Introduction
The tomato (Solanum lycopersicum L.) is one of the most widely cultivated and extensively consumed horticultural crops globally (1). Tomatoes rank second only to potatoes in the global production of all horticultural produce and are consumed worldwide as a raw fruit or in processed products (2). Of the various tomato cultivars, the cherry tomato (Lycopersicon esculentum var. cerasiforme) has the highest fiber, fat and protein contents (3). Tomatoes are a climacteric and short-seasoned fruit and thus have a short shelf life of three days to three weeks depending on the time of harvest, due to its metabolism, high respiration rate and rapid ripening during distribution (4).
Pre-cooling is the rapid removal of heat from freshly harvested agricultural produce, and this process is typically performed as quickly as possible after being harvested. The field temperature of fresh agricultural produce is usually high (5), and excessive field temperature induces the increase of microbial activity, metabolic activity, respiration rates and decay. Immediate cooling after harvest can be used to slow down the metabolism of fresh produce and reduce the growth of microorganisms prior to storage (6). Postharvest recommendations indicate that tomatoes, including cherry tomatoes, should be stored at 10℃ or higher to avoid chilling injury (7,8). Maintaining this temperature can help inhibition of metabolic activity and the growth of microorganisms (6), thus allowing more time for distribution. Nunes et al. (9) demonstrated that perishable produce such as berries should be pre-cooled immediately or not more than 2 or 3 h after harvest to reduce decay and quality loss during storage. Rapid reduction of field temperature and storage near 10℃ can delay the ripening and decay, but tomatoes are generally packed directly in the field or in a packing house without the reduction of field temperature to reduce both cost and distribution time.
Most microorganisms that are initially observed on whole fruit or vegetable surfaces are soil inhabitants, and in most cases, the bacteria present on postharvest fresh produce are similar to those found in the field (10). Likewise, microbial decay of postharvest fruits or vegetables is associated with the survival and death of various microorganisms that originate during harvesting, postharvest handling, or storage and distribution (11). Spoilage refers to any change in the condition of food in which the food becomes less favorable, or even toxic, and these changes may be accompanied by alteration in taste, smell, appearance or texture (12).
Low-temperature storage is a general preservation method that greatly enhances the shelf life and overall quality of fresh produce (13). Low-temperature storage is also a critical factor for controlling the survival of mesophilic or pathogenic bacteria. This condition can inhibit the growth of aerobic microorganisms but provide optimal conditions for the growth of psychrotrophic spoilage bacteria such as Erwinia and Pseudomonas (14). There have been many studies on the effect of storage temperature control on reducing decay and microbial development. However, little information is available about the changes of microbiological and biochemical quality caused by the temperature of postharvest cherry tomatoes prior to storage. Many studies also focused on the prevalence of culturable pathogenic bacteria or fungi on fresh vegetables and fruits (15), and only a few recent studies have examined the initial microbial community composition of tomatoes (16-19). Our understanding of the microbial community diversity in tomatoes remains limited, and little is known about the postharvest management that impactsthe microbial community composition and dynamics. In this study, we analyzed the effects of temperature treatment after harvest through the estimation of decay rate, fruit firmness and electrolytic leakage of the cherry tomato during storage. In addition, the microbiological quality and microbial community dynamics of cherry tomatoes were assessed to explore the difference of the microbial spoilage potential caused by postharvest temperature treatment during storage. This study provides additional depth of understanding regarding the importance of pre-cooling processing for improving freshness of postharvest cherry tomatoes.
Materials and Methods
Cherry tomatoes were harvested from a local farm in November 2016 in Gyeonggi province, Korea. Cherry tomatoes were divided into three groups of approximately 50 kg after harvest. Harvested cherry tomatoes were divided into three groups: T-10, immediately treated at 10℃ after harvest; T-20, treated at 20℃; and T-30, treated at 30℃. The temperature of cherry tomato in each treatment group was recorded using a thermocouple (BTM-4208SD, LUTRON, Taipei, Taiwan). After reaching the target temperature, treatment groups were held for 3 h and then transferred to a refrigerated warehouse in which the temperature was maintained at 10±1℃. Approximately 50 g aliquots from each group (50 kg) were packaged in a 15×11×5 cm PET plastic container. All groups were stored for 34 days at 10±1℃, and microbiological quality and physico-chemical analysis was done at appro×imately 6-day intervals from the initial stage of treatment.
Weight loss was estimated as follows: %weight loss=[(initial weight-fruit weight after storage intervals)/initial weight]×100. Fruit firmness was measured on ten fruits from each sample using a TA-XT2 texture analyzer (Stable Micro System, Surrey, UK) fitted with a 2 mm diameter probe operated at a speed of 0.5 mm/s. The rate of electrolyte leakage (EL) was determined as described by Jeong et al. (29) in triplicated for each replicate. Electrolyte leakage was calculated as: Cherry tomato electrolyte leakage (%): (EL1/EL2)×100. Decay rate was visually evaluated at each sampling time during storage. The decay rate was expressed as the percentage of fruit showing decay symptoms with surface mycelia and soft rot.
The microbiological analysis followed the Korean food code and ISO-4833. Samples were collected from three different temperature treatment and analyzed for total viable cells (TVCs), coliforms, fungi, Enterobacteriaceae and two pathogens (Bacillus cereus and Clostridium perfringens). First, 25 g of each sample were diluted in 225 ml of 0.85% saline and homogenized using a Stomacher®-400 (Seward, Norfolk, UK) for 2 min at 230 rpm. Ten-fold serial dilutions were made in 0.85% saline, and 1 ml of dilution suspension was plated for the enumeration of microorganisms using PetrifilmTM plates (3M Microbiology, St Paul, MN) for TVCs, fungi, and Enterobacteriaceae, and Sanita-kun plates (Sanita-Kun, Tokyo, Japan) for coliforms. These plates were incubated at the optimal temperature for each microorganism, and the typical colonies were enumerated. B. cereus was isolated by inoculating the dilution suspension onto mannitol-egg yolk-polymyxin B agar (MYP, Merck, Darmstadt, Germany) and then incubating these samples at 30℃ for 18 h. C. perfringens was isolated by plating a dilution suspension onto tryptose-sulfite-cycloserine (TSC, Oxoid, Basingstoke, UK) agar without egg yolk and incubated at 37℃ for 24 h under anaerobic conditions using Genbox packages (BioMérieux, Marcy-l’Étoile, France). The presumptive positives of B. cereus and C. perfringens were subcultured on tryptic soy agar (TSA, Merck, Germany) and confirmed biochemically using a VITEK® 2 Compact instrument (BioMérieux, France). The results were expressed as log colony-forming units (log CFU/g).
Each group was analyzed in triplicate at days 0, 13, and 26 to determine the microbial composition. Bacterial strains were identified by MALDI-TOF MS using the VITEX MS system (BioMérieux, France). Specifically, 0.1 mL of dilution suspension used for quantitative microbiological analysis was spread onto plate count agar (PCA, Merck, Germany). The plates were incubated at 37℃ for 24 h, and each colony was then picked from the plates. The picked colonies were transferred to TSA medium (Merck, Germany) and incubated at 37℃ for 24 h. The fresh colonies were directly smeared onto the sample spots on disposable polymeric FlexiMass-DS target slides (Shimadzu-Biotech, Tokyo, Japan) with CHCA matrix solution (10 mg/mL á-cyano-4-hydroxy-cinnamic acid (CHCA, BioMérieux, France) and dried at room temperature (20-25℃). E. coli ATCC 8739 was used as the quality control strain. The data analysis of VITEX MS is based on comparison of the characteristics of the obtained mass spectra in the database within IVD software.
SPSS (IBM© SPSS Statistics ver. 20, New York, USA)was used for statistical analyses. One-way ANOVA was performed to determine the effect of the pre-cooling treatment on microbiological quality and microbial community diversity. For all statistical analyses, a significance level of 0.05 was used.
Results and Discussion
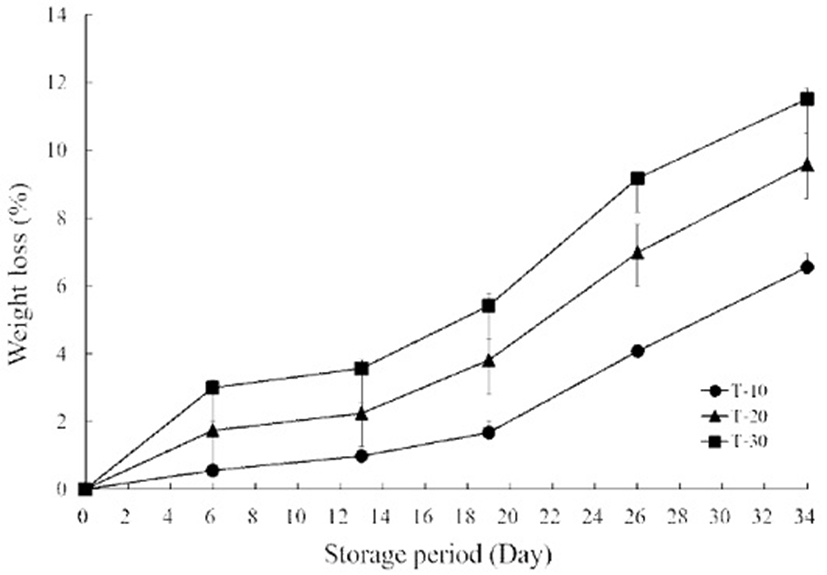
The field temperature of fresh agricultural produce is usually high (8), and excessive field temperature induces fruit softening by the increase in microbial activity, metabolic activity, respiration rates and decay. The change of fruit quality by over-softening increases pathogen susceptibility and the development of undesirable flavors and skin color (21). Thus, the rate of fruit softening determines the postharvest shelf life and rapid cooling to at least 12.5℃ immediately after harvest for tomato is important in delaying softening by reducing field temperature and retarding ripening (22). Weight loss of cherry tomatoes in all treatments markedly increased with the prolonged storage period (Fig. 1). The weight loss in T-10 was slower than those of T-20 and 30, and significant differences were found between each treatment. On day 26, weight loss of T-10 was 4.1%, while this value increased to 5% in both T-20 and 30. Weight loss in T-10 on day 34 increased to 6.5%, while T-20 and 30 exhibited values of up to 10%. Weight loss above 5% is a limiting factor for the postharvest life of fruit crops and is known to be related to temperature and storage time (23). In this study, the weight loss of cherry tomatoes during storage was affected by treatment temperature conditions after harvest. For 26 days of storage, the lowest weight loss was obtained in the 10℃ treatment group, while the highest value was found in the 30℃ treatment group. Javanmardi et al. (24) reported that higher rates of transpiration and respiration in tomatoes stored at 25-27℃ compared to tomatoes stored at 5-12℃ could be the main factor for increased rates of weight loss. Therefore, these results indicated that temperature exposures under 20 and 30℃ prior to storage need to be considered carefully for ensuring the physicochemical quality of cherry tomatoes, regardless of storage at low temperature.
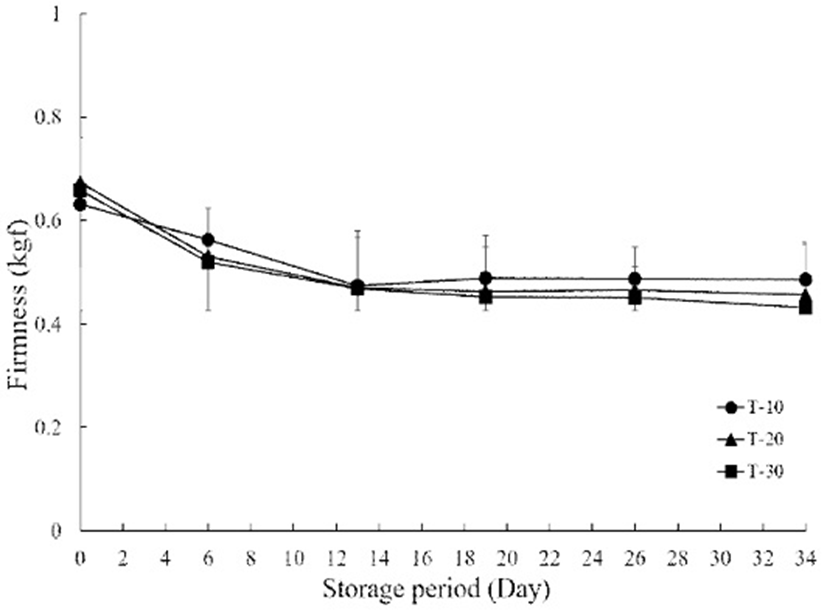
The firmness of T-10, 20 and 30 is presented in Fig. 2. In general, the firmness of cherry tomatoes decreases during storage because the cherry tomato tissue becomes soft due to metabolic changes induced by enzyme action and respiration. The firmness in each treatment group notably decreased with increasing storage time. However, the firmness of T-10 was observed to be higher in value than those at T-20 and T-30 at the assessed time points during storage. On day 13, the firmness in T-10 decreased by 22.2% (488 kgf) while it decreased by 30.1% (462.5 kgf) and 28.7% (462.5 kgf) in T-20 and T-30, respectively. Between 19 and 34 days, T-10 retained 488.6-485.3 kgf of firmness while values of 456.7 kgf and 431.3 kgf were found for T-20 and T-30, respectively. Firmness is one of the most important textural components in the case of fleshy fruit (25). In this study, the firmness of cherry tomatoes showed a remarkable decrease after 6 days in all treatment groups, but a 10℃ treatment after harvest delayed firmness reduction compared to those of the 20 and 30℃ treatment groups. The results of firmness are in agreement with Rab et al. (26), who showed a significantly delayed softening by pre-cooling after the harvest of tomatoes. Nilanthi et al. (27) also found that softening developed in controls without pre-cooling compared with apples pre-cooled to 10℃ after harvest at the end of storage. This may be because of inhibition or inactivation of cell wall hydrolytic enzymes such as polygalacturonase and pectinesterase or suppression of mRNA synthesis coding for wall softening enzymes in tomatoes treated by pre-cooling (28).
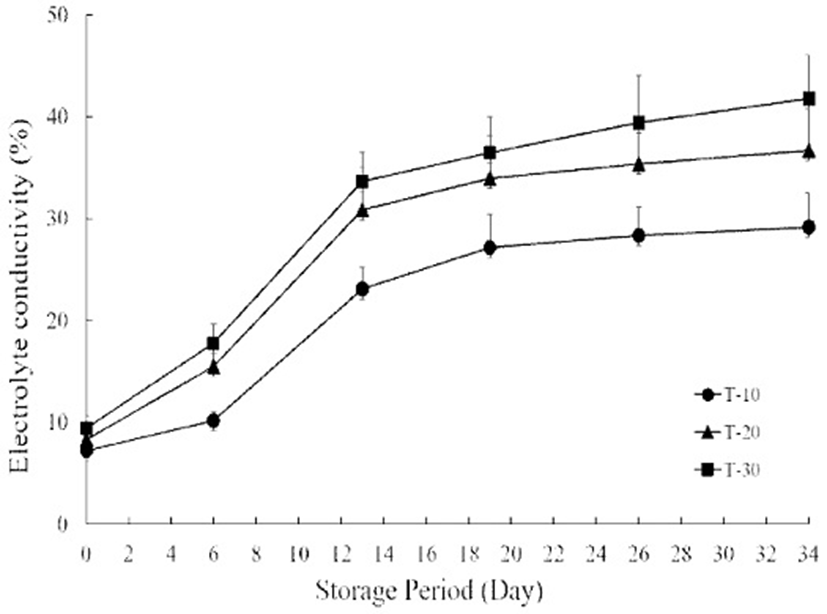
The change of electrolyte leakage (EL) from cherry tomatoes during storage is depicted in Fig. 3. The EL of T-10, 20 and 30 gradually increased until 13 days of storage. The EL on day 6 increased by 28.9, 46.4 and 47.3% in T-10, 20 and 30 compared with those values on day 0. The maximum EL was observed from the T-30 group (17.8%), while the T-10 group showed the lowest value of 10.2% on day 6. The value of T-10 reached 23.1% on day 13, which increased by 68.8% compared with that value on day 0. The EL value was significantly higher in T-20 and 30 compared to those of T-10 on day 13. At the same storage period, the value was observed as 30.8 and 33.6% in T-20 and 30, which increased by 73.0 and 72.1% compared with that value on day 0. The EL value after 13 days was maintained in all treatment groups and showed no significant differences between temperature treatment groups in EL value on day 34. The determination of electrolyte leakage is widely used in fruit stress response and postharvest physiology, since it is considered a good marker for cell membrane disruption upon senescence. A reduction in membrane integrity resulting from lipid peroxidation increases membrane leakage and enhances cell senescence (29). In this study, the electrolyte leakage was strongly affected by treatment temperature after harvest. The electrolyte leakage increased rapidly in all treatment group for 6 days. Nevertheless, the electrolyte leakage of a 10℃ treatment group showed lower values compared with those of 20 and 30℃ treatment groups during the entire storage period. Ion leakage has been used an indicator of damage to the plasma membrane, where low values of electrolyte leakage is evidence of a delay in the loss of membrane integrity. These results were confirmed in other studies performed on sliced tomatoes stored at low temperature (29). A similar response was reported in litchi fruit pre-cooled to 5℃, as the effects of pre-cooling maintain the membrane integrity (30).
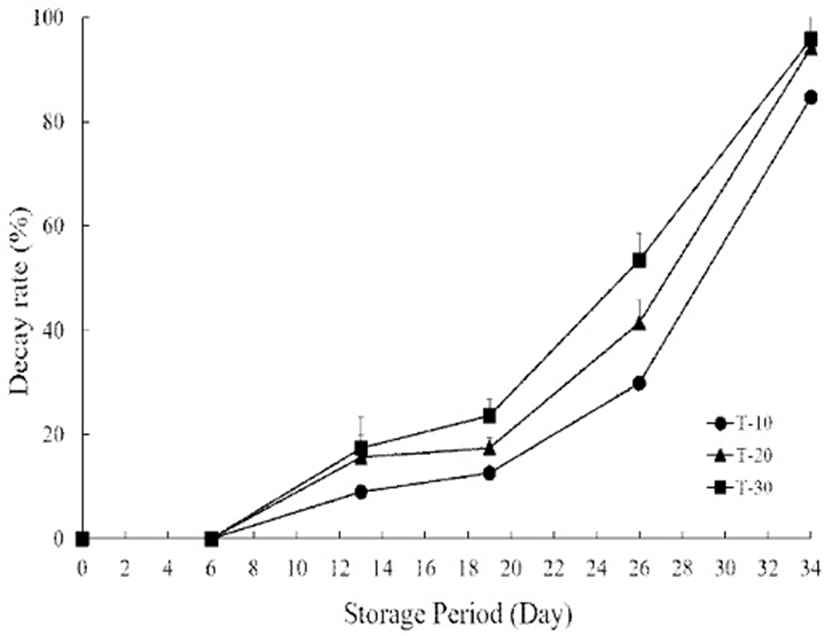
The change of decay incidence did not begin until 6 days in all treatment groups and then it continuously increased with the advancement of the storage period and treatment temperature (Fig. 4). However, the decay rate was significantly lower in T-10 (12.5%) than those in T-20 (17.3%) and 30 (23.5%) on day 19. T-10 showed a significant delay in the increase of decay rate in cherry tomato until 26 days of storage. While T-20 and 30 on day 26 showed decay rate of up to 40%, in particular, the decay rate of T-30 was 53.3%. The decay rate rapidly increased between 26 and 34 days, and reached 84.6% in T-10, 94.2% in T-20 and 95.8% in T-30. Although 10℃ treatment after harvest increased firmness and electrolyte leakage for 6 days of storage, it effectively delayed the decay incidence in the 10℃ treatment group during the early stage of storage. After 26 days, the decay incidence was 29.8% in the 10℃ treatment group while there was up to 40-50% decay in the 20 and 30℃ treatment groups. High temperatures such as 20 and 30℃ are favorable conditions for decay to develop as tomato fruits ripen. Postharvest soft rot of cherry tomatoes is caused mainly by fungal pathogens (31) and thus, mycelial development produces an abundance of extracellular pectinases and hemicellulases that are important factors for fungal spoilage (32). Since the fungi had a slow rate of growth between 6 and 12℃, the higher levels of decay observed in 20 and 30℃ treatment groups after harvest are likely due to elevated metabolism rates of fungi by favorable growth environments under 20 and 30℃.
The microbiological changes mediated by postharvest temperature condition on the initial day of treatment are shown in Table 1. After treatment, the level of TVCs in T-10 was 1.8 log CFU/g, whereas the level in T-20 was 1 log CFU/g higher than that in the T-10. The TVC level was the highest in T-30, which was 3.4 log CFU/g. The Enterobacteriaceae level was 1.5-2.0 log CFU/g higher in T-20 and T-30 than in T-10. For the coliform levels, T-10 and 20 was less than 1.0 log CFU/g, whereas the level in T-30 was 1.5 log CFU/g, which was the highest level of all the groups. The fungi level was higher in T-20 and 30 than T-10, and the fungi level in T-20 and 30 reached the highest level of 3.7 log CFU/g. E. coli, B. cereus and C. perfringens were not detected in any of the groups. Pre-cooling is known to hinder microorganism development (33). In general, total counts of microbiological populations on agricultural products range from 3.0 to 6.0 log CFU/g. However, the aerobic bacteria of tomatoes were below 3 log CFU/g, as tomatoes have smooth and hydrophobic surfaces caused by wax layers that hinder bacterial adhesion (34). Prior to storage, the TVC, Enterobacteriaceae, coliform and fungi levels increased with elevated treatment temperatures. The 10℃ treatment group had the smallest levels of TVCs, Enterobacteriaceae, coliform and fungi compared with other treatment groups. This study also indicated that bacterial growth showed significant differences between treatment groups during storage. The growth rate of bacteria in cherry tomatoes was inconstant flux depending on the treated temperature condition after harvest, but the microorganisms showed a similar value at the end of storage.
Although TVC levels in the 10℃ treatment group were consistently retained during the early stage of storage, long-term storage of over 25 days failed to inhibit the TVC population. In the same storage period, the decay rate also showed similar results to the TVC growth patterns. The decay rate in the 10℃ treatment group stayed below 30% for 26 days and remarkably increased between 26 and 34 days. Under natural conditions, the outer layer of the cherry tomato tissue consists of a hydrophobic surface providing a natural barrier for microorganisms (34). The surface of produce is, however, subjected to external stress factors such as harvesting, processing, distribution and storage, which can all lead to physical damage (35). The soft rot caused by fungal diseases can change the physicochemical properties of the surfaces by producing bio-surfactants (36).
The differences in microbiological quality between groups stored at 10℃ for 34 days are presented in Table 1. The TVCs level in T-10 increased from an initial level of 1.8 log CFU/g to 5.0 log CFU/g on day 34. The TVC level in T-20 did not increase compared to those on day 0 and maintained 2.9 log CFU/g until 19 days. While between 26 and 34 days, TVC levels remarkably increased to 5.6 log CFU/g from 3.7 log CFU/g on day 26. Similar results were observed in T-30, in which 3.4 log CFU/g, the initial level of TVC, was maintained for 26 days and increased to 5.1 log CFU/g on day 34. Though the initial level of TVC in all treatment groups showed significant differences, TVC levels at the latest storage period showed similar values between all treatment groups. Enterobacteriaceae and coliform in T-10, 20 and 30 gradually increased during storage, and this level showed significant differences between treatment groups until 13 days of storage but showed similar levels between all treatment groups after 19 days. The fungi level in T-10 maintained its initial level (2.7 log CFU/g) for 34 days. This level gradually decreased in T-20, and a decrease of 1.5 log CFU/g was observed on day 34 compared with day 0. In T-30, the fungi level decreased from 3.5 log CFU/g on day 0 to below 1 log CFU/g on day 34. Due to damage of the surface, nutrients are released from the plant tissue, which can be used by microorganisms. In this study, TVC levels and decay rates remarkably increased in all treatment groups after 26 days of storage. These results suggest that increased physical damage by mycelial development may be providing a better substrate for microbial growth by releasing nutrients.
The bacterial diversity of 1550 strains isolated from cherry tomatoes exposed to different temperature after harvest was determined by MALDI-TOF MS. Fig. 5 shows the bacterial distribution according to treated temperature condition. The initial dominant strains after temperature treatment were Bacillus pumilus, Bacillus subtilis/amyloliquefaciens, Staphylococcus xylosus and Pantoea agglomerans. However, the composition of these bacterial communities exhibited significant differences between treated temperature conditions. In T-10, S. xylosus had the highest relative abundance (29.4%), followed by B. pumilus (25.9%), B. subtilis/amyloliquefaciens (18.8%) and, to a lesser extent, (<5%) Paenibillus spp., Brevibacillus spp., B. megaterium, Micrococcus luteus/lylae, S. equorum and S. saprophyticus. The most abundant strains in T-20 were S. xylosus (71.6%), follow by P. agglomerans (8.0%), S. saprophyticus (5.4%), B. subtilis/amyloliquefaciens (3.5%) and Enterobacter cloacae/asburiae (2.6%). The microbial community composition in T-30 showed similar pattern with T-20. The most abundant species in T-30 were S. xylosus and P. agglomerans, with the S. xylosus abundance (70.4%) in T-30 being its highest relative abundance among isolated strains. P. agglomerans occurred with a higher relative abundance in T-30 (14.7%) than in T-10 (0%) and T-20 (8.0%).
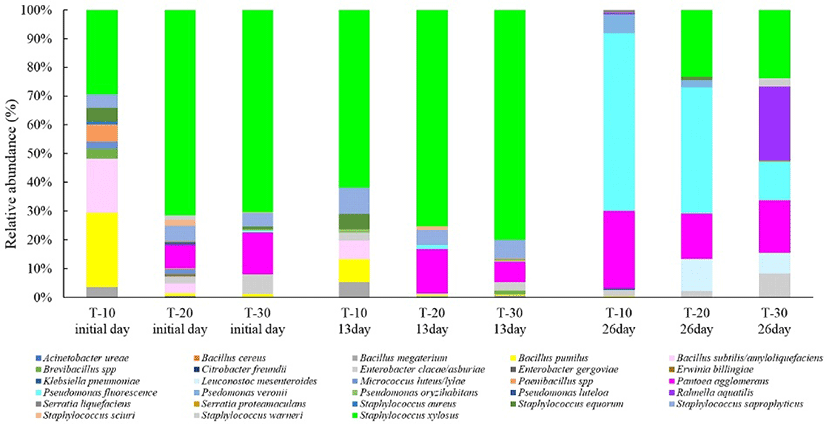
During storage at low temperature, bacterial levels increase and the dominating bacterial population mainly consists of psychrotrophic spoilage bacteria belonging to the Pseudomonadaceae (especially P. fluorescens) and Enterobacteriaceae (especially Erwinia herbicola and Rhanella aquatilis), besides some species belonging to the lactic acid bacteria (especially Leuconostoc mesenteroides) (37, 38). In this study, the elevated TVCs observed in the 10℃ treatment group may result from the survival of psychrotolerant bacteria. According to these results, we determined which bacterial communities respond to altered temperature conditions during storage.
The changes in bacterial community composition during storage are represented in Fig. 5. After 13 days, S. xylosus was the most abundant strain in all treatment group. S. xylosus in T-10 increased from 29.4% on day 0 to 61.8% on day 13 and exhibited its highest abundance of 75.2% in T-20 and 80.1 % in T-30. Conversely, the relative abundance of B. pumilus and B. subtilis/amyloliquefaciens in T-10 decreased. In particular, B. pumilus exhibited a relative abundance of 7.9% in T-10 on day 13 and B. subtilis/amyloliquefaciens decreased to 6.6% on day 13 from 18.8% in T-20 on day 0. In the initial stage of treatment, the bacterial communities in all treatment groups were dominated by S. xylosus or B. pumilus. The 10℃ treatment group showed an especially high abundance of B. pumilus, while S. xylosus was the dominant bacteria in the 20℃ and 30℃ treatment groups. This result is not in accordance with other findings that Pantoea is the most abundant bacterial type associated with field-grown tomatoes (17). The relatively low abundance of Pantoea in this study may be associated with differences in environmental conditions during tomato production that influence the occurrence of these species, especially the difference between greenhouse-grown produce and field-grown produce (17). Additionally, this study determined whether bacterial composition is maintained during storage. Storage for 26 days induced changes in the relative abundance of bacterial groups compared to the initial day. In T-10, Pseudomonas fluorescens was the primary strain, exhibiting the highest relative abundance of 61.9 %. The relative abundance of Bacillus spp. (mainly B. pumilus and B. subtilis/amyloliquefaciens) decreased to less than 1%, while P. agglomerans increased to 27.0% on day 27 compared with the initial day (0%). S. xylosus was found at a very low proportion of less than 0.1% on day 26 in T-10. Cherry tomatoes treated with T-20 and T-30 after harvest had diverse bacterial compositions of E. cloacae/asburiae, P. agglomerans, P. fluorescens, S. xylosus, Leuconostoc mesenteroid and Rahnella aquatilis during storage for 26 days. Interestingly, L. mesenteroides and R. aquatilis prevailed in these groups. In T-20, P. fluorescens showed relatively high abundance (43.7%), and S. xylosus was present in a proportion of 23.3%. P. agglomerans and L. mesenteroid were found in proportions of 15.9% and 11.1%, respectively. In T-30, R. aquatilis were present as the dominant strain (25.8%) on day 26 and S. xylosus still showed a relatively high abundance (23.8%). Compared with its abundance on day 0, P. fluorescens increased to 18.3%, together with Pseudomonas veronii (13.8%) and L. mesenteroid (7.1%). During refrigerated storage, the bacterial composition did not change until day 13, indicating that the microflora affected by the produce temperature was not re-established until day 13 of storage. However, the bacterial composition in all groups showed large differences between day 0 and day 26. A high proportion of spoilage-associated bacteria such as the gram-negative genera Pseudomonas, Leuconostoc, Rahnella and Pantoea were observed at day 26, while the proportions of gram-positive bacteria such as S. xylosus, B. pumilus and B. subtilis/amyloliquefaciens decreased. Interestingly, the composition of spoilage-associated bacteria showed significant differences related to treated temperature. Unacceptable changes of appearance were induced in agricultural produce during storage at 4℃, which corresponded to aerobic psychrotrophic counts exceeding 7-8 log CFU/g (39,40). Pseudomonas spp. are considered to be some of the most important bacteria responsible for spoilage in vegetables and fruits (41). P. fluorescens are psychrotrophic bacteria and are known to cause soft rot symptoms in carrots (41) and tomatoes (42). The occurrence of Pseudomonas in fresh produce thus indicates the beginning of food spoilage. In this study, P. fluorescens was a typically observed spoilage-associated bacterium, and its greatest relative abundance of over 60% was found in the 10℃ treatment group after 26 days and predominated also in the 20℃ treatment group. In addition, the 20 and 30℃ treatment groups did exhibit a high relative abundance of R. aquatilis and L. mesenteroides as spoilage bacteria, and L. mesenteroides was the only species found in the 20℃ treatment group, while R. aquatilis was the only species found in the 30℃ treatment group. Lindberg et al. (43) reported that R. aquatilis showed rapid growth activity at 7℃ and thus can multiply rapidly to high numbers in refrigerated vegetables (38). Furthermore, some strains harbor toxin genes similar to those of enteropathogenic E. coli (43). The occurrence of this strain in cherry tomatoes treated at 30℃ might represent a potential hazard to humans at prolonged storage times. Leuconostoc bacteria are psychrotrophic mesophiles and members of the lactic acid bacteria (LAB) group. Leuconostoc spp. are usually found at low counts on vegetable surfaces (37), but they can proliferate during food storage. L. mesenteroides is a very important spoiler of carrots (37) and has been described as the predominant LAB on tomato fruit surfaces (44). In this study, L. mesenteroides and R. aquatilis were found only in the 20 and 30℃ treatment groups, and the growth of P. fluorescens was limited by 20 and 30℃ treatment during storage. These results suggest that the community of spoilage-associated bacteria observed during cold storage is established differently depending on handled temperature condition after harvest. Bacteria such as P. fluorescens, L. mesenteroides and R. aquatilis have been reported to be responsible for the spoilage and deterioration of fresh produce (42,43). In this study, the level of TVCs on cherry tomatoes treated at 10℃ were below 6 log CFU/g on day 34; however, the decay rate and the relative abundance of P. fluorescens remarkably increased during the same period. Therefore, these results suggest that decay development in the late stage of storage may be associated with psychrotrophic spoilage bacteria.
요 약
Field heat은 수확 후 방울토마토의 연화를 가속화함으로써 shelf life를 감소시키는 중요한 원인이다. 따라서 수확 후 저온 처리는 방울토마토의 수확 후 품질 향상을 위한 방법 중 하나라고 할 수 있다. 수확 후 온도 처리에 따른 방울토마토의 품질 및 미생물 군집에 미치는 영향을 알아보고자 처리 온도와 저장 기간에 따른 품질 변화를 조사하였다. 방울토마토의 품질 특성과 관련하여 수확 후 10℃처리는 중량, 단단함, electrolyte leakage의 감소와 부패율의 증가를 억제하였다. 또한 수확 후 10℃는 20℃ 또는 30℃ 처리에 비해 총균수, 진균류, 장내세균 및 대장균이 1-2 log CFU/g 낮있다. 그러나 저장 26일 후에는 10℃에서도 총균수와 부패율의 급격한 증가가 나타났다. 미생물 군집 변화에 있어서, 처리 직후 방울토마토 표면의 미생물 군집은 Staphylococcus xylosus와 Bacillus pumilus로 나타난 반면 저장 중에는 S. xylosus와 B. pumilus의 비율이 감소하였고 Pseudomonas fluorescens, Rahnella aquatilis 및 Leuconostoc mesenteroid가 저장 후기에 증가하는 것으로 나타났다. 이에 따라 10℃ 처리구에서 총균수와 부패율의 증가는 P. fluorescens의 증가와 관련이 있을 수 있음을 확인하였다. 수확 후 처리하는 온도 조건에 따라 방울토마토의 표면 미생물 군집에 차이가 있었고, P. fluorescens, R. aquatilis 및 L. mesenteroid와 같은 부패 미생물의 증가는 장기간의 냉장 보관 중 발생하는 품질 저하의 원인이 될 수 있다. 본 결과는 수확 후 온도 처리에 따른 부패 미생물의 성장 가능성을 예측하는 새로운 접근법을 제시하였으며 이 같은 특성은 방울토마토의 신선도 연장과 함께 미생물학적 안전성을 향상시키기 위한 방법을 모색하기 위한 중요한 자료로 활용될 수 있을 것이다.










