1. Introduction
Citrus fruit, which contains various biologically active substances such as phytochemical, is a common fruit with the highest production worldwide and is extensively cultivated in tropical and subtropical regions (Park, 2011). Phytochemicals are bioactive nutrients that protect plants from various microorganisms and pests. As the antioxidant, anti-inflammatory, and antibacterial effects of these phytochemicals are well known, research is being conducted to develop them as functional materials (Kim et al., 2013; Shih et al., 2010). Citrus contains both volatile and non-volatile compounds, whose contents differs depending on the variety, which manifests as a difference in color, taste, and aroma of citrus fruits.
Non-volatile components include flavonoids, carotenoids, vitamin C, citric acid, and free amino acids, which are known to have antioxidant, anticancer, and anti-inflammatory effects (Benevent-Garcia et al., 1997; Kim et al., 2019; Whiteman et al., 2005; Yang et al., 2008). Thus far, approximately 60 types of flavonoids are identified in citrus fruits. Among them, nobiletin and tangeretin are polymethoxy flavonoids (PMFs) that exist only in citrus fruits. PMFs exhibit physiological behaviors, such as the inhibition of platelet aggregation, inhibition of lymphocyte proliferation, ulcer prevention, and other anti-inflammatory activities (Fang et al., 2002; Valko et al., 2007). In addition, amino acids, which are non-volatile components, are the building blocks of proteins and are used as nutrients for energy generation during various metabolic processes. There are approximately 20 types of α-amino acids that are used as raw materials to formulate amino proteins, nine of which, namely, Leu, Met, Val, Ile, Lys, Thr, Phe, His, and Trp, are essential amino acids that are not synthesized in the human body and must be ingested through food (Park et al., 2017; Yu et al., 2019).
Volatile compounds are important secondary metabolites found in many plants species, and currently, structures of more than 23,000 terpenoid compounds have been identified (Cheng et al, 2007). Citrus fruits also contain a large number of volatile compounds, and they are not only consumed as raw food or juice, but are also used as in cosmetics and household products (Tomiyama et al., 2012). Research on volatile compounds in citrus fruits started in 1925, and since then, it has been attracting attention in many fields (González-Mas et al., 2019). In citrus fruits, limonene is the main volatile component, but other major compounds such as linalool, β-myrecene and valencene have also been found (Cheng et al., 2012; Cuevas et al., 2017a; Cuevas et al., 2017b). Citrus sinensis, Citrus unshiu, Citrus reticulata, Citrus limon were determined to have health-promoting effects in study on the essentials oil contained in their peels (Nair et al., 2018; Assefa et al., 2017; Barros et al., 2012).
Unlike Onju mandarins, C. hybrid kanpei and C. reticulata natsumi have a late harvest time and are mainly produced in plastic film house facilities that allow for heating in winter owing to their large fruit size and high sugar content. Among the late maturing cotrus fruits, kanpei and natsumi production amount rank 3rd and 5th, respectively, but their prices rank 1st and 2nd. Although kanpei and natsumi have received significant attention from consumers for their high-quality taste and price, there have been no analyses conducted on the fruit components. Therefore, in this study, we attempted to determine the characteristics of these varieties by analyzing the components contained in kanpei and natsumi.
Kanpei is a variety of citrus fruit developed by crossbreeding C. hybrid nishinokaori and C. reticulata ponkan and is commonly known as Redhyang. The harvest period is mid-to-late January, and on Jeju Island, as per the statistics in 2019, 1,445 farms (757 ha) produce approximately 11,061 tons/year. Natsumi, which is an easily peelable citrus fruit, is known by the trade name Karahyang, and its production amounts to 1,573 tons/year at 194 farms (78 ha), typically in late April (Kim et al., 2020; Park et al., 2020). Although kanpei and natsumi account for less than 2% of the total production of 631,310 tons/year of citrus in Jeju, they are attracting considerable attention from consumers. In this study the characteristics of the citrus varieties were investigated by analyzing the content of the non-volatile and volatile compounds in kanpei and natsumi.
2. Materials and methods
Kanpei and natsumi fruits used in this study were collected from the Citrus Research Institute of the National Institute of Horticultural and Herbal Science on harye-ri, seogwipo-si during February to April 2020. Fruit juices extracted from the collected samples were used for the analysis of free sugar, organic acid, free amino acid and aromatic components. Flavonoid components were analyzed using the extract, and which was prepared by separating the flesh and peel, drying comminuting, and extracting three times with 70% ethanol.
Free sugars and organic acids were analyzed using HPLC (Shimadzu Co., Prominence, Japan) after diluting the sample juice 10-fold and filtering it through a 0.45 μm membrane filter. The column for free sugar analysis was ZORBAX NH2 column (4.6×250 mm, 5 μm, Agilent, Santa Clara, CA, USA). Glucose, fructose, and sucrose were used as the standard substance. The column for organic acid analysis was a Shim-Pak GIS column (4.6×250 mm, 5 μm, Shimadzu), and citric acid was used as the standard substance.
For free amino acid analysis, 1/10 of the juice was diluted using tertiary distilled water containing 0.1% formic acid. Methanol (900 μL) containing 0.1% formic acid and 100 μL of diluted sample were mixed and vortexed. After mixing and centrifugation at 18,000 ×g, the supernatant was transferred to a sample vial and used for analysis. For free amino acid analysis, Waters ACQUITY UPLC/Xevo TQ-S (QQQ) (Waters Co. Ltd., Milford, MA, USA) was used, and the column was an IMTAKA Intrada Amino Acid column (3 μm, 50×2 mm). Developing solvent A was a mixture of ACN and 100 mM ammonium formate at a ratio of 20:80 (v/v), B was a ACN, THF, 25 mM ammonium formate, and formic acid at a ratio of 9:75:16:0.3 (v/v/v/v). The analysis conditions were 0 min (A:B=0:100) → 3 min (A:B=0:100) → 6.5 min (A:B=17:83) → 10 min (A) with a flow rate of 0.4 mL/min (A:B=100:0) → 12 min (A:B=0:100) → 17 min (A:B=0:100). As the calibration standard, approximately 20 amino acids were used at varying concentrations of 10, 20, 50, and 100 nmol/mL.
The samples for flavonoid component analysis were extracted using 70% ethanol. Samples were filtered through a 0.22 μm PVDF filter (Millipore) and analyzed using HPLC (e2695 separation module, Waters Co. Ltd., Milford, MA, USA). The column in for the analysis was a YMC-Triart C18 column (250×4.6 mm, S-5 μm, 8 nm), and a UV/Visible detector (Waters 2489) was used. The flow rate was maintained at 1 mL/min and detected at a wavelength of a 280 nm. As standard samples, 10 different flavonoids found in citrus fruits, which were divided into three categories according to their characteristics, were used. Rutin, narirutin, naringin, hesperidin, and neohesperidin were analyzed using the mobile phase conditions of a mixture of acetonitrile and 20 mM phosphate at a ratio of 2:8. The mobile phase conditions used in the analysis of quercetin, naringenin, and hesperetin were a mixture of acetonitrile and 20 mM phosphoric acid at a ration of 4:6, and finally the mobile phase conditions used nobiletin and tangeretin were a mixture of acetonitrile and 20 mM phosphoric acid at a ration of 6:4. A standard calibration curve prepared with a standard substance was used for the quantitative analysis of flavonoid components. The standard calibration curve was prepared using standard substances at concentrations ranging from 15.625 μg/mL to 1,000 μg/mL.
The volatile components of the peel and flesh were analyzed using solid-phase microtextraction (SPME). The SPME device was obtained from Superco (Bellefon, PA, USA) product. The SPME fiber was used to extract the volatile organic compound of flesh and peel, and extraction was perfomed by exposure at 60°C for 15 hr.
After exposure, the SPME fibers were analyzed using an Agilent Technologies 78980A GC System/5975C Inactive XL MSD (triaxial detector) (Agilent, Santa Clara, CA, USA). The analysis column used was DB-17 (0.25 μm×30 m×0.25 mm. ID), and the flow rate of carrier gas was 1.5 mL/min. The temperature of the detector was maintained at 270°C, and the column temperature was maintained at 40°C for 3 min. The oven temperature was heated from 90°C at a rate of 4°C/min and it was heated at a rate of 19°C/min from 210°C/min onward. The volatile compounds were sympathized with the Wiley 275 of GC/MS.
3. Results and discussion
The sugar content of kanpei and natsumi were 12.15±0.61 °Brix and 12.53±0.58 °Brix, respectively, and the corresponding acidity was 0.84±0.03% and 0.88±0.05%. The sugar to acid ratio, one of the main indicators of fruit quality, was 14.46 and 14.26 in kanpei and natsumi, respectively, which was higher than the sugar ratio of 10-12 found in a more common citrus C. unshiu (Lee et al., 2019). In addition, the contents of glucose, fructose, and sucrose, which are some of the main components found in citrus fruits, were measured using HPLC. Free sugar, which determines the sweetness, is one of the key components involved in the taste of food and is a major indicator in predicting changes in food quality as it affects consumer palatability (Jeon et al, 2015; Lee et al, 2019). From the analysis of free sugar, the most abundant component was found to be sucrose, which was contained in kanpei and natsumi at 6.53±0.31 g/100 g and 7.36±0.28 g/100 g, respectively. The glucose content was 1.67±0.04 and 1.57±0.06 g/100 g, respectively, and the fructose content was 1.83±0.09 and 1.75±0.11 g/100 g, respectively, for kanpei and natsumi.
Although the total sugar contents of the fruits were similar, the sucrose (free sugar) content in natsumi was higher than that in kanpei. In addition, analyzing the compositional ratio of free sugars, sucrose, fructose, and glucose levels in kanpei and natsumi were 65%, 18%, and 17% and 69%, 16%, and 15%, respectively (Table 1).
The value shown are the mean±SE (n=10). Means followed by different letters within columns are significantly different according to Duncan’s multiple range test (p<0.05).
Analysis of the free amino acids contained in the juices of kanpei and natsumi showed that the total amino acid content was 221.08 mg in kanpei per 100 g of juice and 120.83 mg in natsumi per 100 g of juice. The amino acids found in kanpei, in the ascending order of their contents, arginine, asparagine, aspartic acid, and serine, corresponding to the amounts of 65.27 mg. 53.56 mg, 30.39 mg, and 15.91 mg per 100 g of juice, respectively. Arginine accounted for 29.52% of the total amino acid content. Natsumi contained arginine, serine, glutamic acid, and alanine in the ascending order, with the content of each being 38.09 mg, 12.38 mg, 10.50 mg, and 9.76 mg, respectively. Arginine was the most abundant, accounting for 31.53% of the total amino acid content (Fig. 1(A)). Free amino acids are known to be involved in imparting sweet, bitter, and umami tastes. Sweet amino acids include Gly, Gln, Thr, Ala, Ser, Asn, Pro, while bitter amino acids include Cys, Arg, Val, Trp, Phe, Leu, Ile, Met, and His. Umami amino acids include Glu and Asp. The content and ratio of various amino acids affect the taste of various foods (An et al., 2020). In kanpei, the ratio of amino acids imparting sweetness, bitterness, and umami tastes was 46.5:33.05:18.78, which contained 102.8 mg, 73.08 mg, and 41.53 mg of amino acids corresponding to each tastes per 100 g of juice. The corresponding ratio in natsumi was 40.13 : 40.49 : 16.02, containing 48.49 mg, 48.93 mg, and 19.35 mg of amino acids corresponding to each tastes per 100 g of juice (Fig. 1(B)).
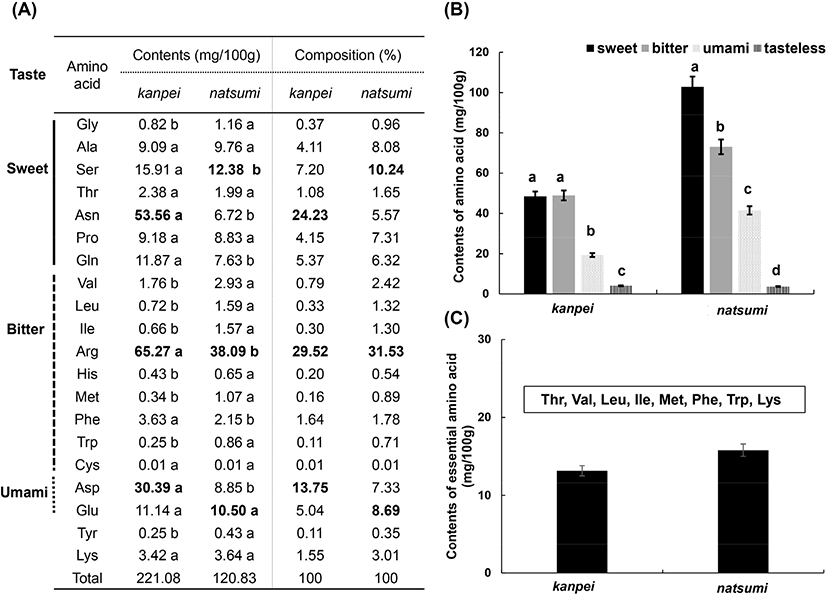
Among the amino acids, the amounts of the eight essential amino acids for adults, Thr, Val, Met, Ile, Leu, Phe, Lys, and Trp, were analyzed. Essential amino acids are necessary for maintaining the body’s balance and growth; however, there are several amino acids that cannot be synthesized in the body and therefore must be ingested through food. The essential amino acid content of kanpei was 13.59 mg/100 g, accounting for 5.95% of the total free amino acids, and for natsumi the content was 17.93 mg/100 g, accounting for 14.3% of the total amino acids (Fig. 1(C)). The total content of amino acids was higher in kanpei, but the content of essential amino acids was higher in natsumi. Therefore, various amino acids, including essential amino acids, were found in both kanpei and natsumi, indicating that they are both nutritionally excellent foods.
Flavonoids contained in kanpei and natsumi were analyzed by considering the flavonoids contained in 10 types of citrus fruits. Flavonoids are abundant in citrus fruits and can be classified into flavanones, flavones, flavonols, isoflavones, and anthocyanidins.
In this study, flavanones and flavones, including rutin, narirutin, hesperidin, neohesperidin, naringin, naringenin, hesperetin, quercetin, nobiletin, and tangeretin were used (Hertog et al., 1993). The main flavonoids in the two varieties of interest were narirutin and hesperidin. The flavonoid contents of kanpei and natsumi were higher in the peel than in the flesh, and the narirutin and hesperidin contents in the kanpei peel were 950.85±36.55 mg and 858.68±53.62 mg in 100 g of extract, respectively. In the natsumi peel, the narirutin and hesperidin contents were 813.94±39.23 mg and 1,967.88±67.49 mg in 100 g of extract. The hesperidin content was significantly higher in natsumi (Table 2). Hesperidin was first isolated from the albedo of oranges by the French chemist Lebreton in 1828 and it was later discovered in lemons and citrus fruits (Manthey and Grohmann, 1998). Hesperidin, one of the main components of Jinpi has been traditionally used to treat inflammation, allergies, and liver disease, and recently, it was demonstrated to improve cardiovascular disease (Yamada et al., 2006; Li and Schluesener, 2017). Narirutin possesses numerous medicinal benefits, such as antioxidant and anti-inflammatory properties as well as the abillity to improve the alcoholic fatty liver function, similar to as heperidin (Tripoli et al., 2007; Park et al., 2012). In addition, 100 g of ‘Kanpei’ peel extract contained 14.65±0.87 mg of naringin and 129.92±49.68 mg of neohesperidin, and 100 g of natsumi peel extract contained 26.57±2.17 mg of naringin. The main flavonoids of kanpei and natsumi were hesperidin and narirutin, which was similar to the composition of C. unshiu (M’Hiri et al., 2017). In additional, nobiletin and tangeretin were found in amount of 370.13±24.99 mg/100 g and 100.97±8.11 mg/100 g, respectively, in kanpei peel extracts. This was more than the respective amounts of 102.10±7.42 mg/100 g and 57.43±2.77 mg /100 g found in natsumi peel extracts (Li et al., 2018). The nobiletin and tangeretin of polymethoxylated flavones (PMFs), which are known to have antiviral, anticancer, anti-inflammatory, and antioxidant properties, have been found only in citrus fruits. Neohesperidin, a flavonoid characteristic of oranges identified in kanpei is considered to be a characteristic of a cultivar developed by crossbreeding C. hybrid nishinokaori (C. hybrid kiyomi × C. sinensistrovita) and C. reticulata (Singh B et al., 2020).
The values shown are the mean±SE (n=5). Means followed by different letters within columns are significantly different according to Duncan’s multiple range test (p<0.05).
Citrus fruits contain major flavonoids that are characteristic of each variety, which shows the differences between various breeds. According to Yang et al. (2019) most citrus fruits as a high content of hesperidin and narirutin; grapefruit and sour oranges possess a high naringin content and pomelo has high naringin and hesperidin contents.
Sweetness, sourness, saltiness, bitterness, umami, and the aromatics result in an infinite combination of tastes. The scent perceived by the nose is a combination of various aromatic ingredients, and each fruit has its own unique scent. In previous studies, most of the volatile components in citrus fruits were terpenoids, alcohols, aldehydes, acids, and esters (Pichersky et al., 2006; Schwab et al., 2008). Terpenoids, most commonly found in citrus, are an important volatile component of citrus and are necessary for the growth and development of plants. Monoterpene and sesquiterpene play an important role in secondary metabolism for the interaction of plants with environmental factors (Chappell, 1995; De-Oloveira et al., 1997; Dudareva et al., 2004; Rodriguez et al., 2011). The volatile components of citrus differ according to variety and parts, but the content of limonene, which is an aromatic component representing the flavor of citrus, is always the highest. The limonene content in the peel and flesh of the kanpei fruit accounted for 57.229% and 74.293% of total amount of volatile components, respectively, while the corresponding amount in the peel and pulp of natsumi fruits were 66.907% and 83.777%. In the case of kanpeiγ-terpinene was the second most abundant vilatile component, accounting for 8.554% and 8.238% in the peel and flesh, respectively, followed by linalool at 8.019% and 3.607%, respectively. In natsumi, γ-terpinene accounted for 9.619% and 8.097% of the peel and flesh, followed by β-myrcene with 5.313% and 2.284%, respectively. The content of volatile components of kanpei and natsumi differed between the peel and flesh from breed to breed. α-Thujene, trans-sabinene hydrate, cis-sabinene hydrate, and metha-1,4,8-triene were only identified in the peel, whereas α-terpinene was identified only in the flesh. Furthermore, octanal and pentylcyclopropane were identified only in kanpei, and cyclooctanal and α-cubebene were identified only in natsumi (Table 3). As for volatile components, hydrocarbons accounted for more than 80% of the total volatile components in the peel and flesh of kanpei at 84.54% and 93.48%, respectively, and the corresponding percentage were 96.196% and 97.856% in natsumi, indicating that the main volatile components were hydrocarbons. By analyzing the various useful components contained in kanpei and natsumi, the difference in the composition and content of the constituent compounds between the two varieties was confirmed, and it is considered that the bioactive compounds contained in both the fruits can be used as functional food materials.
RT, retention time.
Area%, relative peak area percentage.
4. Conclusions
In this study, non-volatile components, such as free sugar, organic acids, free amino acids, and flavonoids, as well as volatile components were analyzed to investigate the component characteristics of the kanpei and natsumi citrus varieties. From the analysis of free sugar in kanpei and natsumi, the highest free sugar content for both was sucrose at 6.53±0.31 g and 7.36±0.28 g per 100 g of juice, respectively. In addition, the analysis of approximately 20 free amino acids revealed that arginine was the most abundant in both varieties, and essential amino acids accounted for 5.95% and 14.3% of total amino acids in kanpei and natsumi, respectively. Through flavonoid analysis, narirutin and hesperidin were identified as the major flavonoids in both varieties. In the volatile component analysis, kanpei was found to contain limonene, γ-terpinene and linalool, whereas natsumi contained limonene, γ-terpinene and β-myrcene as major volatile components. Through component analysis, the main components of kanpei and natsumi were identified, and the results were used to evaluate the differences and characteristics of each variety.










