorded by Wirenfeldt et al. (2024), where brown algae Ulva sp. exhibited higher fucoxanthin levels when freeze-dried compared to microwave and conventional drying methods. Fucoxanthin is highly susceptible to oxidation due to exposure to heat, air, light, and strong acids or bases. This sensitivity is attributed to its highly unsaturated chemical structure, containing an allenic bond, a 5,6-monoepoxide group, and nine conjugated double bonds (Baek et al., 2019). On the other hand, the elevated fucoxanthin levels in microwave-dried samples compared to hot-air dried samples can be due to the short exposure time to high temperatures, which promotes the extraction of fucoxanthin from the seaweed structure while minimizing exposure to air and light.
Due to its numerous health-enhancing properties, including anti-inflammatory, anti-diabetic, anti-cancer and antioxidant, fucoxanthin is a potential functional ingredient for the nutraceutical, cosmetics, and food industries. Currently, fucoxanthin is primarily extracted from phaeophytes like Eisenia bicyclis, Laminaria japonica, U. pinnatifida, and Hizikia fusiformis. As stated by Lourenço-Lopes et al. (2022), fucoxanthin extracted from U. pinnatifida has been recognized as safe for human consumption. Moreover, the growing demand for fucoxanthin exceeds its current production, prompting researchers to explore economical and environmentally sustainable methods to enhance its production.
Chlorophylls are green pigments primarily responsible for photosynthesis in plants and algae. These pigments are non-polar, with a structure featuring a magnesium atom bound to a central porphyrin or hydroporphyrin ring. Among the algae, chlorophylls a, b, c, and d are commonly found. However, in brown algae, chlorophylls a and c are predominant, with minimal amounts of chlorophyll d, while chlorophyll b is typically not present (Garcia-Perez et al., 2022).
The chlorophyll content in U. pinnatifida subjected to three various drying conditions is shown in Table 2. Among the chlorophylls, chlorophyll a was the most prevalent, accounting for 86-92% of the total chlorophyll content. Chlorophyll a serves as the primary pigment for photosynthesis. Chlorophyll c contributed 6-12%, while chlorophyll d comprised only 2-3% of the total chlorophylls.
In terms of drying conditions, the greatest chlorophyll retention was observed in freeze-dried samples, with chlorophyll levels 2.2-fold higher than hot-air dried samples and 2.8-fold higher than microwave-dried samples. Similar to carotenoids, chlorophyll pigments are highly prone to degradation when exposed to oxygen, light, and elevated temperatures. According to Silva et al. (2019), the chlorophyll content in the seaweeds Ulva rigida, Gracilaria sp., and Fucus vesiculosus decreased as the drying temperature increased, particularly until 60°C.
Chlorophyll has been widely recognized and used as a natural food colorant for many years. However, its remarkable functional properties, including anti-inflammatory, anti-cancer, antioxidant, and wound-healing effects, have driven an increasing demand for its production. Nowadays, chlorophyll is also extensively utilized in the pharmaceutical and cosmetics industries.
Polyphenols are a varied collection of naturally occurring substances present in plant-based food products. They exhibit various health-promoting properties, such as anti-inflammatory, antimicrobial, antiviral, antioxidant, and anti-allergic effects. These compounds can neutralize ROS or donate electrons to free radicals, which reduces oxidative stress and helping prevent various diseases. In the food industry, polyphenols play a important role in preventing the oxidative degradation of lipids, which enhances the shelf life of products while promoting their health benefits (de Araújo et al., 2021).
The total polyphenol contents (TPC) of dried U. pinnatifida were significantly influenced by drying conditions (Table 3). The highest TPC was observed in freeze-dried samples (1,360.60±9.47 μg GAE/g), while hot-air drying resulted in a substantial 63% reduction (507.89±21.29 μg GAE/g). Similarly, microwave drying reduced the TPC by 44%, yielding 767.35±10.72 μg GAE/g. This indicates that polyphenols are highly sensitive to heat, particularly during prolonged exposure. Alean et al. (2016) also reported that polyphenols, being heat-sensitive compounds, undergo degradation primarily due to high temperatures and extended drying durations.
Between the two heat-drying methods, microwave drying retained higher TPC compared to hot-air drying. A similar trend was observed by Badmus et al. (2019), who reported that Hallidris silicosa, a brown seaweed with the highest TPC among five edible brown seaweeds analyzed, retained more TPC after microwave drying than hot-air drying at 40°C and 60°C. This was attributed to shorter heat exposure during microwave drying.
Seaweeds are valuable sources of polyphenols, which are secondary metabolites synthesized in response to harsh environmental conditions. These compounds serve a protective role, enabling seaweeds to adapt to environmental stress while offering potential health benefits when used as a functional ingredient.
Phlorotannins are phenolic compounds unique to brown algae, constituting approximately 5-12% of their dry mass. These hydrophilic compounds vary in molecular size from 126 Da to 126 kDa and are formed through the polymerization of phloroglucinol monomers via the acetate-malonate (polyketide) pathway. Phlorotannins are well-known for their diverse bioactivities, including antioxidant, anti-inflammatory, anti-diabetic, anti-allergic, and skin-protective properties (Venkatesan et al., 2018).
The phlorotannin content of dried U. pinnatifida varies significantly depending on the drying method employed (Table 3). Freeze-dried samples exhibited the highest phlorotannin content, measuring 1,887.10±22.97 μg PGE/g, which was 2.6 times higher than that of hot-air dried samples and 1.4 times higher than microwave-dried samples. Studies suggest that freeze-drying is the most efficient method for phenolic compound retention, likely due to their heat sensitivity. For instance, Subbiah et al. (2023) reported significantly higher phlorotannin levels in five brown algae species from the Australian coast, when freeze-dried in comparison with hot-air and vacuum-dried samples.
In contrast, hot-air dried samples contained 723.06±14.73 μg PGE/g, while microwave-dried samples had 1,384.51± 23.39 μg PGE/g. These results are consistent with trends observed in total polyphenol and flavonoid contents, further emphasizing the susceptibility of phlorotannins to thermal degradation during prolonged heat exposure in hot-air drying.
Phlorotannins are increasingly utilized in various industries as functional bioactive components. Presently, the European Union has approved their use in dietary supplements, reflecting the growing potential of marine-derived compounds in health and nutrition products (Zheng et al., 2022).
Flavonoids are a group of polyphenolic compounds defined by a benzo-γ-pyrone structure and are commonly found in fruits and vegetables. They can be categorized according to their chemical structure, level of unsaturation, and the oxidation state of their central carbon ring. To date, more than 10,000 flavonoid compounds have been isolated and characterized from diverse natural sources, including flavans, anthoxanthins, and flavanones. Flavonoids are renowned for their numerous health benefits, such as anti-inflammatory, anti-carcinogenic, antioxidant, anti-malarial, and neuroprotective properties. These compounds are extensively utilized in nutraceutical, pharmaceutical, food, and cosmetic industries (Ullah et al., 2020).
The total flavonoid content (TFC) of U. pinnatifida dried under various conditions is presented in Table 3. Among the drying methods tested, freeze-dried samples exhibited the highest TFC at 14.35±0.14 μg QE/g. Heat drying significantly reduced flavonoid levels, with hot-air dried samples showing a 67% reduction (4.73±0.02 μg QE/g) and microwave-dried samples showing a 54% reduction (6.62±0.02 μg QE/g). According to Speisky et al. (2022), the hydroxyl groups in the structure of flavonoids make them highly prone to autoxidation. This susceptibility is further increased when flavonoids are exposed to prolonged heat, oxygen, or light.
For heat drying, the TFC exhibited a pattern similar to the TPC, with higher levels detected in microwave-dried samples compared to those dried using hot air. This is consistent with the observation of Dang et al. (2016), wherein TFC in the brown alga Hormosira banksii was greater in microwave-dried samples than in those hot-air dried at 40°C, where extended heat-drying durations led to significant degradation. Brown algae are well-known sources of secondary metabolites, including flavonoids, which exhibit strong antioxidant activity and may help prevent various diseases.
The pigments and bioactive compounds present in U. pinnatifida are renowned for their antioxidant properties. Reactive oxygen species (ROS) are naturally generated as by-products of cellular aerobic metabolism. These include both radical and non-radical oxygen species, such as superoxide anion (O2-), hydrogen peroxide (H2O2), and hydroxyl radical (HO•), which arise from the incomplete reduction of oxygen. While ROS are essential for certain cellular processes, overproduction can trigger oxidative stress, potentially resulting in cellular damage, programmed cell death (apoptosis), or necrosis. The antioxidant defense system is necessary in maintaining the balance between ROS generation and elimination, thereby protecting cells from oxidative damage (Kozlov et al., 2024).
The antioxidant properties of U. pinnatifida were assessed by analyzing its DPPH and ABTS radical scavenging activities (Fig. 2). Freeze-dried samples (226.39±12.06 μg GAE/g) exhibited the strongest DPPH radical scavenging activity, followed by microwave-dried (102.83±1.18 μg GAE/g) and hot-air dried samples (83.26±1.61 μg GAE/g). A comparable pattern was seen for ABTS radical scavenging activity, where freeze-dried samples demonstrated the strongest activity (1.19 ±0.01 mg AAE/g). No significant difference was observed between hot-air dried and microwave-dried samples, both yielding values of 0.56±0.01 mg AAE/g.
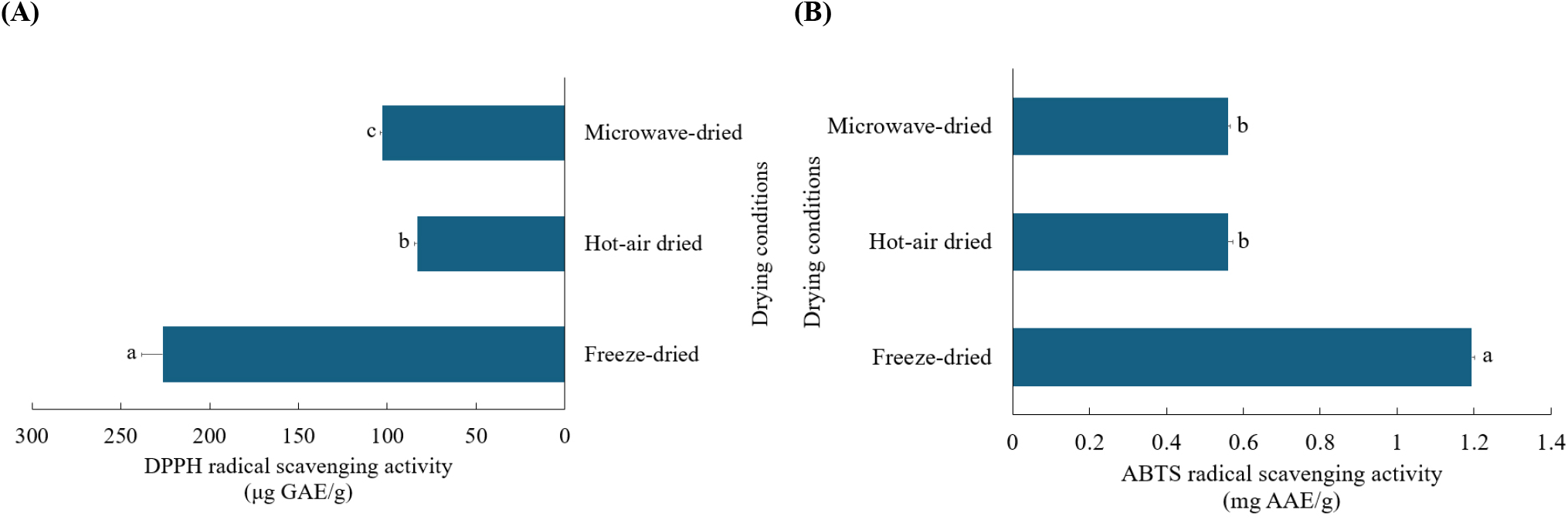
Based on the results from both scavenging assays, heat drying significantly reduced antioxidant activity by up to 55% in microwave-dried samples and 63% in hot-air dried samples, indicating a substantial impact on the antioxidant properties of the samples. Wanderley et al. (2023) mentioned that high temperature, combined with extended heat exposure and drying time, can impact a product’s antioxidant capacity. Several studies have explored the antioxidant properties of U. pinnatifida. However, most have focused on fresh samples or specific compounds extracted from it.
The results of this study are consistent with Zhao et al. (2022), who reported that freeze-dried S. fusiforme showed the highest DPPH and ABTS radical scavenging activities compared to samples dried using microwave, hot-air, or sun-drying methods. Furthermore, their results demonstrated that microwave drying yielded higher DPPH radical scavenging activity than oven drying, while no significant difference was recorded between microwave and hot-air drying in ABTS radical scavenging activity.
As shown in Fig. 3, a strong positive correlation was observed between the functional pigments (carotenoids, fucoxanthin, and chlorophylls), total polyphenols, phlorotannins, flavonoids, and antioxidant activities (DPPH and ABTS radical scavenging activities).
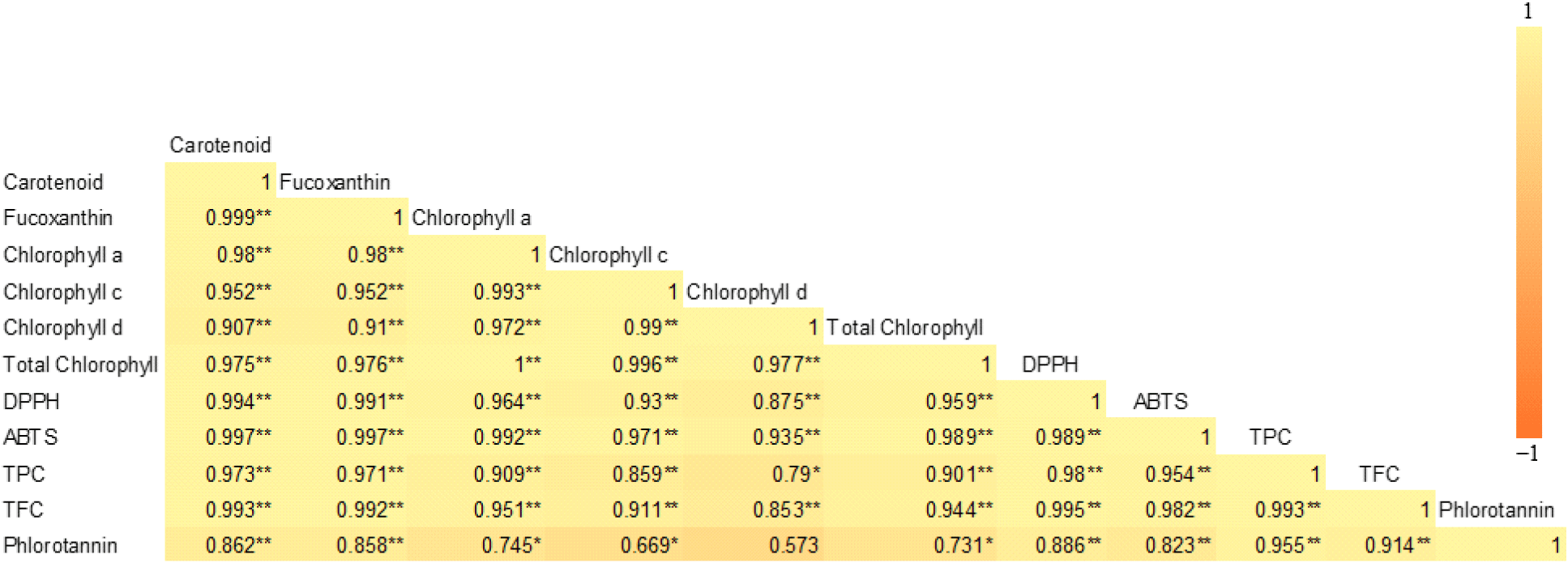
Fig. 3 illustrates a strong positive correlation between functional pigments (carotenoids, fucoxanthin, and chlorophylls), total polyphenols, phlorotannins, flavonoids, and antioxidant activities, including DPPH and ABTS radical scavenging activities. All of these compounds have the capacity to scavenge ROS, thereby reducing oxidative stress and enhancing antioxidant capabilities. Moreover, all functional compounds evaluated exhibited heat sensitivity, showing reduced levels under heat-drying conditions.
4. Conclusions
Due to the high moisture content of seaweed U. pinnatifida, drying is a critical step before industrial production. However, drying can significantly affect product quality. This study evaluated the influence of various drying conditions (freeze-drying, hot-air drying, and microwave-drying) on the functional pigments and antioxidant properties of U. pinnatifida. Results revealed that freeze-drying retained the highest levels of carotenoids, fucoxanthin, chlorophylls, total polyphenols, phlorotannins, flavonoids, as well as antioxidant activities, showing the sensitivity of these compounds to heat. Among heat-drying methods, hot-air drying caused greater reductions in compound retention compared to microwave-drying, which can be attributed due to prolonged exposure to heat, air, and light. Correlation analysis showed strong positive relationships among all the evaluated compounds, which are recognized for their antioxidant properties. Overall, considering the high operational costs of freeze-drying, microwave-drying presents a cost-effective and viable alternative, exhibiting better retention of functional pigments and compounds and shorter drying time compared to hot-air drying.










