1. Introduction
Plants been used for centuries to obtain the vital nutrients they contain for health and vitality, and they have garnered significant attention of researchers and intended consumers with respect to associated health benefits (Ameer et al., 2017a; Ameer et al., 2017b). Watermelon (Citrullus lanatus), a member of the Cucurbitaceae family, is related to squash, cucumber, pumpkin, and gourds and is botanically designated as a common fruit for human consumption. The Cucurbitaceae family ranks high among plant families in terms of total number of species used for food (Edwards et al., 2003). China is the leading watermelon producer (Lin et al., 2014; Naz et al., 2013). The critical elements determining superior watermelon varieties are sugar content and sweetness (Okonmah et al., 2011). Cultivars may have yellow or red flesh color. Due to their high water activity (0.97 to 0.99) and acidic pH (5.2 to 6.7), products, such as melon and watermelon, are described as perishable foods and are usually prone to food-borne pathogen exposure and disease (Harris et al., 2003; Melgar et al., 2008). In terms of composition, watermelon is composed of approximately 92% water (Leskovar et al., 2004). Watermelon also contains bioactive phenolic compounds (Jaskani et al., 2005; Kaur et al., 2001).
Watermelon is processed to manufacture a range of value-added food products. Thus, the food industry is encouraged to develop products through watermelon processing. Watermelon is divided into major three parts: the flesh, peel, and rind. The rind is considered a waste product; nevertheless, modern studies have reported the manufacture of various products from watermelon rind, such as candies and jam. Watermelon can also be utilized to formulate cakes, ice-cream, and jellies (Banureka et al., 2011; Iqbal et al., 2017; Madhuri et al., 2003; Mohamad et al., 2012; Muhammad et al., 2015; Peter-Ikechukwu et al., 2018; Sindhuja et al., 2005; Ubbor et al., 2009). Watermelon can be used for fresh fruit salads, smoothies or salsa, sandwiches, snacks, desserts, and garnishes. In Namibia, watermelon juice is fermented into a stimulating and energetic alcoholic drink (Okomnah et al., 2011). Pre- and postharvest factors influence the nutritional profile of watermelon produce. Genotypic variations exist within every group of commodities, with variation in postharvest life composition and quality (Rashid et al., 2020; Raza et al., 2019). A multidisciplinary method should be applied to emphasize nutritional and quality enhancement, exhibiting significant influence on human wellness and nutrition. Ripe fruits may yield inferior flavor quality. Over-ripe fruits are expected to develop mealy and tasteless flavors quickly after harvesting (Khan et al., 2020; Zhao et al., 2021). Picking fruit too late or prematurely increases the likelihood of postharvest physiological issues, compared with fruit picking at the proper maturity and harvesting stage (FAO, 2021).
This review summarizes the watermelon nutrition profile and factors affecting its nutritional concentration and antioxidant activity.
2. Watermelon fruit
The scientific name of watermelon is C. lanatus, which originates from Latin and Greek. Citrullus is derived from the Greek word “citrus,” and the Latin word “lanatus” indicates a connotation of being woolen, describing the minor hairs on the trunks and plant foliage. Watermelon is a part of the Plantae kingdom, Cucurbitaceae family, order Cucurbitales, and Citrullus genus. Tarbooz (Hindi and Urdu), Kallangadiballi (Kannada), Tormuj (Bengali), Indrak (Gujarati), Tarbuj (Manipuri), Kaduvrindavana (Marathi), Eriputccha (Telegu) are common names for watermelon in India (Pons et al., 2020).
An ordinary watermelon comprises about 68% flesh or pulp, 30% rind, and 2% seeds. The watermelon has grey or light green perpendicular streaks on its thick, smooth, deep green outward rind. Black seeds are located in the middle third of the flesh in structural configuration, and the fruit inside is red (Pons et al., 2020; Wehner et al., 2001). Seedless varieties may have no seeds at all or only very trivial and shrill, jelly-like white seeds, while seeded watermelons have dark brown or black elliptical seeds (Okomnah et al., 2011).
The first watermelon harvest was documented in Egypt 5,000 years ago. It has since spread far to other parts of the world. At present, watermelon can also be cultured in tropical countries, and it is even found in the Kalahari Desert of Africa (Naz et al., 2013). Currently, China is the top watermelon producer, followed by Turkey, the United States, Republic of Korea, and Iran (Revee et al., 1995). Global watermelon production is estimated at 29.6 million tons and has pronounced economic importance (Rashid et al., 2020). Watermelons were often placed in the interment tombs of kings to nurture them in the eternal life. The first Asian country to culture watermelons was Vietnam; however, some scholars dispute this claim (Ikechukwu et al., 2018). Early American colonizers were growing the fruit by the 17th century, and some speculate that New World pioneers brought the plant to Native Americans in the first decade of the 16th century. When the Moors established trade with the China, watermelon agronomy spread across Asia, the Persian Gulf, and onward to Europe (Jensen, 2012).
3. Varieties of watermelon
Globally, there are approximately 1,200 commonly consumed watermelon cultivars (Chalabi et al., 2004). Certain cultivated varieties are differentiated based on flesh color. Differences between cultivars may also be based on genotype, environmental situations, preharvest and postharvest factors, and maturity stage (Leskovar et al., 2004; Perkins-Veazie et al., 2012; Perkins-Veazie et al., 2001).
4. Cultivars
Red flesh watermelons contain less citrulline than yellow flesh watermelons (Rimando et al., 2005). Elevated levels of lycopene and varying amounts of β-carotene are present in red flesh watermelons. Ascorbic acid content is usually concentrated in the outermost part of the red flesh watermelon, and they are higher in the ripe stage of the red flesh watermelon. Madera, Fiesta, Oasis, Royal Sweet, Star Bright, Crimson Sweet, King of Hearts, Queen of Hearts, Royal Charleston, Sugar Baby Icebox, Summer Festival, Sweet Beauty, and Sweet Favorite are some popular cultivar names (Jensen, 2012).
Yellow-fleshed watermelons contain carotenoids varying from rich to trace amounts (Davis et al., 2009). The authors reported that ascorbic acid content is considerably lower than that in red-fleshed watermelons. Sunshine, Golden Midget, Yellow Baby, Yellow Doll, Golden Crown are some popular yellow watermelon cultivars.
Seedless cultivars include 313 Hybrid, Scarlet Trio, Sweet Triple, Tiffany, and Crimson Trio (Jensen, 2012). Watermelon varieties suggested for common cultivation include the following:
Sugar Baby: This cultivar has red flesh, small-sized fruit, and it can be planted just 4 feet apart.
Sweet Beauty: This cultivar was urbanized in Americas in 2004. It has red flesh, and it ripens in 80 days.
Golden Midget: This watermelon variety has pink flesh and a honey taste. It ripens in 70 days and it has elfin yellow skin.
Compared with other parts of world, per capita watermelon consumption is 3 times higher in Asian societies (Dermesonlouoglou et al., 2007). Watermelon can be used to prepare snacks, fresh salads, desserts, and decorations. Watermelon flesh may be baked as breakfast cereal with maize, usually including green vegetables, fruits, and tender young leaves. This product is a treasured stock feedstuff, particularly in times of famine. For cooking or storage of berries, hollowed fruit can be used as an ampule. The pulp and seeds are prepared in numerous altered ways for consumption (Alka et al., 2018). The rind is dried, sliced, cooked, and consumed in several parts of Africa. Pickled watermelon rind is widely consumed in certain regions of the USA (Oyeleke et al., 2012). The rind is useful as a feed or as fertilizer. Sometimes watermelon rind is employed for edible purpose. The rind is boiled slowly, stir-fried, or more frequently pickled in China. The de-fruited and de-skinned rind is stir-fried with chili peppers, scallions, garlic, olive oil, sugar, and rum. In the Southern US, Ukraine, Russia, Bulgaria, and Romania, pickled watermelon rind is also consumed ordinarily (Ramindo et al., 2005). As reported by Bradie et al. (2012), watermelon seeds provide various nutrients, such as proteins and minerals, including copper, manganese, potassium, iron, zinc, magnesium, sodium, and phosphorous, as well as B vitamins, fats, and phytochemicals. Seed powder is converted into flour to cook snacks consumed with sauces. Seed oils are also used to manufacture cosmetic oils (Jensen et al., 2011). Watermelon fruit has moisturizing, palliative, antiaging, and antioxidant properties, which aid its use in cosmetics manufacturing. Watermelon has high content of vitamin C in its composition. Vitamin C is a powerful antioxidant that is very valuable in photoaging treatment with its radical scavenging action (Chiu et al., 2003).
The watermelon nutritional profile per 1 cup serving (152 g) is mentioned below in detail. With a total carbohydrate value of 11.6 g, watermelon contains organic sugars, such as sucrose (1,863 mg), glucose (2,433 mg), fructose (5,174 mg), and maltose (92.4 mg). Watermelon has a protein content of 0.9 g per serving (Okonmah et al., 2011). Concerning vitamins, watermelon contains β-carotene (0.467 mg), β-cryptoxanthin (0.12 mg/100 g), lycopene (6.9 mg), vitamin C (0.0125 mg), betaine (0.5 mg), pantothenic acid (0.3 mg), choline (6.3 mg), and folate (0.0046 mg) (Nisa et al., 2012). Monounsaturated and polyunsaturated fatty acids are found in trace amount of 0.1 g each, whereas the phytosterol content is 3.1 mg (Jaskani et al., 2005; Kaur et al., 2001). Concerning minerals, calcium (10.8 mg), magnesium (15.4 mg), phosphorus (16.9 mg), potassium (173 mg), sodium (1.5 mg), zinc (0.2 mg), copper (0.1 mg), selenium (0.0006 mg), and fluoride (0.0023 mg) are prominently found in watermelon (Ahmad et al., 2020; Jiang et al., 2020; Johnson et al., 2012).
The fruit sampling area and genotypic differences influence the lycopene, flavonoid, total vitamin C, total phenol, and docosahexaenoic acid composition, as well as the lipophilic and hydrophilic properties of antioxidants in the watermelon flesh (Tlili et al., 2011). Fresh watermelon is known as a significant lycopene source with high bioavailability for humans. Lycopene bioavailability from fresh watermelon juice resembles that of processed tomatoes (Edwards et al., 2003; Lee et al., 2018), including substantial phenol concentrations (Brat et al., 2006; Perkins-Veazie et al., 2002). These important secondary metabolites exert extremely beneficial peroxyl radical scavenging actions, demonstrating potential pharmacological benefits (Larson, 1998; Manach et al., 1998). Moreover, Perkins-Veazie et al. (2006; 2007) highlighted the importance of sampling areas and genotype and assessed soluble solid and lycopene contents in watermelons. They also recommended documented and standardized sampling technique implementation when evaluating watermelon quality characteristics. Reproducible sampling methods must be established to test flesh quality, feasible in large fruits, such as watermelons. In a study by Tlili et al. (2011) lycopene content reached the maximum quantity (102.4 mg/kg FW).
Environmental factors are usually referred to as the dominant climate circumstances during the growing season, including air humidity, light, and temperature. These factors exert a significant effect during watermelon fruit development, affecting photosynthesis, cell cycle duration, transpiration, metabolism, respiration, and phloemic transport, consistently altering fruit internal and external qualities, size, and storage ability (Ladaniya, 2008). Vitamin C, comprising dehydroascorbic and ascorbic acids, is a vital nutritional factor of quality in numerous horticultural crops. It performs various biological activities in the human body. Various factors, such as preharvest climate circumstances, genotypic differences, cultural practices, harvesting, postharvest handling methods, and maturity procedures, influence vitamin C content in fruits and vegetables. More intense light during growing season increases the vitamin C content in plant tissues. Temperature management after harvesting is an essential factor to maintain fruit and vegetable vitamin C content, which decreases at high temperatures and with longer storage durations (Lee and Kader, 2000).
High application rates of nitrogen fertilizers reduce vitamin C content in numerous fruits and vegetables. Vitamin C content in numerous crops can be enhanced through normal irrigation (Lee and Kader, 2000). C. lanatus pulp contains mineral and organic sugar concentrations. Ripe C. lanatus contained maximal carbohydrate (6.5%) quantity, compared with unripe fruit (3.5%). Moisture was most prevalent in the fruits at both stages (ripe 91.5% and unripe 94.8%). Lastly, some mineral quantities were measured. Unripe fruit contained 0.31% calcium, 0.81% potassium, and 0.004% iron, while ripe watermelon contained 0.005% iron, 0.29% calcium, and 0.89% potassium (Gwana et al., 2014).
The application of organic fertilizers to cultivate watermelon allows rendering of nutrient and organic matter supply. The effect of organic fertilizer application rate on watermelon growth has been investigated in previous studies. Mixed organic fertilizer and mixed expeller cake are applied to maintain appropriate soil nutrient quantities and enhance marketable watermelon yield (Uhm et al., 2012). Calcium and gypsum application are required to increase the concentration of elements in the rind and leaves (Scott et al., 2019). Plant physiological functions improve with increasing fertilizer application rates and soil moisture content.
Okonmah et al. (2011) reported that watermelon is best suited to quench the thirst and fulfill the caloric requirement of consumers owing to its nutritional components. Based on wet weight, moisture content makes up 92% of the watermelon composition (Anon, 2008). Furthermore, Nisa et al. (2012) reported that watermelon provides abundant valuable antioxidant compounds to the consumer, such as polyphenols, anthocyanins, and flavonoids (Jaskani et al., 2005; Kaur et al., 2001). Moreover, it does not contain fat and protein and has been regarded as source of vital minerals, such as magnesium, selenium, and zinc. Other significant bioactive compounds in watermelon include glycosides, alkaloids, saponins, triterpenoids, phytates, and tannins, prominently found in watermelon rind. However, Johnson et al. (2012) reported that phytonutrients, such as oxalates, are deficient in watermelon rinds.
Plant secondary metabolite production is conceivably affected by genetics (Cartea et al., 2011). Studies have revealed that the phytochemical content of individual cultivars can fluctuate meaningfully due to other factors as well. In plants, factors that affect total phytochemical contents include soil type, water content, light, temperature, and mineral composition (Hansen et al., 2010; Johnson, 2002; Rao et al., 2010). Furthermore, fruit quality can also be affected by agronomic practices (Rosen et al., 2005). For example, total glucosinolate level is considerably disturbed by high sulfur rates and low nitrogen application (Bleesington et al., 2010).
Postharvest handling, processing, and storage can promptly lead to fluctuations in phytochemical profile and plant quality. Health-promoting components may be affected due to such fluctuations (Jones et al., 2007). Non-enzymatic browning and lipid oxidation are the two foremost chemical fluctuations triggering deterioration, which can lead to changed color and flavor throughout food processing and storage. Oxygen level, light, temperature, water activity, and presence of catalysts, such as iron and copper transition metals, influence lipid oxidation. Non-enzymatic browning decreases throughout the storage of concentrated and dried foods. These factors disturb phytochemicals. Throughout the altered vegetable processing steps, heat can lead to significant losses in carotenoid bioactivity. Oxidation is the foremost reason for carotenoid deprivation in foods. Overly extensive storage at high temperature causes a gradual decrease in phenolic compounds and other flavonoids. In plants, phenols are simultaneously present in free and conjugated forms. Peroxidase and polyphenol oxidase enzymatic oxidative reactions are the foremost reasons of postharvest loss of phenolic compounds (Piljac-Zegarac et al., 2011).
Phytochemical deterioration can decrease as a result of proper packaging and lower temperature. In fruits, higher antioxidant activities were sustained at 48°C storage, compared with 25.8°C (Sabir et al., 2011).
CA and MAP are operational practices for sustainability and marketability of fresh produce goods. Compared with packaged samples or conventionally stored packages, MAP and CA technologies can preserve bioactive compounds, decrease respiration rate, and increase the shelf life of vegetables and fruits (Hanekon et al., 2010; Koyuncu et al., 2010).
Postharvest storage technology for fresh products extensively uses 1-methylcyclopropene (1-MCP)-type chemicals. To slow down the ripening of vegetables and fruits and preserve their freshness, 1-MCP is used as a plant growth regulator (Chriribogan et al., 2008).
Plant foods are often processed to fulfill the diverse consumer needs. Usually, the processed products have comparatively lower nutritional values than the analogous fresh produce, mainly due to nutrient loss throughout processing. Nutrient content, conformation, and bioavailability of compounds, such as phytochemicals, in fruits and vegetables, is usually affected by industrial processing and cooking methods, such as sterilizing, freezing, blanching and canning, microwave-heating, boiling, and steaming (Podsedek et al., 2007; Podsedek et al., 2008).
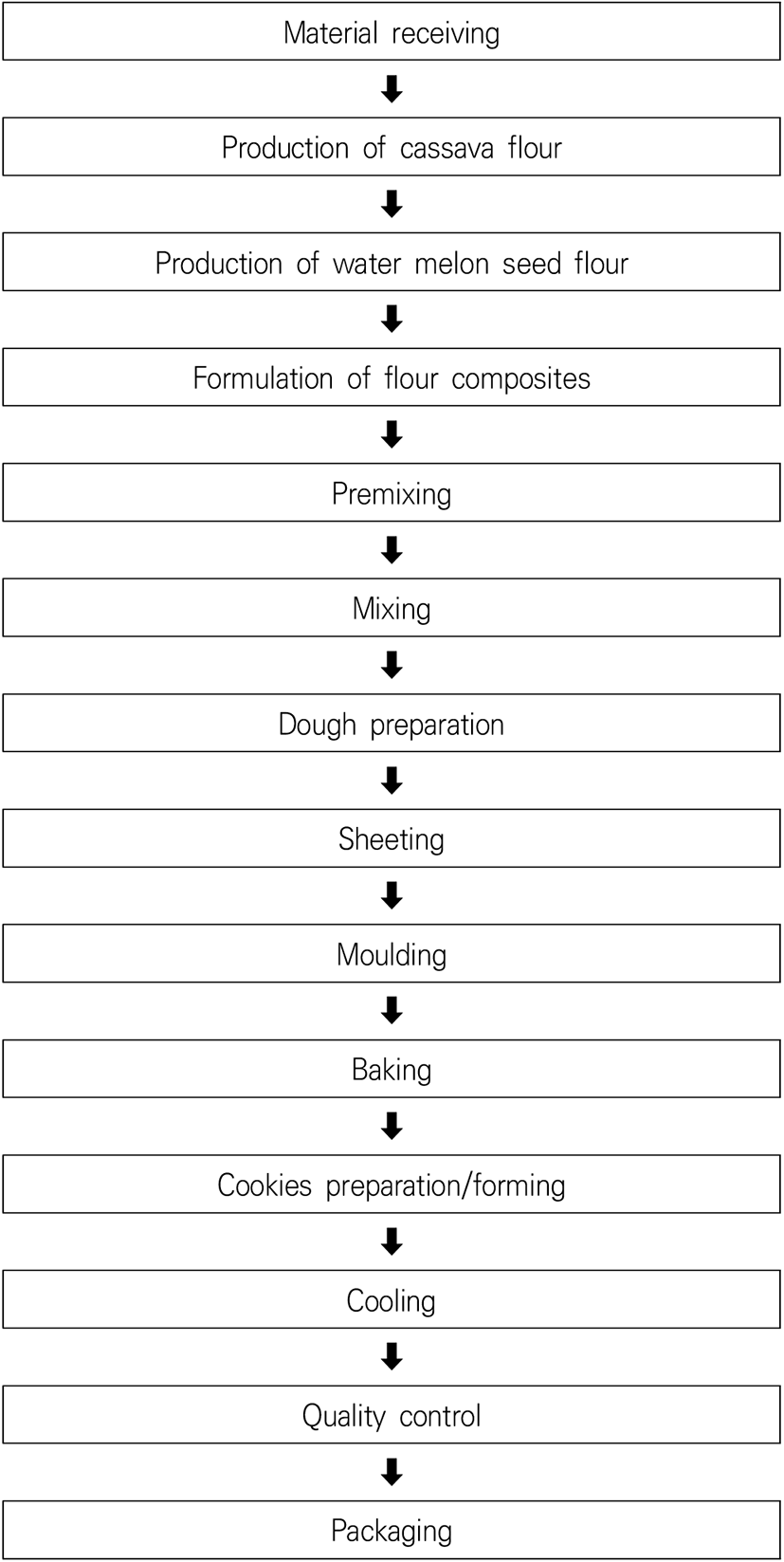
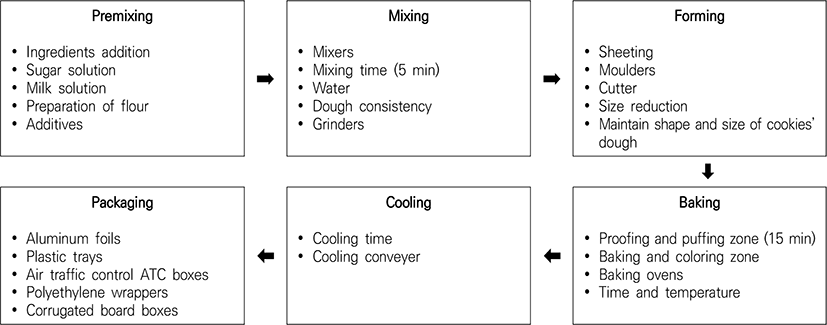
Composite flour obtained from watermelon seeds is used to produce watermelon cookies. A combination of cassava and watermelon seed flours is used to formulate cookies, with 20% wheat added for texture. These cookies are rich in protein, as watermelon seeds are a well-defined source of proteins and amino acids (Ubbor et al., 2009), as shown in Fig. 1. Major stages in watermelon cookie manufacturing are shown in Fig. 2.
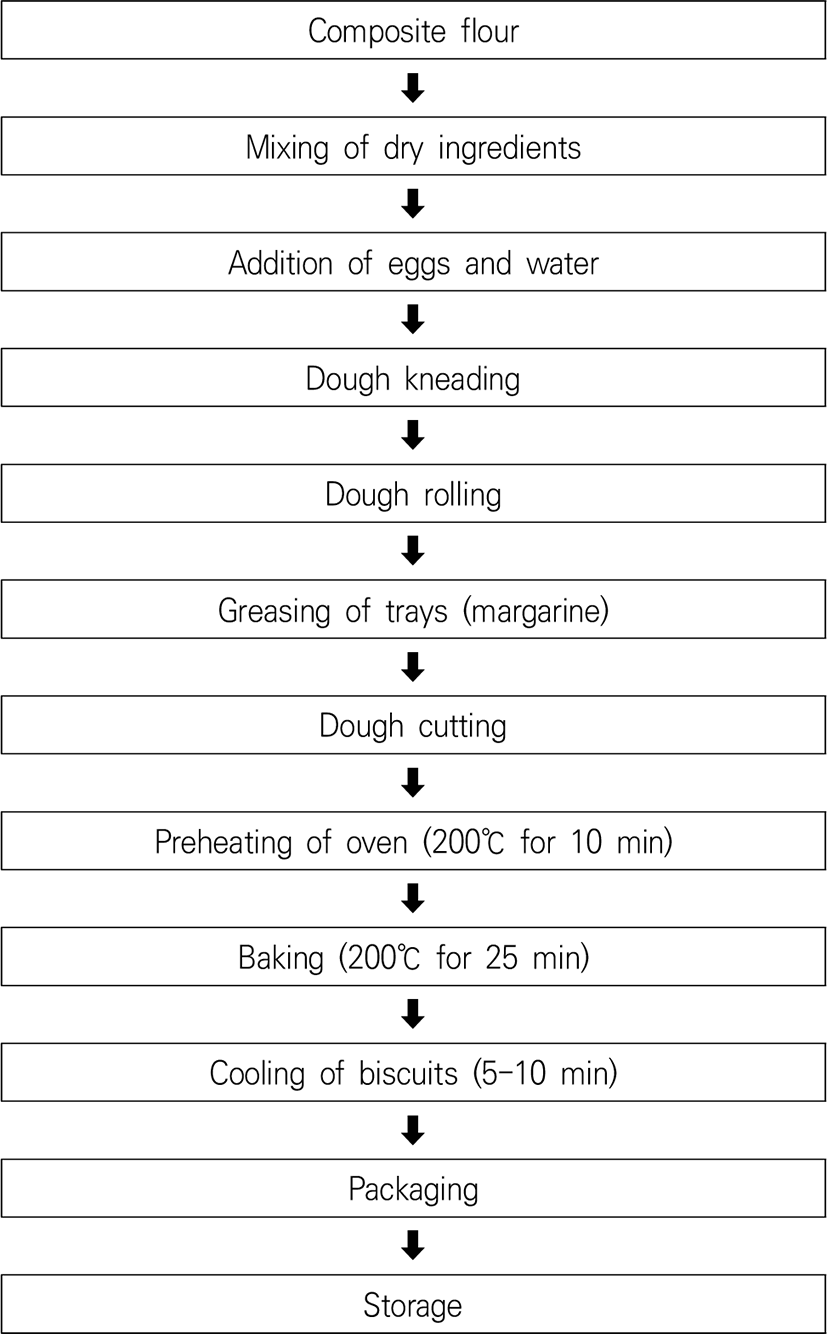
Cereal snack foods, such as wafers, bread, and biscuits (also called cookies) have gained popularity particularly among children. Numerous attractive characteristics are associated with biscuits, such as a broad consumer base, comparatively longer shelf life, and good sensory quality (Banureka et al., 2011). Toasted watermelon seeds and wheat flour are used to produce biscuits. Watermelon seeds are extracted, toasted, and converted into seed meal after grinding. The obtained seed meal is later utilized to prepare flour. Toasted watermelon seed meal contains increased crude fiber, ash, protein, and fat (Peter-Ikechukwu et al., 2018). A flow diagram of watermelon biscuit preparation is shown in Fig. 3.
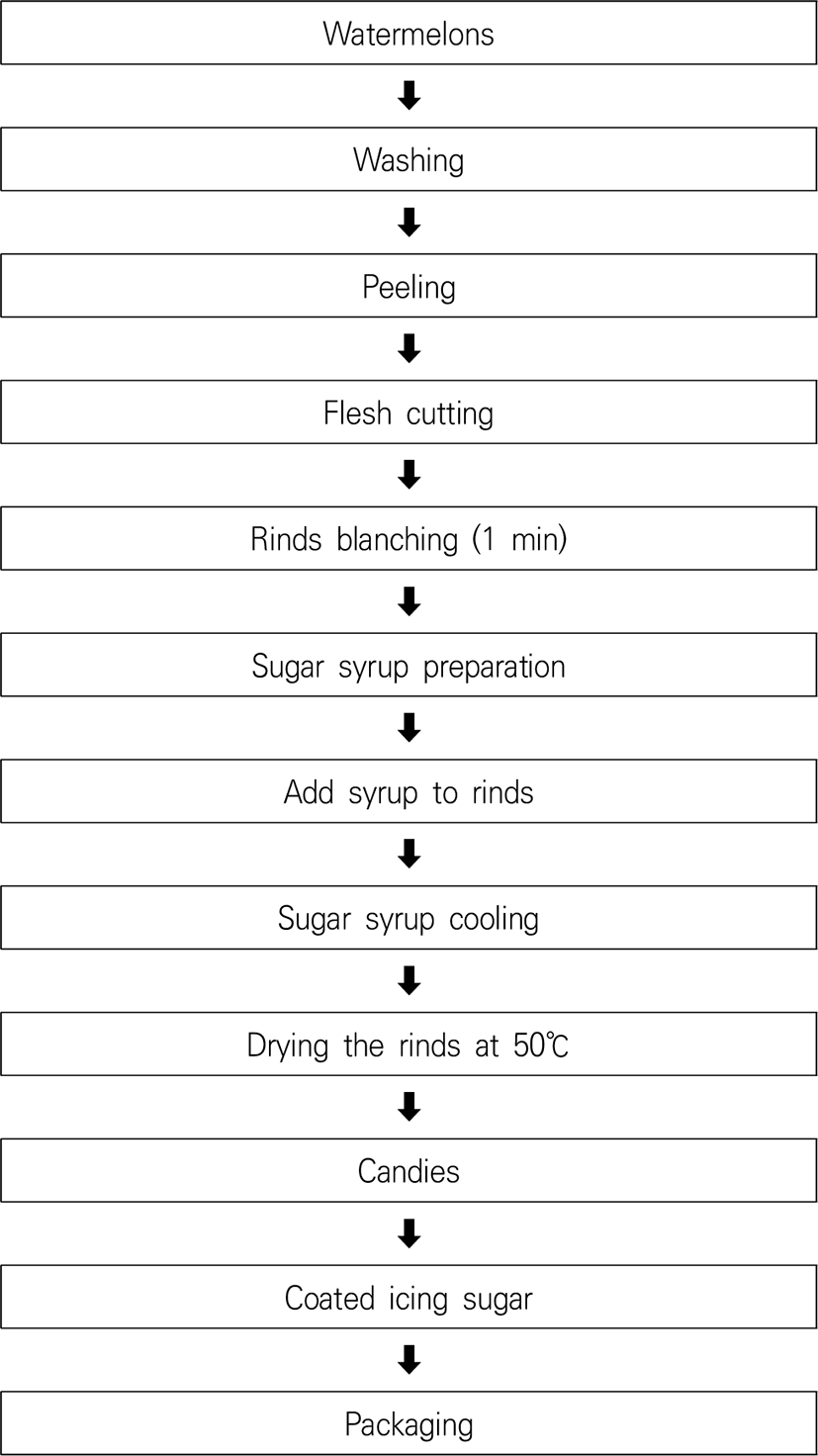
Flesh, rind, and seeds are the three major categorized watermelon components. The rind is commonly used as feed or applied as fertilizer; however, it is discarded in most cases. Advanced processing reveals that the rind can be used as an ingredient of food items, such as pickled products, vadiyam, cheese, and candy (Madhuri et al., 2003). Osmotic dehydration is used to formulate dehydrated candy from watermelon rinds, using slow syrup saturation. The optimal candy dehydration time is 14 h, and relished candy is prepared from watermelon rinds (Muhammmad et al., 2015). A flow diagram of watermelon candy preparation is given in Fig. 4.
Watermelon waste materials are some of the most essential food-grade agro-waste products. Watermelon jam is mostly prepared in Southeast Asia, especially in Malaysia. Watermelon rinds are used to prepare jams with different and unique flavors (pineapple, vanilla, lemon, and strawberry). Jams with strawberry flavor are the most popular and liked by consumers (Iqbal et al., 2017). A flow diagram of watermelon jam preparation is given in Fig. 5.
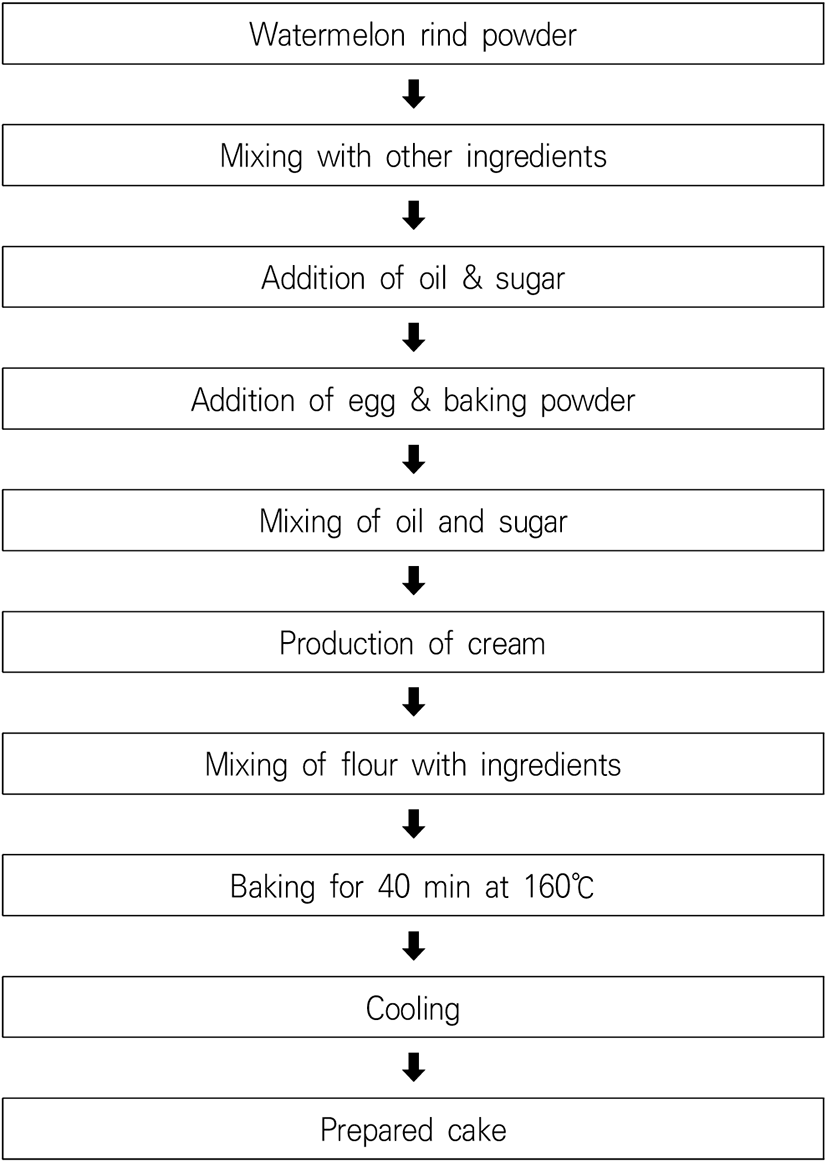
The largest and best-organized food industry around the globe is the bakery industry. Cake production is the most popular production practice. Cakes are palatable and relished bakery items prepared using sugar, essence, flour, baking powder, shortening, and eggs as primary ingredients (Sindhuja et al., 2005). Watermelon rind is added during cake processing, as well as composite rind and wheat flour. In addition, a low quantity of watermelon rind flour significantly enhanced the cake volume (Iqbal et al., 2017). Flow diagram of watermelon cake preparation is given in Fig. 6.
The food industry aims to provide fresh and nutritional fruit juices, and consumer demand is increasing, leading to non-thermal processing of fruit juices to retain bioactive compounds and ensure safety. The Unites States Food and Drug Administration has declared watermelon products as potentially hazardous foods, owing to their slightly acidic content, with a pH ranging from 5 to 6. This highlights the dire need for watermelon juice processing with minimal microbial spoilage and maximum retention of bioactive compounds for extended periods of time. Non-thermal processing of watermelon juice reportedly allows the production of juice with minimal enzymatic and microbial activities, and non-thermally processed juice resembles freshly obtained watermelon juice, with respect to sensory and nutritional characteristics. Microbial load may be present in fresh juice after exposure to aerial microbes. Non-thermal processing techniques, including pulsed electric field, high-pressure processing, ultraviolet irradiation, and ultrasound-assisted extraction, have been extensively utilized by researchers to inactivate microorganisms, such as Listeria monocytogenes, E. coli, L. innocua, and Salmonella enteritidis. Synergistic treatment with non-thermal methods with mild heat treatment has also led to promising results in watermelon juice processing (Bhattacharjee et al., 2019). Processing and operational conditions for juice processing must be optimized and scaled up from the laboratory and pilot scales. Effective microbe inactivation has been reported, but issues pertaining to food-borne viruses still need to be addressed. There is also a need to fundamentally understand non-thermal process design and desired standard products. The application of these non-thermal processes is limited to the pilot and lab scales, as large-scale industrial application incurs high costs, due to the use of industrial thermal devices. Non-thermal processing methods are advantageous due to microbial inactivation and enhanced shelf life and nutritional characteristics, but their high fixed cost limits their applicability in fruit juice processing. Further research is needed in this regard to increase the scale of these non-thermal methods and optimize costs to fulfill both consumer and industrial needs (Bhattacharjee et al., 2019).
11. Conclusions
This review discusses the relevant literature on watermelon processing and phytochemical potential. The review focused especially on watermelon characteristics, varieties, and processing. Watermelons can be processed into a variety of products. Watermelon is usually consumed fresh. However, it has a relatively short shelf life in its fresh state, ranging from days to weeks. Fresh watermelon frequently presents complications in storage and shipping, and significant income loss may be incurred as the product degenerates. To increase income and reduce degeneration losses, watermelons are processed into numerous products with extensive diversification. Many food items are prepared from watermelon flesh and non-flesh processing, including cookies, jam, jellies, ice cream, cake, and biscuits.