1. Introduction
Recently, with rising household incomes and improved living standards, people have shown a growing interest in adopting a healthy lifestyle, particularly regarding disease prevention and anti-aging (Fang et al., 2023). As a result, there has been an increased focus on more nutritious food choices, with many studies exploring natural functional foods and their physiological benefits (Hopwood, 2020). Diseases such as cancer, heart disease, and aging are linked to oxidative stress caused by reactive oxygen species (Samak et al., 2009). Various plant species and parts have been studied for their antioxidant properties, particularly their ability to remove free radicals (Bahorun et al., 2004).
Asparagus (Asparagus officinalis L.), a herbaceous plant from the Asparagaceae family, includes over 300 species, but only A. officinalis is commonly consumed (Seong et al., 2006). Asparagus comes in several varieties, including purple, greenish-purple, white, and green, with green and white being the most popular worldwide (Kulczyński et al., 2016). Asparagus is valued for its health benefits due to its rich content of vitamins, minerals, and bioactive compounds. It is especially high in flavonoids like rutin and quercetin, with green and purple varieties containing significant amount of polyphenols (Kobus-Cisowska et al., 2019). Although white asparagus has a slightly lower nutritional profile compared to green asparagus, it possesses a high sugar content and milder texture and flavor (Maeda et al., 2005).
Originally, asparagus was mainly cultivated in the United States and Europe, but since 1966, it has also been grown in various regions of Korea, including Jeju Island and Gangwon Province (Seong et al., 2006). By 2019, asparagus exports reached 25 tons, generating $185,000, with production in 2020 estimated at 70 hectares and 400 tons (Kwon et al., 2020). As production and consumption of Korean-grown asparagus continue to rise, research into its functional properties is crucial for expanding its market potential and increasing its value as a functional food ingredient.
Fresh asparagus is about 90% water, with the rest comprising carbohydrates, proteins, free amino acids, minerals, vitamins, and dietary fiber (Lee et al., 2015). Research has shown that asparagus is rich in bioactive components, such as polyphenols, saponins, and ascorbic acid (Kulczyński et al., 2016; Negi et al., 2010). Asparagus extract has been found to have antioxidant, antibacterial, antifungal, antitumor, and anticancer properties (Jang et al., 2004; Negi et al., 2010).
Environmental factors, such as cultivation region, may influence the bioactive compound composition of asparagus, with potential differences between asparagus grown in Jeju and Gangwon Province (Kim and Ji, 2021). In Korea, studies have explored various aspects of asparagus, including its use in traditional soy sauce, its anti-inflammatory and anti-gout effects through asparagus root extract, and the quality of cookies enriched with asparagus powder (Yang et al., 2010). Other research has examined the functional properties of asparagus stem and root extracts (Han et al., 2021), its potential to alleviate hangovers and gout (Seo et al., 2022), and its antioxidant properties in sponge cakes made with asparagus powder (Zhang et al., 2015). Most studies have focused on green asparagus, with fewer investigating the bioactive components and antioxidant properties of white asparagus, especially with respect to different extraction methods. Most studies have relied on DPPH and ABTS assays, without comprehensive evaluations using multiple extraction solvents and additional assays like FRAP and SOD. To address this gap, this study systematically evaluates the antioxidant activity of both green and white asparagus extracts from Korea, using 65°C hot water and 70% ethanol, which differ in polarity and extraction efficiency. A variety of antioxidant assays are used to provide essential data for developing Korea-grown asparagus as a functional food ingredient.
2. Materials and method
The following were purchased from Sigma Chemical Co.: DPPH, ABTS, L-ascorbic acid, gallic acid, and quercetin. Folin-Ciocalteu phenol reagent was purchased from Fluka Co.
Greenhouse-grown green asparagus and white asparagus, cultivated in Yanggu, Gangwon Province, were used in the experiment, with the asparagus harvested in June 2022. Green asparagus and white asparagus were initially dried at 50°C for 48 h using a hot air dryer (CF-21WF, Gongju Jeil Instrument & Tech, Gongju, Korea). The dried asparagus was ground using a grinder. Thereafter, 100 g of ground asparagus was extracted with 2 L of 70% ethanol in a constant-temperature water bath (WSB-45, Daihan Scientific Co. Ltd., Cheongju, Korea) at 25°C for 12 h, and the extract was filtered through filter paper (No. 2, Advantec Toyo Kaisha, Ltd., Tokyo, Japan). The hot water extract was prepared by adding 2 L of 65°C water for 12 h. Extraction temperature and duration time were selected based on previous studies, which reported that moderate temperatures in the range of 60-80°C are suitable for effectively extracting phenolic compounds from plant materials while minimizing thermal degradation (Fan et al., 2014) and that an extraction time of 12 h ensures sufficient compound release without causing thermal damage. Each extract was concentrated in a rotary vacuum evaporator (Laborota 4000-efficient, Heidolph Instruments GmbH & Co., Schwabach, Germany), lyophilized in a freeze dryer (JP/VD-400F; Tietech Co., Osaka, Japan), and frozen at -18°C for experimental use.
The total polyphenol content in each asparagus extract was measured using the Folin-Ciocalteu assay (Singleton and Rossi, 1965). In brief, 1 mL of each extract was combined with 5 mL of distilled water and 0.5 mL of Folin-Ciocalteu reagent. The mixture was stirred for 8 min, followed by the addition of 10 mL of 7% Na2CO3 solution, and the volume was adjusted to 25 mL with distilled water. The mixture was then incubated at 25°C for 2 h, and the absorbance was measured at 750 nm with a spectrophotometer (UV 1800, Shimadzu Co., Kyoto, Japan). Gallic acid was used as the standard, and the results were expressed as mg of gallic acid equivalents per gram of dry matter (mg GAE/g).
The total flavonoid content was assessed following the procedure outlined by Moreno et al. (2000). Briefly, 0.5 mL of each extract was combined with 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 M aqueous potassium acetate, and 4.3 mL of 80% ethanol. The resulting mixture was left at room temperature for 40 min, after which its absorbance was recorded at 510 nm using a spectrophotometer. Quercetin served as the reference standard for generating the calibration curve. The results are reported as milligrams of quercetin equivalents per gram of dry weight (mg QE/g).
The DPPH radical-scavenging activity was assessed following the method described by Blois (1958). In brief, 1 mL of each extract was mixed with 2 mL of 7.5×10−5 M DPPH solution and incubated for 30 min at 37°C. The absorbance was measured at 517 nm using a spectrophotometer. L-ascorbic acid was used as the reference standard for comparison. The percentage of DPPH radical-scavenging activity was calculated using the following equation:
The ABTS radical-scavenging activity was measured using the method of Re et al. (1999). In brief, 7 mM ABTS solution and 2.45 mM potassium persulfate solution were mixed in equal volumes and incubated at 30°C for 12 h in the dark. The resulting mixture was diluted with 5 mM potassium phosphate buffer (pH 7.4) to adjust the absorbance to 0.7 at 413 nm. Next, 4 mL of ABTS+ reagent and 40 μL of each extract were combined and allowed to react for 1 min. The absorbance was then measured at 413 nm. L-ascorbic acid was used as the reference standard for comparison. The ABTS radical-scavenging activity was calculated using the same formula applied to the DPPH radical-scavenging activity.
The ferric reducing antioxidant power (FRAP) assay was conducted according to the procedure described by Benzie and Strain (1996). To prepare the FRAP reagent, 100 mL of 300 mM acetate buffer (pH 3.6), 10 mL of tripyridyltriazine (TPTZ) solution, and 10 mL of 20 mM FeCl3 ․ 6H2O were mixed. Then, 3 mL of FRAP reagent was added to 0.1 mL of each sample and reacted at 37°C for 4 min. The absorbance of the samples was measured at 593 nm. A calibration curve was obtained from the standard curve generated using FeSO4 ․ 7H2O, and the results are expressed in FeSO4 eq mM.
The SOD-like activity was determined using a modified version of the method described by Marklund and Marklund (1974). Each extract was prepared at a concentration of 100 μg/mL. Then, 2 mL of each extract, 0.3 mL of Tris-HCl buffer solution (50 mM Tris and 10 mM ethylene-diamine-tetraacetic acid, pH 8.5), and 0.2 mL of 7.2 mM pyrogallol were mixed and incubated at 25°C for 10 min. The reaction was terminated by adding 1 mL of 1 N HCl, and the degree of pyrogallol oxidation was assessed by measuring the decrease in absorbance at 420 nm. The SOD-like activity was expressed as the percentage reduction in absorbance relative to the blank.
All experiments were conducted in triplicate or more. Statistical analysis was performed using one-way analysis of variance (ANOVA) with IBM SPSS Statistics (version 25, IBM Corp., Armonk, NY, USA). The results of each experiment are expressed as the mean±SD, and the significance of differences among the mean values of the experimental group were tested using Duncan’s multiple range test at p<0.05 after ANOVA.
3. Results and discussion
The total polyphenol content of the 65°C hot water and 70% ethanol extracts of green asparagus (termed WEGA and EEGA, respectively) and white asparagus (termed WEWA and EEWA, respectively) are shown in Table 1. The total polyphenol content of WEGA and EEGA was 31.80 and 38.96 mg GAE/g, respectively, whereas that of EEWA and WEWA was 24.57 and 18.13 mg GAE/g, respectively. The hot water and 70% ethanol extracts of green asparagus had a higher polyphenol content than those of white asparagus. Moreover, the 70% ethanol extracts of green and white asparagus exhibited a significantly higher total polyphenol content compared to the hot water extracts. Kulczyński et al. (2016) found that green asparagus has the highest phenolic acid content, followed by purple and white asparagus. Another study indicated that plants require exposure to light to accumulate phenolic compounds (Kolb et al., 2001).
1) WEGA, 65°C hot-water extract of green asparagus; EEGA, 70% ethanol extract of green asparagus; WEWA, 65°C hot-water extract of white asparagus; EEWA, 70% ethanol extract of white asparagus.
Phenolic compounds in the plant kingdom are broadly divided into phenolic acid, coumarins, flavonoids, and tannins, and they are known to exert antioxidant activity by scavenging free radicals from phenolic hydroxyl groups (Madsen et al., 1996). Lee et al. (2011) found that the water extract displayed a lower total polyphenol content than the ethanol extract when assessing the total polyphenol content of cypress tree stem extract using different solvents. Therefore, the difference in the phenolic compound content according to extraction solvent appeared to be consistent with that found in the present study.
The total flavonoid contents of WEGA, EEGA, WEWA, and EEWA were 24.18, 26.11, 9.09, and 16.61 mg QE/g, respectively (Table 1). Most flavonoids are insoluble in water but dissolve more readily in organic solvents like acetone and ethanol (Ham et al., 2016). As a result, in the current study, the 70% ethanol extracts appeared to have a higher total flavonoid content than the hot water extracts owing to differences in flavonoid solubility. Stoffyn et al. (2012) revealed that rutin had the highest content among flavonoids in asparagus, and it varied according to the time and place of harvest. Moreover, Maeda et al. (2005) found that green asparagus contained higher levels of rutin than white asparagus. Consequently, in the present study, it can be inferred that the total flavonoid content varied among the different types of asparagus.
To measure the antioxidant activity of WEGA, EEGA, WEWA, and EEWA, the electron-donating activity of DPPH was measured at different sample concentrations (Fig. 1). At 50, 100, 200, 300, 400, and 500 μg/mL, WEGA displayed 17.33%, 25.38%, 40.64%, 62.45%, 84.14%, and 90.60% activity, whereas EEGA exhibited 9.79%, 32.11%, 36.08%, 56.83%, 75.57%, and 86.14% activity, respectively. At the same concentrations, WEWA exhibited 15.35%, 27.82%, 34.17%, 61.53%, 66.26%, and 79.13% activity, whereas EEWA displayed 17.68%, 36.02%, 46.88%, 52.57%, 68.28%, and 73.53% activity, respectively, indicating a significant increase in radical-scavenging activity as the sample concentration increased. The IC50 values for DPPH radical scavenging activity of the samples were determined as follows: 257.81 μg/mL for WEGA, 255.52 μg/mL for EEGA, 285.61 μg/mL for WEWA, 218.18 μg/mL for EEWA, and 33.42 μg/mL for ascorbic acid. Among the extracts, EEWA exhibited the highest antioxidant activity, while ascorbic acid showed the strongest radical scavenging ability. The asparagus extract demonstrated dose-dependent antioxidant activity, with the IC50 values highlighting its promising potential as an antioxidant substance. This finding was also noted by Kim et al. (1994), who studied the antioxidant activity of medicinal plants. At a concentration of 1.0 mg/mL, angelica, licorice, and jade porridge exhibited 15.8%, 13.3%, and 5.4% activity, respectively. As previously mentioned, asparagus extract has higher antioxidant activity than medicinal plants. If a sample exhibits high free radical-scavenging activity, it is expected to possess antioxidant properties and be potentially useful in suppressing free radical-induced aging in the human body (Lee et al., 2001). Asparagus extract exhibits high electron-donating ability and has been considered to possess excellent antioxidant effects. Bahorun et al. (2004) found a strong correlation between the total phenol and total flavonoid contents and DPPH radical-scavenging activity, and polyphenols are key contributors to the antioxidant properties of plants. The squares of the correlation coefficients (R2) between DPPH radical-scavenging activity and total polyphenol and total flavonoid contents were 0.964 and 0.914, respectively. Compared with white asparagus extracts, the higher antioxidant activity of green asparagus extracts possibly originated from their significantly higher total polyphenol and total flavonoid contents. According to Kim et al. (2011), Opuntia humifusa ethanol extracts demonstrated higher DPPH radical-scavenging activity compared to hot-water extracts, displaying a similar trend to the findings of the current study. Based on the above results, the DPPH radical-scavenging activity of asparagus appears to be affected by the extraction solvent, and these results indicate that the asparagus ethanol extract possesses relatively high antioxidant activity, rendering it a potentially suitable functional antioxidant material.
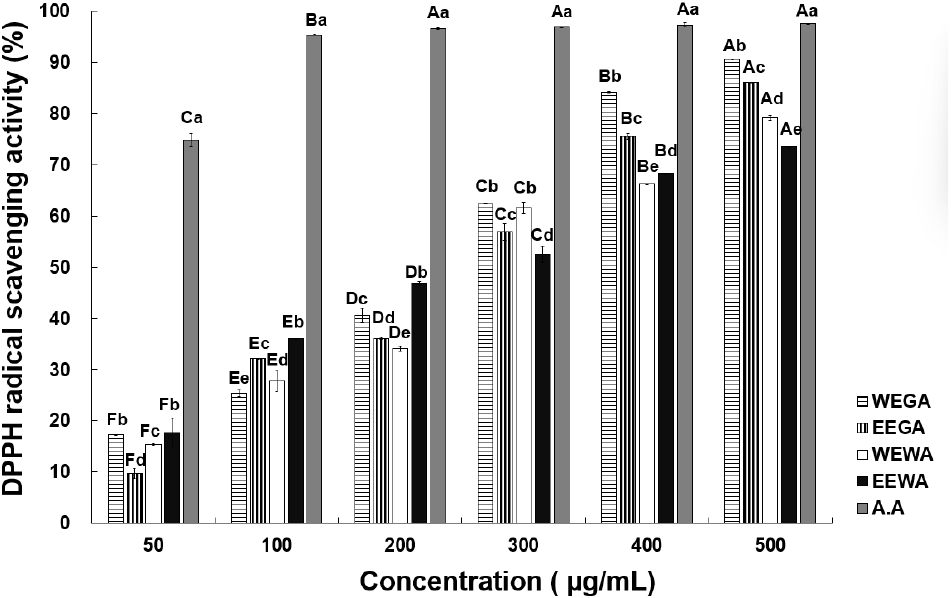
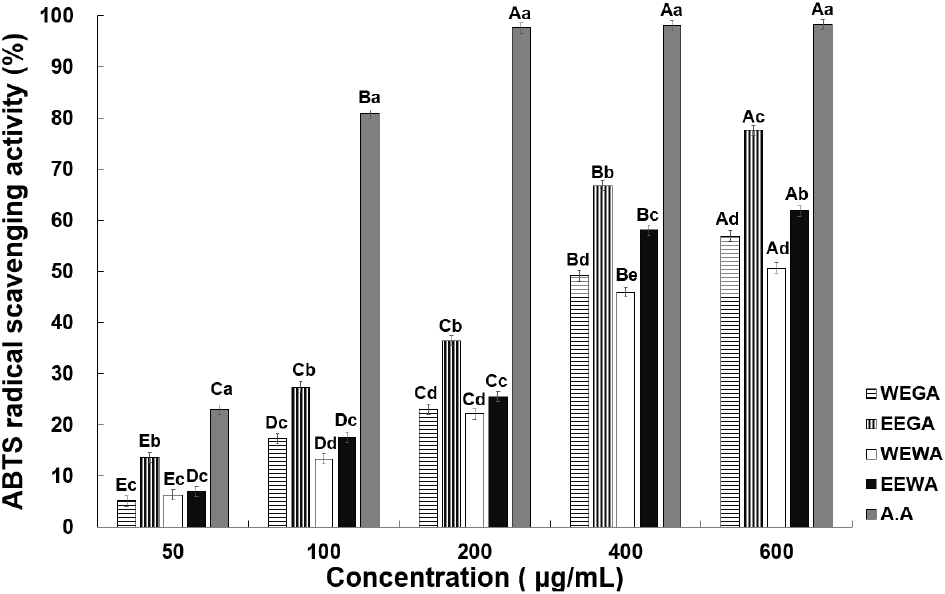
The ABTS radical-scavenging activities at various concentrations of asparagus extract are presented in Fig. 2. At 50, 100, 200, 400, and 600 μg/mL, WEGA exhibited 5.06%, 17.24%, 22.96%, 49.13%, and 56.91% activity, whereas EEGA displayed 13.54%, 27.36%, 36.40%, 66.76%, and 77.58% activity, respectively. WEWA exhibited 6.24%, 13.29%, 22.08%, 45.89%, and 50.65% activity, whereas EEWA displayed 6.92%, 17.51%, 25.48%, 58.09%, and 61.08% activity, respectively, indicating a significant increase in ABTS radical-scavenging activity. These results are consistent with those observed for the DPPH radical-scavenging activity, demonstrating that the radical-scavenging activity increased with increasing asparagus extract concentration. Lee and Lee (2020) compared the ABTS radical-scavenging activity between the water and ethanol extracts of Sigesbeckia glabrescens Makino. They found that the ethanol extract of S. glabrescens Makino showed superior ABTS radical-scavenging ability compared with the water extract. Choi et al. (1992) examined the antioxidant activity of plants extracted with ethanol and water and also found that ethanol extracts displayed stronger antioxidant activity than water extracts, which is consistent with the findings of the current study. Overall, the ABTS radical-scavenging activity of green asparagus extracts was higher than or equal to that of white asparagus extracts. As mentioned previously, this may have resulted from the increased accumulation of phenolic substances in green asparagus, owing to sunlight exposure, which resulted in increased antioxidant activity.
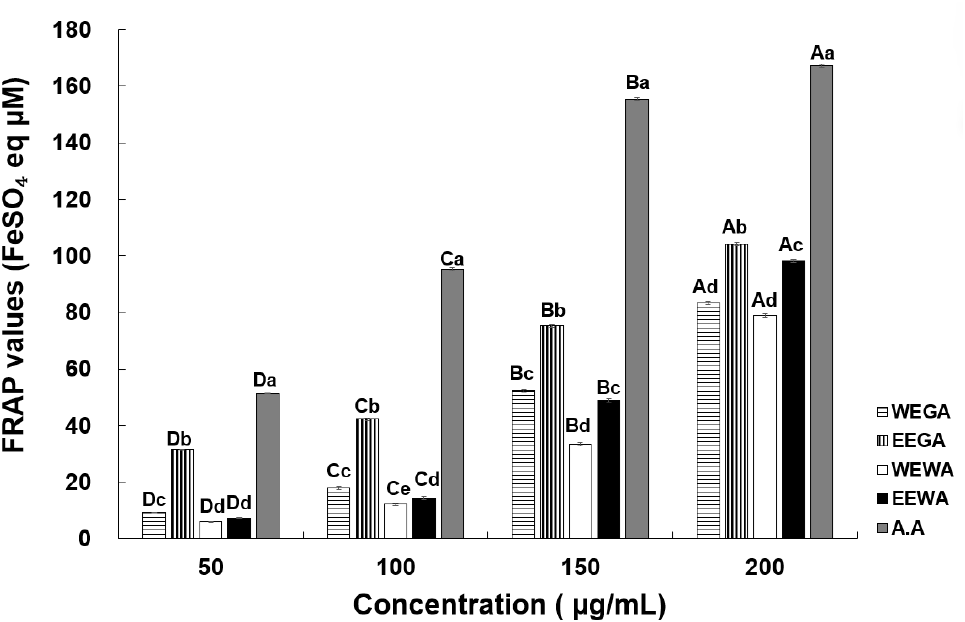
The FRAP assay relies on the rapid reduction of ferric-TPTZ by antioxidants in the samples, resulting in the formation of ferrous-TPTZ, a blue-colored product (Benzie and Strain, 1999). The reducing power values of WEGA, EEGA, WEWA, and EEWA are shown in Fig. 3. At concentrations of 50, 100, 150, and 200 μg/mL, WEGA exhibited a reducing power of 9.22, 17.22, 52.39, and 83.35 FeSO4 eq μM; EEGA showed a reducing power of 31.56, 42.26, 75.23, and 104.07 FeSO4 eq μM; WEWA displayed a reducing power of 6.01, 12.20, 33.47, and 78.84 FeSO4 eq μM; and EEWA showed a reducing power of 7.37, 14.37, 48.94, and 98.17 FeSO4 eq μM, respectively. The FRAP values increased significantly with higher concentrations of asparagus extracts. Moreover, the reducing power of the ethanol extract exceeded that of the water extract. According to Ryu et al. (2011), the FRAP values of the water and ethanol extracts of Artemisia annua L. increased significantly with sample concentration and were dependent on the total polyphenol and total flavonoid contents of mugwort extract, which aligns with the findings of the present study. Additionally, a strong correlation between the FRAP assay results and DPPH radical-scavenging activity was observed (Moon et al., 2003). Compared with other plant extracts, the ethanol and 65°C hot water extracts of green asparagus showed greater reducing power. Therefore, asparagus extracts are considered as potential natural antioxidants. In Korea, due to high temperature and humidity conditions during the summer and heavy rainfall during the monsoon season, asparagus is primarily cultivated in greenhouses (Seong et al., 2001). According to the study by Kim et al. (2016), asparagus grown in rain-sheltered greenhouses showed higher physiological activity than asparagus grown in open field. Therefore, it can be inferred that the greenhouse grown asparagus used in this study also exhibits high physiological activity.
SOD is an enzyme involved in reducing O2− (superoxide) to normal oxygen in vivo. The SOD-like activity of asparagus extracts was assessed to identify a small molecular, non-enzymatic material that plays a similar role to that of SOD in inhibiting oxidative stress in the body. The SOD-like activities of the 65°C hot water and 70% ethanol extracts from both green and white asparagus are shown in Table 2. At 100 μg/mL, the SOD-like activity of WEGA, EEGA, WEWA, and EEWA was reduced to 33.30%, 55.26%, 37.40%, and 42.27%, respectively. Therefore, the SOD-like activity of asparagus is relatively higher compared to other medicinal plants, including umbrella sprouts, which exhibited a SOD-like activity of 1.49%-11.27% at a concentration of 0.1-1.0 mg/mL (Lee et al., 2009). Moreover, according to Kim et al. (1995) who observed a correlation between SOD-like activity and total polyphenol content, green asparagus, which has a comparatively high total polyphenol content, possessed higher SOD-like activity than white asparagus. This suggests that the antioxidant activity of asparagus may be attributed to key polyphenol compounds, such as rutin. Rutin, a well-known flavonoid, has been shown to possess significant antioxidant properties (Magalingam et al., 2013), which could further enhance the overall SOD-like activity of green asparagus. Therefore, the extract of asparagus grown in greenhouses in Korea can exhibit higher antioxidant activity and appears to demonstrate the immense potential as a functional food in the future.
| WEGA1) | EEGA | WEWA | EEWA | F-value | |
|---|---|---|---|---|---|
| SOD-like activity (%) | 33.30±1.242)a3) | 55.26±1.02c | 37.40±2.04a | 42.27±2.12b | 182.34***4) |
1) WEGA, 65°C hot-water extract of green asparagus; EEGA, 70% ethanol extract of green asparagus; WEWA, 65°C hot-water extract of white asparagus; EEWA, 70% ethanol extract of white asparagus.
4. Conclusions
We measured the antioxidant properties of water and ethanol extracts from both green and white asparagus grown in Korea using various methods to explore their potential health benefits as functional foods. The polyphenol contents were 31.80 mg GAE/g in hot water extract of green asparagus (WEGA), 38.96 mg GAE/g in 70% ethanol extract of green asparagus (EEGA), 18.13 mg GAE/g in hot water extract of white asparagus (WEWA), and 24.57 mg GAE/g in 70% ethanol extract of white asparagus (EEWA). The flavonoid contents for WEGA, EEGA, WEWA, and EEWA were 24.18 mg QE/g, 26.11 mg QE/g, 9.09 mg QE/g, and 16.61 mg QE/g, respectively. The DPPH and ABTS radical scavenging activities of the green asparagus extracts were higher than those of the white asparagus extracts. The SOD-like activity of WEGA, EEGA, WEWA, and EEWA at a concentration of 100 μg/mL was 33.30%, 55.26%, 37.40%, and 42.27%, respectively. The high antioxidant activity of the ethanol extract from green asparagus is considered to result from the accumulation of bioactive compounds like polyphenols due to more light exposure during growth and the differences in the degree of solvent extraction of functional substance. These results confirm that the ethanol extract of green asparagus can be a promising functional food ingredient with excellent antioxidant properties.










