1. Introduction
Eggs are an excellent protein sources and are consumed daily worldwide. Several factors, such as egg size, egg shape, eggshell color, and eggshell quality influence consumers’ acceptability and preference. In the egg industry, the internal qualities of eggs such as egg white cleanliness and viscosity, size of the air cell, yolk shape, and yolk strength are considered important parameters, which indicate egg freshness (Mehdizadeh et al., 2014). The freshness of eggs is influenced by several factors, such as temperature, humidity, storage time, and age of the hen. Among these factors, the storage time and temperature play important roles in maintain the freshness of eggs, since the internal quality of eggs starts deteriorating immediately after the eggs are laid (Severa et al., 2010; Singh et al., 2014, Yimenu et al., 2017). Currently, many studies have focused on evaluating the freshness of eggs via non-destructive and non-invasive techniques because the traditional destructive Haugh unit method requires a large number of eggs. The higher the HU value, the better is the egg freshness because higher quality eggs always have thicker egg whites.
The brown color of eggshells is due to the deposition of protoporphyrin, formed due to the degradation of hemoglobin in blood (Yang et al., 2009). This brown pigment is included in the white calcium carbonate shell typically during the last phase of egg shell formation. The intensity of this brown color varies with diet, age and strain of the hens (Alasahan et al., 2016; Ingram et al., 2008; Seo et al., 2010). The brown layer is still calcium based and the eggshell color irreversibly changes upon washing (Leleu et al., 2011).
The difference in darkness intensity is characterized by International Commision on Illumination (CIE) L* (blackness, 0 or whiteness, 100), a* (+: redness or −: greenness), and b* (+: yellowness, −: blueness) values (Baylan et al., 2017; Kim et al., 2015; Luvkanov et al., 2015). Moreover, the brownness of eggshells is quantitatively determined as the brightness value, which is measured as luminance (CIE Y, cd/cm2) (Withouck et al., 2013). CIE is a color matching system which quantitatively links wavelength distribution in the visible spectrum with the colors perceived by human vision. This color matching process provides a mathematical generalization of the parameters recorded as points across the visible spectrum. Detection using an instrument perceives the reflected light at a specific wavelength as numerical values. The color information from the spectral data can be mapped as a flat and tongue-shaped CIE chromaticity diagram, which is an internationally accepted method of color identification. The CIE system characterizes colors by the luminance parameter Y and two color coordinates x and y, which specify the position in the chromaticity diagram.
The non-destructive visible-infrared transmittance spectroscopy is used to estimate the freshness of eggs and the resulting spectral data shows an increased transmittance value as the freshness of eggs decreases with aging (Giunchi et al., 2008; Kemps et al., 2006; Yao et al., 2014). The brightness of the eggshells is an important parameter in optical detection because darker the eggshells, lesser is the amount of light transmitted. Previous research has shown that the transmission of incident light reduces as the intensity of the brown color, i.e., brownness (darkness) increases. In contrast, the brighter eggshells allow a higher degree of light transmission at wavelength λ=625 nm (Lorentec etal., 2019; Shafey et al., 2004). Thus, it is assumed that most of the incident light was absorbed by the darker eggshells, thus reducing transmission of the incident light.
Furthermore, the egg white viscosity (thinning) also changes with age, leading to deterioration in the internal quality of eggs. To note, a longer storage time increases the pH inside the egg and decreases the egg white viscosity. Moreover, aeration through the eggshell also increases the pH level and causes thinning of the gel-like thick albumen inside the egg by disrupting the linkages between ovomucin and lysozyme (Chen et al., 2019; Mehdizadeh et al., 2014). The changes in transmittance and viscosity value of the egg white are often used to determine egg freshness and are known to be directly related to the shelf life of eggs.
The non-destructive spectroscopic method is a promising technique for investigating eggshell quality and freshness. This method provides rapid and accurate results, and thus, is suitable for industrial applications (Loffredi et al., 2021). However, the use of eggshell color variability for real-time detection of the freshness of eggs under different storage conditions is still a concern (Akowuah et al., 2020; Dai et al., 2020; Hansoongnoen et al., 2012).
In the present study, the freshness of eggs was investigated via non-destructive optical detection using ultraviolet-visible (UV/VIS) transmission spectroscopy with eggs collected daily from the farm. The color parameters of diverse brown eggshells were summarized using the CIE 1931 color system, and the correlation between luminance (brightness, CIE Y) and transmittance value was obtained and analyzed using a linear regression model, to minimize the interference of eggshell brightness in the transmittance of the incident light. Lastly, the viscosity of egg white was also measured to determine the freshness of brown eggs during storage and verify the decreased transmittance value. Therefore, the aim of this study was the real-time detection of the freshness of brown eggs having various shell brightness, to separate old eggs from the fresh ones in a farm. This study provides a novel, rapid, and simple method for the non-destructive and on-site testing of egg freshness.
2. Materials and methods
Fresh brown eggs were obtained from grass-fed Hy-Line hens (<50 week-old) raised in an organic farm (Rfood Agricultural Corporation, Chungbuk, Korea). The farm management provided an additional dietary supplement to the hens, composed of 80% corn, and 20% complex of protein, amino acids and vitamins. After collecting the eggs, they were washed thoroughly using the farm’s automatic washer (Nine Systems, Chungnam, Korea) equipped with a spray nozzle and brushes and dried. Following this, the cracked and abnormally shaped eggs were removed using an automatic crack detector. A total of 1,000 eggs were used to determine the degree of darkness, among which 54 eggs were chosen by random sampling and used to determine the relationship between eggshell brightness (CIE Y) and transmittance value of incident light (T%). For freshness measurement, 70 eggs were collected on the day they were laid and divided into 7 groups (10 eggs/group). The eggs were then stored at 20°C with 55% RH (relative humidity) for 7 days.
Eggshell color information was collected using UV/VIS spectrophotometer (SE2020-050-VNIR, OTO Photonics, Hsinchu, Taiwan) in the wavelength range of 350-1,020 nm with a resolution of 0.38 nm. The light source was a halogen lamp equipped with an infrared filter (150 Watt, GSS5-1000-F, Fiber Optic, Cheonan, Korea) and connected to an illuminator (FOK-150W, Fiber Optic), which provided continuous light supply during the measurement. The spectrum of light transmitted through the egg white was obtained using this instrument daily for 7 days, to determine the freshness of eggs. The transmittance value was measured daily from eggs in each group (1 group/day) and once an egg was used for analysis, it was discarded. The measurement was repeated during the 7-day cycles. The software SpectraSmart® provided by the manufacturer was used to control the color information and spectral acquisition as shown in Fig. 1. The experiment for freshness measurement was conducted twice with 140 eggs, to obtain statistically significant data. The transmittance value on Day 7 was set as the criteria to identify the stale eggs. To confirm the accuracy of determination, tests were conducted with a total 120 eggs mixed with the already known 15 stale eggs. At the end of the test, all the 15 stale eggs were successfully by measurement with 0% error. To further validate the results, the eggs were cracked and egg white thinning was evaluated. After the spectral assessment, the eggs were carefully broken and the egg white component was separated by removing the yolk and chalaza, for daily viscosity measurement. The egg white viscosity measurement was performed at 25±0.5 °C using the Brookfield viscometer (DV2T, Brookfield Engineering, Middleboro, USA) with spindle SC4-18 at 60 rpm at a shear rate of 79.2/s. The measurements were conducted for 30 s at intervals of 5 s. In addition, shear rate-dependent changes in viscosity were obtained for different shear rates from 10 to 100/s.
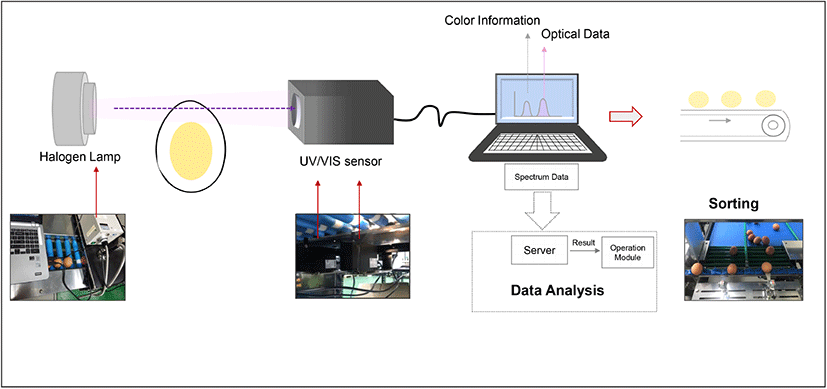
The eggshell CIE 1931 color parameters were obtained via UV/VIS spectrophotometer in transmittance mode with a standard light source and viewing angles. The two-chromaticity coordinates (x, y) and luminance CIE Y values were obtained from 54 eggs under illuminant A at a specific viewing angle of 2°. The tristimulus values of XYZ were calculated using x, y coordinates and the CIE Y values from the following equation (Westland, 2003):
Diversity in the eggshell darkness was measured, and the color components of the brown eggshells (L*, a*, b*) were calculated to correct certain experimental errors due to diverse eggshell surface darkness. The brightness of each brown eggshell was calculated in terms of CIE L*, a*, b* coordinates from equation (2) (Westland, 2003):
where Xn, Yn, Zn are the tristimulus values, viz. 109.85, 100.00, and 35.58, respectively, of a specific white color stimulus of the illuminant A. In addition, the color difference value (ΔE) was calculated from equation (3) (Eleroğlu et al., 2016):
The obtained spectral color parameters of CIE XYZ under illuminant A were converted to the CIE standard illumination D65 through a mathematical transformation using the Bradford matrix as shown below (Pascale, 2003), to map the coordinates in the CIE 1931 chromaticity diagram:
The seven stimulus colors (yellow, orange, red, aqua, pink, purple, and blue) were presented in the CIE 1931 chromaticity diagram to compare the physiologically perceived brown eggshell color via human vision with quantitative description of each color. The CIE xy coordinates of the stimulus color were adapted from McCamy et al. (1976). The CIE xyY values (illuminant C) were converted to those of illuminant D65 by mathematical conversion using Bradford matrix to compare the parameters reported by Xiao et al. (2011) and showed close approximation.
The CIE color data were collected from 54 eggs chosen by random sampling. The mean and the corresponding dispersion of brownness of eggshells (standard deviation) were evaluated. The measurement precision was determined as coefficient of variation (CV) using Microsoft Excel. The data showing a correlation between transmittance and viscosity values as of storage period represented mean± standard error and were analyzed on Microsoft Excel using one-way analysis variance (ANOVA) with a 95% confidence level to estimate the significance of the results.
3. Results and discussion
The freshness of brown eggs during storage was evaluated by the non-destructive UV/VIS spectroscopic technique. In optical detection, the eggshell brightness was considered as an important parameter because darker eggshells allowed lesser transmission of the incident light than the brighter ones did. Thus, the brightness of the eggshell color was investigated prior to spectroscopic measurements. The brown eggs collected in the current study showed diverse eggshell brightness and the color ranged from light yellow-brown (brightest, bottom) to dark red-brown (darkest, top) in human visual perception (Fig. 2(A), inset). The CIE 1931 (x, y) coordinates of the brightest (0.51, 0.42) and the darkest (0.64, 0.35) eggshell color were obtained through spectral assessment. These coordinates were equivalent to the stimuli color positions of orange (0.54, 0.40) and red (0.55, 0.33), respectively, as previously reported by Xiao et al. (2011). The (x, y) coordinates were then mapped in a 2D CIE 1931 chromaticity diagram and a cluster was built near the border of the spectral locus. This clustered region was assigned to orange and red spectral regions which were characterized by distinctive stimuli colors in the CIE 1931 chromaticity diagram (Fig. 2(A)). The darkness of the eggshells was measured and obtained as quantitative luminance (CIE Y, cd/cm2), a photometric value, signifying the luminous flux of perceived power of light per unit projected area (Withouck et al., 2013). The luminance values of the 54 eggs ranged from 7.77 cd/cm2 (dark) to 49.01 cd/cm2 (light) (Table 1). A higher luminance value indicated a brighter eggshell, and the CIE coordinate (x, y) tended to shift towards the orange stimulus region in the chromaticity diagram (Fig. 2(B)).
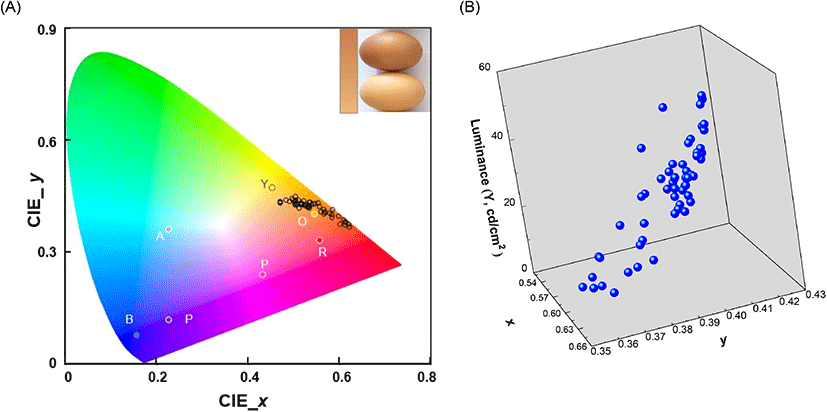
In addition, the different degrees of brownness of the eggshells were further measured quantitatively as changes in the L*, a*, b* values. The characterized color information of the eggshell in the form of CIE L*, a*, b* and color difference (ΔE) are summarized in Table 1. As mentioned earlier, the eggshell color was light yellow-brown to dark red-brown and these colors were quantitatively characterized using the CIE color system. Brown is mixture of three primary colors (red, yellow, and blue) and the range of brownness is affected by the ratio of these three components. These three primary colors were expressed as CIE a* and b* values and their quantitative relation generated different degrees of color. In addition, the degree of brightness was represented as L* value from the darkest (0) to the brightest (100). The gradient of brownness of the eggshells was inversely associated with the L* and a* value. The ratio between the red and green component was displayed a* value and a shift towards a higher a* value was represented as deep red.
The spectral measurements and analysis results showed that the eggshell lightness, L* value was 33.49 (minimum, dark) and 75.46 (maximum, light). The range of L* value indicated that brown eggshells exhibited medium brightness. The decrease in the L* value for the dark brown eggshells has been directly related to increase in the a* value, which represented redness. The light yellow-brown color is the combined brighter and greenish property, represented as large L* and small a* value.
In contrast, dark red-brown color indicates dark and reddish, resulting in a small L* and large a* value.
Experimental results clearly demonstrated that an increase in the L* value correlated with a decrease in eggshell redness, i.e., the a* value (Fig. 3(A)). The largest a* value (49.30) was observed when the L* value was the lowest (33.49). The smallest a* value (19.47) was linked with the highest L* value (75.46). The minimum and maximum eggshell color tone (b* values) were 46.39 and 97.95, respectively. The eggshell color showed diverse brownness ranging from light yellow-brown (brightest) to dark red-brown (darkest) under human visual perception and spectrophotometric quantitation displayed as changes in the L*, a*, b* values. Results displayed relatively higher a* and b* values than those reported previously (Eleroğlu et al., 2016; Li et al., 2016; Seo et al., 2010). These values possibly resulted from the different strains of egg-laying hens, and the corresponding dietary condition (Ingram et al., 2008; Seo et al., 2010). Seo et al. (2010) showed that lightness (L*) decreased from 52.2 to 49.6, while the redness (a*) increased from 14.7 to 15.7 significantly when Fe-soy protein (Fe-SP) supplementary treatment (Fe-SP 100 and Fe-SP 100+MgO) was provided to the Hy-Line brown egg-laying hens. In the present study, the measured L* and a* values for the grass-fed, egg-laying hens were higher than those reported by Seo et al. (2010). Thus, different dietary conditions induced varied eggshell colors although the eggs were collected from the same strain of Hy-Line hens.
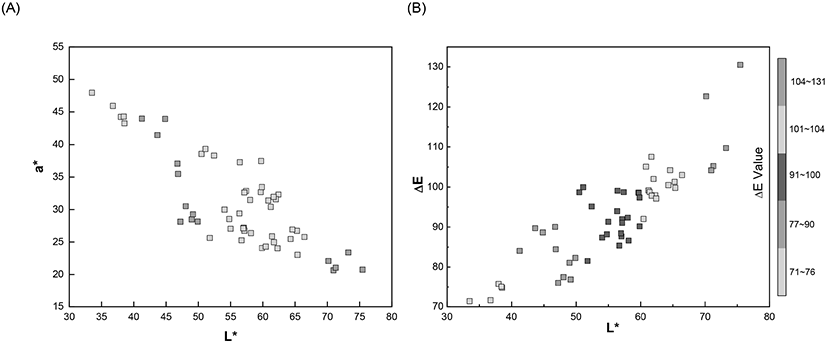
The color difference (ΔE) values were calculated based on the obtained CIE L*, a*, and b* values. The ΔE value utilizes a generally known linear formula to calculate the distance between two color points and a lower ΔE value indicates a darker color. In Fig. 3(B), the ΔE values showed a linear increased with the L* values, representing brightness. The darkest eggshell showed the smallest ΔE (71.10) and L* (33.49) values and increased gradually until it reached the maximum value of ΔE (130.51) and L* (75.46). In the plot, most of the ΔE values were scattered between 80 and 110, signifying intermediate to a lighter level of brownness of the eggshells because the darker eggshells should exhibit lower ΔE values (Eleroğlu et al., 2016).
The internal quality of the brown eggs in terms of altered freshness was measured spectrophotometrically and the maximum absorbance (λmax) was observed at 622 nm (Fig. 4(A)). The optical measurement of the transmittance value of egg white was aimed to determine the actual amount of light passing through ignoring the effect of different eggshell darkness. As stated above, the intensity of brownness (brightness) of the eggshells was diverse in range. The correlation between the transmittance and luminance values (CIE Y) was determined prior to the transmittance values of the eggs was quantified because the brighter eggshells allowed more transmission of the incident light. The relationship between transmittance and luminance (CIE Y) showed a correlation coefficient of 0.92 in linear regression (Fig. 4(B)). The actual quantity of the incident light transmitted through the eggs was obtained from the following equation (6):
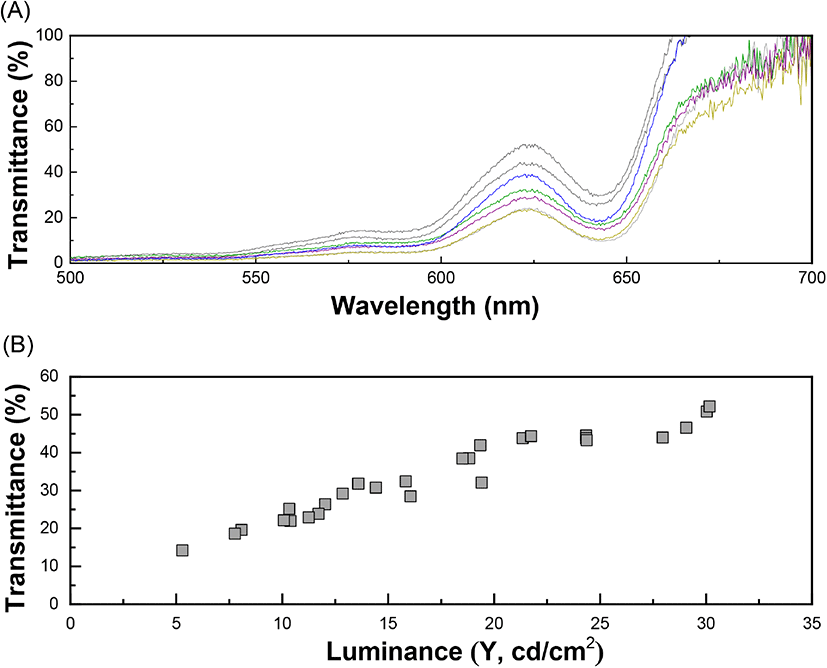
where, YT = transmittance of incident light passing through an egg and x = luminance (CIE Y) of the eggshell. The variation in the transmittance values with respect to storage time was further measured to investigate the freshness of the eggs up to 7 days from laying. The obtained spectrum of the transmittance values from the brown eggs (λmax = 622 nm) were corrected using the linear regression model (Equation 6) to adjust the brightness of each eggshell. The longer the storage time, the greater the transmittance value, due to decreased viscosity of the liquid-like egg white protein inside the egg. The transmittance increased gradually from 36.0% (day 1) to 38.8% (day 3), followed by a much steeper slope after day 3, and finally reached 50.8% on Day 7 (Fig. 5(A)). The increased transmittance value is related to a loose egg white protein network allowing more incident light than that with a dense structure. Additionally, the egg white freshness, calculated based on the transmittance value of day 1 as 100%, showed a negative relation with the storage time. Results showed that the freshness of the eggs decreased gradually to 59% on day 7 (Fig. 5(B)). The relation between the freshness value of the eggs (YF) and the storage time (x) can be represented as follows (correlation coefficient of 0.8433):
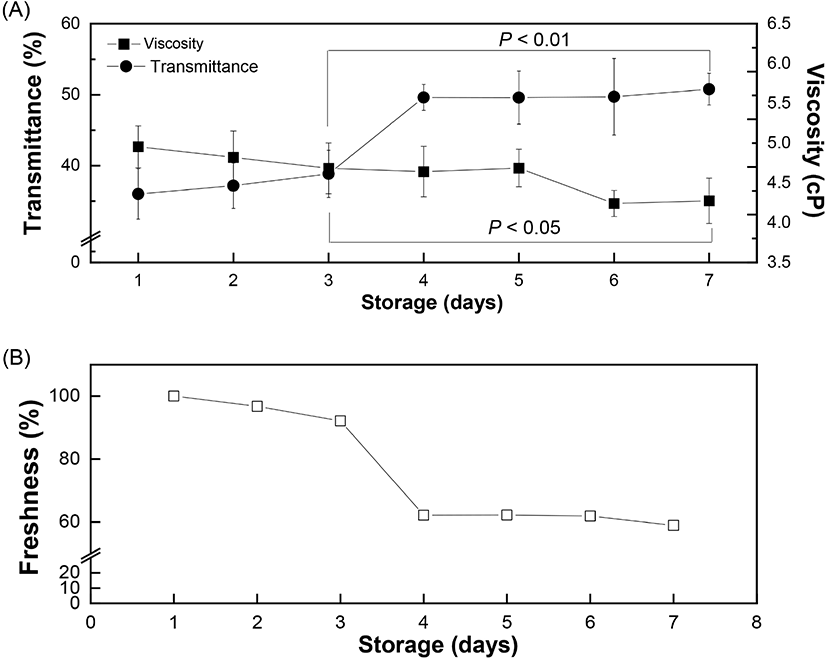
where, YF = freshness of egg followed by normalized transmittance value (%), x = storage time (day). The transmittance values from different storage days were normalized using the day 1 value as the standard. The difference in the transmittance value with respect to day 1 was directly proportional to the storage time. Therefore, the increased quantity of incident light passing through the egg white reflected that the eggs became aged and thus their freshness declined. Spectrophotometric measurement of the internal quality of the brown eggs in terms of altered freshness showed the main peak position at 622 nm (λmax=622 nm), as reported previously (Lorentec et al., 2019). The actual amount of incident light passing through the eggshells was measured by establishing the linear regression model between the luminance (CIE Y) and transmittance values. After correcting for the brownness of the eggshells, the actual light transmitted through the egg white was measured and showed a significant increase for a longer storage period (Fig. 5(A)). This could be attribute to increased passage of light through a loose and less dense egg white protein network. It is known that decreased CO2 concentration during storage induces a shift in egg white pH towards alkalinity (maximum - 9.5) (Yao et al., 2014). This increase in pH results in the gel-like thick egg white albumin losing its viscosity (egg white thinning) caused by partial disruption of the egg white protein network which allows more light to pass through.
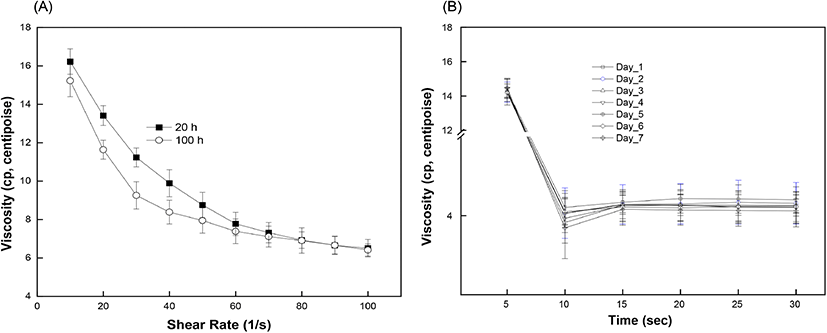
The effect of storage time on the viscosity of egg white is shown in Fig. 6(A). At a constant shear rate, the viscosity of the egg white decreased with increasing storage time. The measured viscosity of egg white was 4.95 centipoise (cp) on day 1 and gradually decreased to 4.69 cp on day 5. The viscosity value decreased more rapidly after day 5 and reached 4.28 cp on Day 7. Egg white is an aqueous solution with a gel-like structure and exhibits a non-Newtonian, pseudoplastic flow behavior which decreases the viscosity with increasing shear rate and time. The egg white viscosity was determined as a function of shear rate (10 to 100/s) and time (up to 30 s). Results clearly demonstrated that the shear-thinning behavior of egg white led to a gradual decrease in viscosity with increasing shear rate (Chen et al., 2019). Initially, the viscosity values for short (20 h) and long storage time (80 h) were 16.4 cp and 15.2 cp, respectively, and the difference in viscosity was maintained up to a shear rate of 80/s (Fig. 6(A)). The two plots showed gaps which indicated different viscosity values during gradual decrease until they merged after a shear rate of 80/s. Such shear-thinning behavior can originate from the breakdown of linkages between protein molecules. Further shearing would stabilize the internal protein structure by completing rearrangement and forming a loose structure. Egg white samples from different storage periods exhibited a time-dependent non-Newtonian thixotropic behavior showing a decrease in viscosity with respect to shearing time at a fixed centrifugation speed (Fig. 6(B)). Viscosity showed a time-dependent decrease, signifying thixotropic behavior (Atilgan and Unluturk, 2008; Singh et al., 2014).The rapid decrease in viscosity at the beginning of shearing however became constant after 15 s. This can be explained as the shear-induced rearrangement of the internal structure towards the direction of shear causing a decrease in flow resistance. The storage time is closely related to changes in the egg white viscosity. Our results also confirmed a significant reduction in viscosity with increased storage time at a constant shear rate, as reported earlier (Severa et al., 2010; Singh et al., 2014). The decrease in egg white viscosity with an increase in storage time reflects the disruption of linkages between protein molecules, which unwind and become relatively flexible. As eggs age, water gradually evaporates through the eggshell and loose inter-molecular linkages rupture, thereby causing a decrease in flow resistance by the progressive liquefaction and thinning of the egg white component (Cancer and Yüceer, 2015). In some portions, the loose inter-molecular linkages collapse and rearrange towards the shear direction (Chen et al., 2019; Spada et al., 2012). As a consequence, the loose and partially disrupted protein network in aged egg white facilitates the transmittance of incident light through the eggs. A prior study also reported a negative linear correlation between the freshness of eggs and the storage time (Liu et al., 2007). Our results prove the inverse relation between viscosity and transmittance value with increase in storage time and provide evidence that the freshness of brown eggs decreases within the storage time up to 7 days.
4. Conclusions
In the current study, non-destructive transmittance detection, using the direct radiation of incident light through the short axis of the eggshell surface, and the viscosity value of egg white were used to evaluate the change in egg freshness during 7 days. Our results prove the inverse relation between viscosity and transmittance value with increase in storage time and provide evidence that the freshness of brown eggs decreases within the storage time up to 7 days. This novel optical-based technique to measure egg freshness has potential applications in designing an automatic egg selection machine, which can separate stale eggs from the fresh ones on-site in small and medium egg farms by simply measuring the transmittance of eggshells of varying darkness.










