1. Introduction
Wild indigo (Baptisia tinctoria), also called Sophora tinctoria (Duke et al., 2002; Felter and Lloyd, 1898), is a herbaceous plant belonging to the legume family (Fabaceae) and is widely used in traditional medicine (Bone, 2003). Its roots are used in traditional South American Indian medicine to treat wounds, ulcers, and inflammation. Currently, it is considered an immunomodulatory drug and has been introduced into homeopathy since the middle of the 19th century (Nikishin et al., 2016). It is a perennial plant with a height of 60-90 cm, which a small narrow stem, alternate, trifoliate leaves with rounded apices, and canary yellow flowers (Hutchens, 1991; Tobyn et al., 2016).
Cultivated B. tinctoria plants have been used to treat pneumonia, influenza, and tuberculosis. Tea made from this herb was used to wash smallpox lesions internally and externally (Hutchens, 1991). An infusion of B. tinctoria and Juniperus utahensis has been used as a drug for kidney disease (Nikishin et al., 2016). Moreover, B. tinctoria poultices have been used to treat snake bite injuries (Chevallier, 2001). Well-known anticancer agents include vitamin C, vitamin E, carotenoids, and flavonoids. Recently, peptides with antioxidant properties have been identified in various plant and creature sources (Borrello et al., 1984). The course of oxidative stress reactions and the production of reactive oxygen species depend on the absolute or relative content of endogenous antioxidants in tissues; therefore, an increase or decrease in their concentrations can affect the level of oxidative stress (Halliwell and Gutteridge, 2015).
Based on signs, such as wrinkles, spots, sallowness, extreme wrinkles, laxity, and weathered appearance, elastase and collagenase can accelerate skin maturation. Hyaluronidase (HAase) is an enzyme that catalyzes the breakdown of hyaluronic acid (HA), the primary structural substance in connective tissue. When HA is hydrolyzed to glucosamine and glucuronic acid, its viscosity decreases and the permeability of connective tissue increases (Jiratchayamaethasakul et al., 2020). Tyrosinase is an enzyme related to the production of melanin, a pigment widely distributed throughout the skin, hair, and eyes of living organisms. Melanin is synthesized in the human body in the melanosomes of the epidermal layer, where tyrosine is used as a precursor by tyrosinase to produce 3,4-dihydroxyphenylalanine (DOPA). Since melanin produced in the epidermal layer darkens the skin, experiments on physiologically active substances that effectively inhibit tyrosinase activity are considered very useful for finding whitening effects (Cha et al., 2010). The anti-inflammatory and analgesic mechanisms of nonsteroidal anti-inflammatory drugs are associated with the inhibition of cyclooxygenase, which promotes the synthesis of prostaglandins that are primary mediators of inflammation and pain. In addition, they affect the synthesis and transformation of other substances involved in the occurrence and spread of inflammation and pain in the body (Halliwell, 2011).
The antioxidant and enzyme inhibitory activities of B. tinctoria roots had not been investigated previously. Therefore, this study aimed to confirm that B. tinctoria extract could exhibit antioxidant activities, such as DPPH and ABTS radical cation scavenging activities, as well as the activities of HAase, tyrosinase, elastase, and collagenase, as a natural anti-inflammatory substance.
2. Materials and methods
B. tinctoria specimens used in the study were cultivars grown in Fujian Province, China (27°32′36″-27°55′15″ north latitude; 117°24′12″ east longitude-118°02′50″). The plants were obtained from Joryherb Biotechnology Co., Ltd. (Shaanxi, Xi’an, China). Brown-colored B. tinctoria roots were stored for drying. After drying in an oven (JeioTtech, Daegu, Korea) at 45°C, the roots were ground at 25,000 rpm using a high-speed grinder at 25,000 rpm (RT-08; Rong Tsong Precision Technology, Taichung, Taiwan). The root material was pulverized using a 40-mesh sieve, and the powdered roots were stored at 4°C.
In our study were used some steps to prepare B. tinctoria extracts. The B. tinctoria extract powder (1 g) was added to 10 flasks. In the first flask, the powder was dissolved in 200 mL distilled water (DW) and mixed thoroughly, and the resulting solution was boiled until the volume reached 100 mL. To each of the remaining 10 flasks, 100 mL ethanol solution, ranging from 10% to 100% in concentration, was added, and the solution stirred at 120 rpm under the conditions of rotary vacuum evaporation in a shaking incubator for 24 h. The 50% ethanol extract was found to contain the highest phenol content for extraction using 95% purity level of ethanol. Continuous assays and extraction were performed using the 50% ethanol. For example, 100 mL of 50% ethanol solution was added as a solvent to 1 g of sample powder, followed by stirring at 120 rpm for 24 h. All extracts were filtered through Whatman no. 1 filter paper (Whatman Inc, Piscataway, NJ, USA) and subjected to rotary vacuum evaporation (Eyela NE, Tokyo, Japan). Phenol content of the ethanol extracts (10-100%) was determined to select an ethanol concentration with the optimum TPC. The water and ethanol extracts were then divided into two portions; the first was used as a phenolic extract while the second was incubated and freeze-dried using a freeze dryer (FDS8518, Ilshin Bio-Base Co. Ltd., Dongducheon, Korea) at −80°C for 4 days until use. The resulting powder was used as a solid sample. Next, four different concentrations (50, 100, 150, 200 μg/mL) were prepared for both the phenolic extract and the freeze-dried solid samples.
HPLC analysis was performed as described previously by Park et al. (2018). Quantitative analysis of phenolic compounds was performed using an Agilent 1100 series HPLC system (Agilent Technologies Inc., Waldbronn, Germany) equipped with a diode array detector (DAD) and autosampler and column compartment. The system was equipped with a ZORBAX Eclipse XDB-C18 column (4.6 × 250 mm, 5 μm) (Agilent Technologies Inc., Santa Clara, CA, USA), and the column temperature was set at 30°C. Elution was performed using 70% mobile phase A (0.1% acetic acid in HPLC water), 17% B (methanol), and 13% C (acetonitrile), the flow rate being 1.0 mL/min, and detection being performed at 278 nm under ultraviolet light for phenolics and flavanols. The total running time was 45 min. The water and ethanol extracts of B. tinctoria were filtered through a 0.20 μm SM13P020NL filter (Hyundai Micro, Seoul, South Korea) and injected into the HPLC system (injection volume, 10 μL).
The TPC was determined using a mixture of 1 mL of 95% ethanol and 1 mL of the extract. First, 5 mL of DW was added to 1 mL of sample extract soution, followed by 0.5 mL 1 N Folin-Ciocalteu reagent (Junsei chemical Co. Ltd., Tokyo, Japan), and the solution was mixed well in a vortex mixer KMC-1,300V (Vision Scientific Inc., Daejeon, Korea). The mixture was allowed to stand for 5 min, after which 1 mL of Na2CO3 was added. Subsequently, a UV spectrophotometer (Optizen 3220, Merasys Co. Ltd, Seoul, Korea) was used to measure the optical density (OD) at 725 nm. TPC was estimated using a standard curve with gallic acid as the standard (mg/g) (Folin and Denis, 1912).
DPPH radical scavenging activity was determined as described previously by Blois (1958), with certain modifications. A 60 μM DPPH working solution was prepared with 50% ethanol to obtain an OD of 0.70±0.10 at 517 nm. Initially, 1 mL of B. tinctoria extract (50-200 μg/mL) was prepared in test tubes and mixed with 3 mL of 60 μM DPPH. All mixtures were allowed to stand for 15 min, after which the absorbance was measured at 517 nm to determine the DPPH radical scavenging effects.
The ABTS radical cation scavenging activity was determined as described previously (Fellegrini et al., 1999). First, 5 mL of 7 mM ABTS was mixed with 88 μL of K2S2O8 (potassium persulfate) solution and stored in a refrigerator at 4°C for 12-16 h in the dark. Subsequently, 88 mL of ethanol was added to 1 mL of the stock solution and mixed thoroughly; 2,6-di-tert-butyl-4-methylphenol (BHT) (Sigma-Aldrich Inc., St. Louis, MO, USA) was used as a positive control. The ABTS solutions were adjusted to an OD of 0.70±0.02 based on the absorbance at 734 nm. Finally, 1 mL of ABTS solution was added to 50 μL of each sample, mixed in a vortex mixer, and the absorbance measured at 734 nm.
Antioxidant PF was determined using the method described by Andarwulan and Shetty (1999). First, 1 mL of 30 mg/mL β-carotene was diluted with 50 mL of chloroform. The solution was placed in a water bath at 40°C for the chloroform to evaporate. Subsequently, 184 μL of Tween 40 was added to 50 mL of hydrogen peroxide, and 20 μL of linoleic acid was added during mixing, to form an emulsion. Next, 100 μL of sample solution was mixed with 5 mL of the emulsion. After the sample solutions were thoroughly mixed and cooled, they were placed in a dark room at 50°C for 30 min. Absorbance was measured at 470 nm and the PF value was calculated as absorbance of the control versus that of the reaction.
Production of TBARS was measured as described by Buege and Aust (1978). An emulsion was prepared by adding 1% tween 40 and 1% linoleic acid to a flask and mixing thoroughly. Subsequently, 0.2 mL of sample solution was added to 0.8 mL of emulsion and incubated at 50°C in a water bath for 12 h. Next, 4 mL of 2-thiobarbituric acid was added to the experimental solution and the mixture was boiled in a water bath for 15 min followed by cooling in an ice bath for 10 min. The reaction solution was centrifuged at 492 ×g for 20 min and then allowed to stand for 10 min. The supernatant was collected thereafter and its absorbance was measured at 532 nm. The TBARS assay value was calculated as the difference in absorbance between the experimental sample solution and control solution.
The elastase inhibitory activity was measured as described previously (Kraunsoe et al., 1996). Briefly, 5 g of 0.8 mM N-suc-(Ala)3-p-nitroanilide were added to 0.8 mM substrate in 1 mL of 0.2 M Tris-HCl buffer (pH 8.0) and mixed thoroughly. Subsequently, 0.1 mL of 1.0 U/mL porcine pancreatic elastase (Sigma-Aldrich Co., St. Louis, MO, USA) was added to each sample with 50-200 μg/mL phenol content. Additionally, 0.1 mL of DW, instead of the sample, was added to one solution as a negative control. Finally, the amount of p-nitroaniline in the solution was determined by measuring the absorbance at 410 nm. Ursolic acid was used as the positive control.
The collagenase inhibitory activity was measured as described by Wunsch and Heidrich (1963). First, 0.3 mg/0.15 mL of 4-henylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg was added as a substrate to 0.2 mg/mL collagenase (Sigma–Aldrich) to reach a volume of 0.25 mL, and was then dissolved. Subsequently, 0.1 mL of each sample solution, with a phenol content of 50-200 μg/mL, was added. The reaction was terminated by adding 0.5 mL of 6% citric acid and 2 mL of ethyl acetate, and absorbance was measured at 320 nm. Epigallocatechin-3-gallate (EGCG) was used as a positive control. The collagenase inhibition activity was associated with a decrease in absorbance of the reaction and control sample solutions.
The tyrosinase inhibitory activity was determined as described by Vincent and Hearing (1987). The reaction mixture consisted of 2.3 mL of 0.1 M sodium phosphate buffer (pH 6.8), 0.4 mL of 1.5 mM substrate L-tyrosine solution, 0.1 mL of mushroom tyrosinase solution (250 U/mL; Sigma–Aldrich), and 0.2 mL of sample. In the control, 0.2 mL of DW was added instead of the sample. The reaction was performed at 37°C for 20 min. Absorbance was measured at 475 nm and kojic acid was used as a positive control.
HAase inhibitory activity was determined as described previously (Dorfman and Melvin, 1948). The glucoxazoline derivative was indicated by p-dimethylaminobenzaldehyde production, and absorbance was measured to confirm HAase activity. Subsequently, 0.05 mL of HAase (7,900 unit/mL) dissolved in 0.1 M acetate buffer (pH 3.5) was added to 0.1 mL of sample and pre-incubated at 37°C for 5 min. Next, 0.05 mL of 12 mg/mL melted HA, dissolved in 0.1 M buffer (pH 3.5), was added as a substrate, and the mixture was incubated at 37°C for 45 min. pH of the albumin mixture solution was decreased to 3.75 using 5 M HCl. Albumin mixture solution properad with 2.28 g of acetic acid added in 1.63 g sodium acetate and dissolved in 500 mL DW. In the mixture added 0.5 g albumin (Thermo Fisher Scientific Korea Ltd, Seoul, South Korea). Next, the mixture was cooled, and the absorbance measured at 600 nm. As a positive control, ammonium pyrrolidinedithiocarbamate (PDTC) was used in the same concentration as that of the sample.
All tests and experiments were performed in triplicate (n=3). Statistical analysis was performed using IBM SPSS Statistics Client Documentation 25.0 for Windows (Statistical Package for Social Science, Chicago, IL, USA). Duncan’s multiple range test was performed to analyze the differences. A p<0.01 indicated a significant difference.
3. Results and discussion
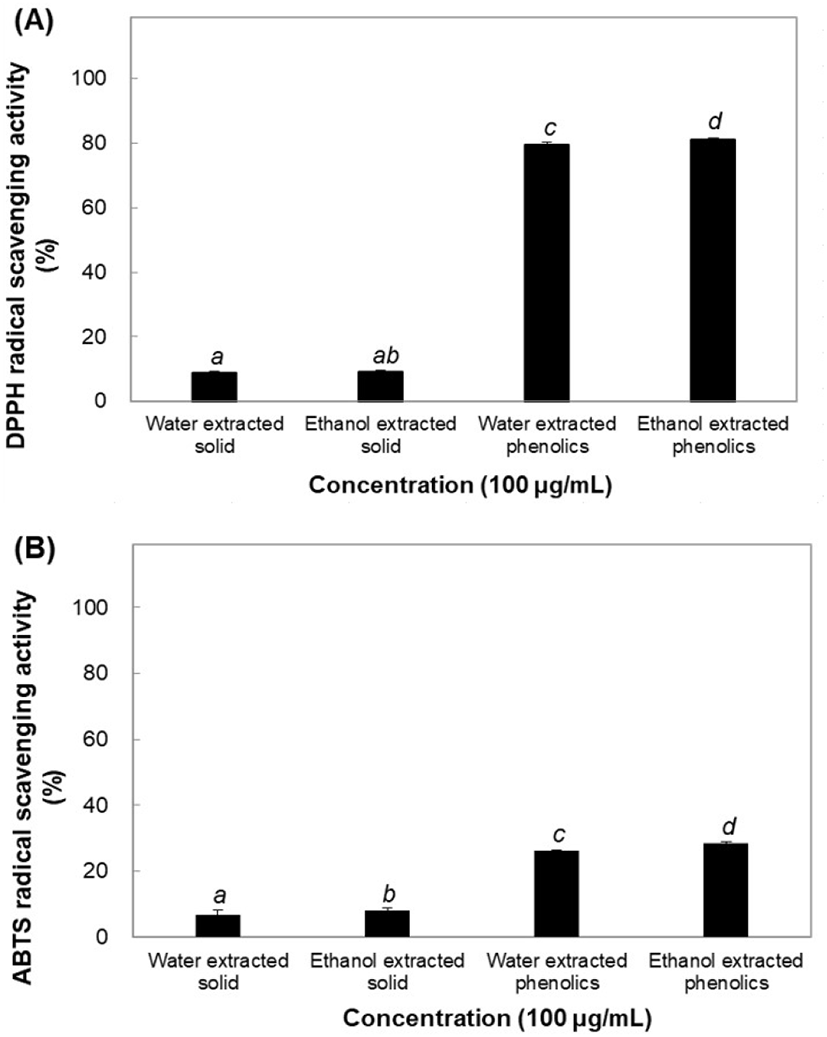
Antioxidants and pro-oxidants maintain balance and homeostasis in the body. However, this balance may be disrupted upon production of excess levels of free radicals. Oxidative reactions damage various cellular components, such as nucleic acids, proteins, and lipids; the damage is commonly referred to as oxidative stress. Antioxidant activities of the solid and phenolic extracts were compared using ABTS and DPPH assays. Furthermore, both methods were validated and their effectiveness was compared in terms of recovery, precision, detection, and quantification of antioxidant effects (Ratnam et al., 2006). The DPPH radical scavenging effect was measured to compare the physiological activities of both solid and phenolic components of B. tinctoria. Solid water and ethanol extracts (100 μg/mL) produced 8.73% and 9.24% reductions in radical effects, respectively, and ABTS oxidation remained almost the same, decreasing by 6.82% and 8.07%, respectively, owing to the physiological activity of the solid extracts. The antioxidant and physiological activities of phenolic water and ethanol extracts were determined by a DPPH assay, and significantly high radical scavenging activity of 79.49% and 81.16% was seen (Fig. 1(A) and (B)). Additionally, the ABTS assay showed a lower antioxidant capacity of water- and ethanol- extracted phenolics than the DPPH assay, scavenging activity being decreased by 26.18% and 28.32%, respectively. The results indicated that phenolic components have the highest activity in both water and ethanol extracts. One of the most critical properties of phenolic and polyphenolic compounds is their involvement in redox reactions and in neutralizing reactive oxygen species (Biswas et al., 2012).
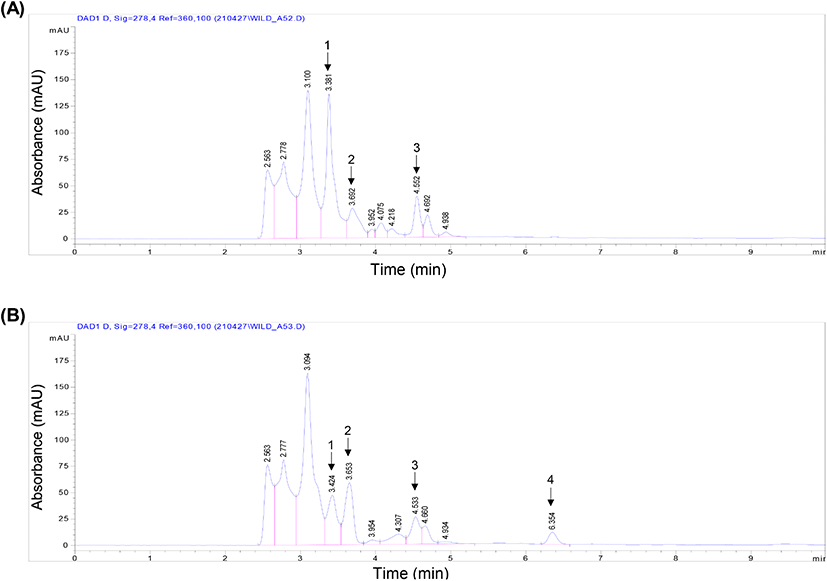
Eleven peaks were detected from the 50% ethanol and water extract chromatograms. Comparison of both the extracts revealed the colorless phenol content, which was analyzed using HPLC-DAD (Borbalan et al., 2003). Post-column methods can be used to identify the phenolic compounds by combining an HPLC system with additional detectors and syringe pumps (for reagents) (Burnaz et al., 2017). The water extract showed a high first peak of 136.2 mAU at 3.381 min, whereas it decreased to 47.1 mAU from 3.424 min in the ethanol extract. After extraction of B. tinctoria powder using ethanol, most of the interrelation of the compounds changed; for example, the second peak of the ethanol extract increased to 58.8 mAU from 3.653 min. In the water extract, another round of second peak significantly decreased to 28 mAU from 3.692 min, and in the same water extract, the third peak increased to 38.7 mAU from 4.552 min (Fig. 2(A)). However, in the ethanol extract, the peak significantly decreased to 26 mAU from 4.533 min. Furthermore, the ethanol extract showed a new fourth peak to 11.4 mAU at 6.354 min (Fig. 2(B)). To establish the phenolic profiles, two different ripening stages, other than the harvesting stage, were selected for water and ethanol extract phenolics; however, according to previous studies, the extracts may contain a high level of phenolics (Perestrelo et al., 2012).
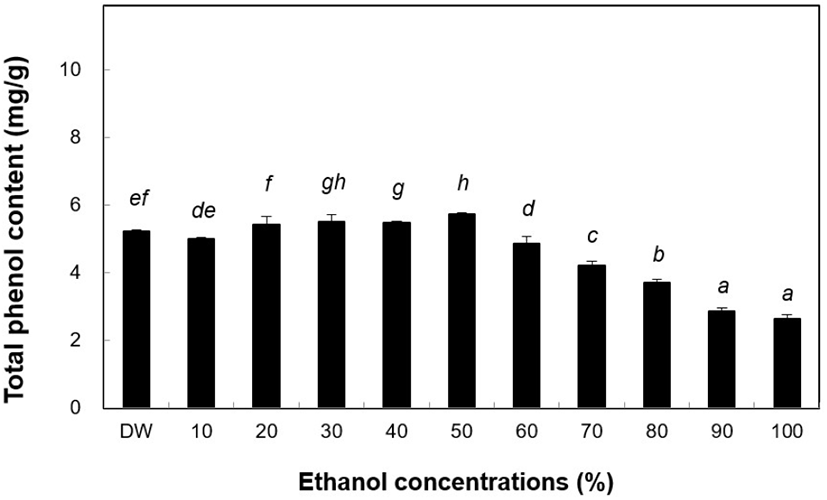
TPC generally contributes to the antioxidant activity in vegetables and cereals. During storage, the quality and color of these food products may change, which could be associated with the quality-quantity balance of antioxidants, and under acidic conditions during the phenolic reagent reaction (Sanchez-Rangel et al., 2013). In the present study, phenol content of the ethanol extract of B. tinctoria was high. The highest amount of phenolic compounds (5.74 mg/g) was found in the 50% ethanol extract (Fig. 3) when comparing ethanol concentrations between 50% and 100%. The DW extract had a phenol content of 5.22 mg/g. In the following experiments, water and 50% ethanol extracts were used in all assays. Antioxidant activity varies widely across the different phenolic compounds (Kevin et al., 1999). The ethanol extracts of tongue fern plant powder were dissolved in 80% ethanol solution and phenol content was determined to be 29.38 mg/g (Akhmadjon et al., 2020). The activities of ethanol extracts of B. tinctoria differed slightly.
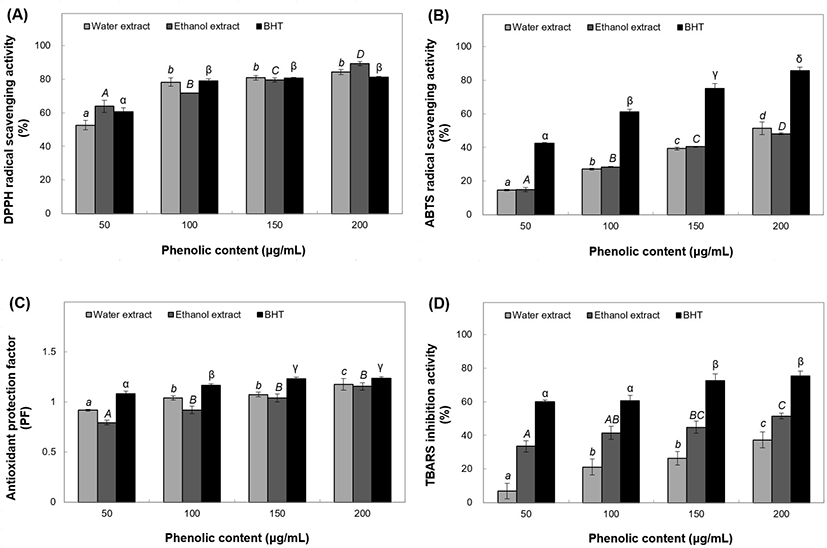
The extracts or powders of many herbs, plants, fruits, and vegetables have antioxidant and antimicrobial properties (Yuan et al., 2005). We tested four concentrations of phenolic extracts by antioxidant activity assays. The DPPH radical scavenging activity of water extracts at 50-200 μg/mL concentrations ranged from 52.61% to 84.23% (Fig. 4(A)). Ethanol extracts at the same concentrations showed radical scavenging activity ranging from 63.80% to 89.30%, aalong with significant increase in DPPH radical scavenging activity of BHT ranging from 60.57% to 81.07%. ABTS radical cation scavenging activity of the 50 μg/mL water extract was found to be 14.75%, which sharply increased to 27.13-51.39% at 100-200 μg/mL concentrations (Fig. 4(B)). The ethanol extracts showed scavenging activity of 14.89-48.10% within the same concentration range, whereas the BHT positive control showed antioxidant activity of 42.44-85.65% with 50-200 μg/mL phenol content. The antioxidant PF of the water extracts ranged from 0.92% to 1.18% at 50-200 μg/mL while that of the ethanol extracts significantly increased from 0.79% to 1.16% at the same range of phenolic concentrations (Fig. 4(C)). PF of the positive control rose from 1.08% to 1.24% at 50-200 μg/mL concentrations. TBARS inhibitory activity in the positive control reached a peak of 59.95-75.26% for 50-200 μg/mL phenol content. Furthermore, the water extracts showed a sharp increase from 6.84% to 37.31% (Fig. 4(D)) while the ethanol extracts showed a remarkable increase from 33.55% to 51.43% over 50-200 μg/mL concentrations.
Extracts, such as of L-ascorbic acid and butylated hydroxyanisole (BHA), are less effective in scavenging the radical cation ABTS. In contrast, ascorbic acid and BHA have shorter reaction durations than the positive control concentrations. Based on the number of electrons available to inactivate the model free radical systems DPPH and ABTS, differences in kinetic activity of antioxidant compounds may be considered to be related to stoichiometric reactions (Meyer, 1947).
HA is a macromolecular polysaccharide consisting of glucuronic acid and glucosamine (Girish and Kemparaju, 2007). In the polymeric form, HA reduces inflammation by reducing macrophage phagocytic activity. Inflammation is caused by small molecules during the wound healing phase (English et al., 2006). B. tinctoria extracts were tested for their anti-inflammatory effects by assessing their HAase inhibitory activity. Ethanol extracts of B. tinctoria inhibited HAase activity by 0.77% to 14.3% at 50-200 μg/mL. Water extracts showed a higher inhibition rate of 20.02% at 200 μg/mL concentration, compared to the positive control PDTC (Fig. 5(D)) Positive control treatment at 50-200 μg/mL resulted in an increase in HAase activity inhibition, up to 46.39%, and the effects of all concentrations were significant, with inhibition increasing with concentration. B. tinctoria has long been used as an anti-inflammatory medicine for treating illnesses such as sore throat and osteomyelitis (Yuan et al., 2005), and the 50% ethanol extract was found to have the best anti-inflammatory effect, based on previous studies. HA polymers are known to enhance the moisture content of skin (Kaatz et al., 1988). According to the above experimental results, B. tinctoria extracts showed HAase inhibitory activity, and hence may be applied in beauty foods owing to their anti-nflammatory effects.
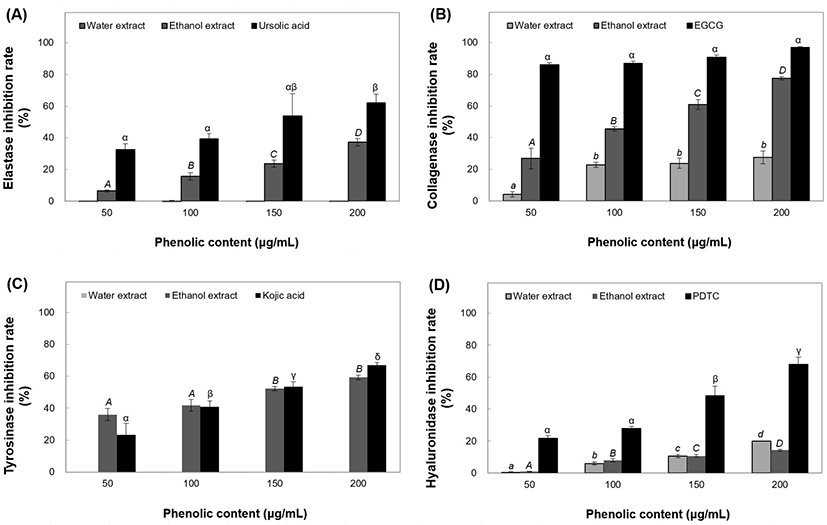
Melanin is generated to protect the skin from UV radiation and is responsible for the development of skin color (Wakamatsu and Ito, 2002). The factors that affect skin color include variations in melanocyte counts, aberrant production, and melanin structures in melanosomes (Ko et al., 2009). Melanin is synthesized by L-tyrosine-based tyrosinase (Pak et al. 2016). Thus, we measured the tyrosinase inhibitory activity of B. tinctoria extracts to determine their skin-whitening effects. The ethanol extracts exhibited significant tyrosinase activity inhibition of 36.01-59.26% at concentrations of 50-200 μg/mL (Fig. 5(C)). However, the water extracts showed no tyrosinase inhibition effects. An inhibition rate of 66.33% was achieved using 200 μg/mL kojic acid as a positive control.
For measuring elastase inhibitory activity, water and ethanol extracts were divided into four concentrations (50-200 μg/mL). The water extracts did not exhibit elastase inhibitory activity; the ethanol extracts demonstrated considerable elastase inhibitory activity of 6.50-37.21%. The elastase inhibitory activity of ursolic acid (positive control) peaked at 32.63-61.97% at 50-200 μg/mL concentrations (Fig. 5(A)). Elastase inhibitory effects of 150 medicinal plants had been studied previously, of which 70 were found to not inhibit porcine pancreatic elastase activity (Lee et al., 1999).
Estrone decreases collagen production and breakdown by stimulating fibroblasts in the dermal layer. Estrogen production slows down during natural aging, thereby increasing endocrine aging (Liyanaarachchi et al., 2018). In this study, the collagenase inhibitory activity of B. tinctoria extracts at concentrations of 50-200 μg/mL was measured. B. tinctoria water extracts showed collagenase inhibitory activities of 4.25-27.60%, and ethanol extracts showed inhibitory activities ranging from 26.86% to 77.39% (Fig. 5(B)). Elastase inhibitory activity of the EGCG positive control ranged from 85.87% to 97.03%. Although the activity of B. tinctoria extract was lower than that of EGCG, which is already used as an anti-wrinkle cosmetic material, it did show high collagenase inhibitory activity.
HAase inhibitors are effective regulatory agents that maintain a balance between HA anabolism and catabolism, thereby keeping the skin both moist and smooth. Enzymatic L-tyrosine oxidation during melanin synthesis is of particular interest, because melanin plays various roles in the development of many diseases where melanin synthesis may be altered. The use of tyrosinase inhibitors in medical and cosmetic products has been increasing, with several anti-melanogenic reagents, such as monobenzone and hydroquinone, currently in use (Kubo et al., 2000).
4. Conclusions
We aimed to evaluate the physiological activities of B. tinctoria root extracts for use in antioxidants, cosmetics, and functional foods. Different physiological activities, such as antioxidant activity and inhibition of elastase, collagenase, tyrosinase, and HAase activities, were evaluated using phenolic extracts to test the utility of B. tinctoria as a functional material. The 50% ethanol extract showed a high TPC of 5.74 mg/g, which was higher than that of the water extract. The DPPH and ABTS radical cation scavenging activities, antioxidant PF, and TBARS inhibitory activity of phenolic compounds in B. tinctoria extracts were measured at concentrations ranging from 50 to 200 μg/mL. The elastase and collagenase inhibitory effects were 37.21% and 77.39%, respectively, for ethanol extract (200 μg/mL) and 63.38% and 80.07%, respectively, for water extract (200 μg/mL). Compared to ursolic acid and EGCG, used as positive controls, B. tinctoria extracts showed relative elastase and collagenase inhibitory activities in the phenol concentration range of 100-150 μg/mL. Therefore, B. tinctoria extracts were considered to have excellent inhibitory effects on the enzymes involved in physiological activities, hence being considered highly usable as a functional material. The ethanol extract exhibited tyrosinase inhibitory activities ranging from 36.01% to 59.26% at 50-200 μg/mL concentrations, which might produce a skin-hitening effect. While measuring the HAase activity inhibition (related to anti-inflammatory effect) by B. tinctoria extract, the water and ethanol extracts at 200 μg/mL phenol concentration showed 20.02% and 14.30% inhibition, respectively. As a result, B. tinctoria extract can be applied as a functional material in cosmetics and food supplements for its antioxidant and skin health promoting activity.










