Introduction
Perilla frutescens, an annual herb with a distinctive aroma and taste, has been cultivated for centuries in eastern Asia(1). Traditionally, it was consumed as an herbal medicine to treat sweating, cough, diuresis, food poisoning, stomachache, and anodynia. However, recently, P. frutescens leaves have been used as a flavor enhancer, pickled vegetable, and for beverages or tea(2). Asif (2012) reported that P. frutescens comprises of several phenolic compounds including rosmarinic acid, luteolin, apigenin, vanilic acid, cimidahurinine, and caffeic acid(3). The plant leaves have been used as a traditional Chinese herbal medicine for treating cold, cough, and food poisoning(3). Further, numerous studies have been shown that P. frutescens exert anti-inflammatory effect in murine macrophage(4), antibacterial effect in Staphylococcus aureus(5), antioxidant effect(6), antidepressant-like effect(7), anti-liver injury in d-galactosamine-sensitized mice(8), and antimicrobial activity(9). However, only few studies have reported anticancer effects of P. frutescens and its fractions.
Melanoma, a skin cancer type, usually arises from melanocytes in the epidermis(10). It is one of the most common cancer type in western countries, especially North America, Europe, and Latin America; due to the climate(11). Melanoma could occur not only of the skin, but also of the eyes or any area that is comprised of melanocytes. There are 2 major causes of melanoma: genetic factors and exposure to sun’s ultraviolet (UV) light. Recently, melanoma’s incidence rate has been increasing in East Asia, including South Korea(12). The early stage of melanoma could be treated by surgery; however, chemo- and immune-therapy might be required after the melanoma cells have spread to other organs. Recently, many studies have reported that natural products could effectively inhibit melanoma tumor growth(13,14). However, mechanisms underlying the effect of P. frutescens in inducing melanoma cell death remains unknown.
Therefore, the study was aimed to determine the anticancer activity of P. frutescens methanol extract and its fractions on B16 and SK-MEL-2 melanoma cells. Additionally, the findings propose that P. frutescens and its fractions could be developed as functional food materials for melanoma cancer prevention.
Materials and methods
Samples were prepared form P. frutescens as previously described(15). The above-ground portion of plant was extracted with methanol at room temperature for over 3 days. After filtration, the methanolic extract (PE) was evaporated and partitioned sequentially with 2 organic solvents: ethyl acetate (PEEF), and butanol (PEBF). The final portion derived after all the partitioning was the aqueous residue fraction (PEWF).
Total phenolics were determined using the Folin-Ciocalteau reagent(16). P. frutescens methanolic extract fraction (0.1 mL) were diluted to 0.9 mL with distilled water and to it, Folin-Ciocalteau reagent (0.1 mL) was added. After 5 min, 7% sodium carbonate (1 mL) was added and the contents were thoroughly mixed. After color development, absorbance measured at 760 nm, by a UV/Vis spectrophotometer (Mecasys, Daejeon, Korea) after 120 min using gallic acid as a standard. The total phenol concentration was expressed as μg/100 mL of dried PE and its fractions.
B16 mouse melanoma cell lines were obtained from the Korea Cell Line Bank (Seoul National University, Seoul, Korea), whereas, SK-MEL-2 human melanoma cell lines and HaCaT human keratinocyte cells were procured from American Type Culture Collection (ATCC, Rockville, MD). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU/mL), and streptomycin (100 μg/mL) and RPMI-1640 medium containing d-glucose, 2 mM l-glutamine was supplemented with 10% FBS, and 1% (v/v) penicillin–streptomycin, in a humidified atmosphere of 5% CO2, at 37℃.
Cell viability was determined by following previously described procedures(17). Several PE and PEEF concentrations were added for 24 h. The cells were fixed with 50% trichloroacetic acid (Sigma-Aldrich, St. Louis, MS, USA) to terminate the reaction. The cell suspension was added with 0.4% SRB (Sigma-Aldrich) in 1% acetic acid (Daejeong, Kyunggi-do, Korea). After 1 h of incubation, the plates were washed, and the dyed cells were dissolved with 10 mM Tris buffer (pH 10.5, Sigma-Aldrich). The 96 well plate was read using a micro-plate reader (540 nm) to obtain the absorbance.
The cells were seeded at a density of 1×106 cells in 6-well plates and cultured for 24 h in DMEM. Following this, the cells were treated with indicated sample concentrations for 24 h. For measuring the sub-G1 populations, the cells were collected and fixed in ice-cold 70% ethanol in media and stored at 4℃ overnight. After re-suspension, the cells were washed and incubated with RNase (1 μL, 1 mg/mL), propidium iodide (20 μL, 1 mg/mL), and phosphate-buffered saline (PBS, 500 mL) at 37℃ for 30 min. Following the staining process, FACSan flow cytometer (Becton Dickinson, Mountain View, CA, USA) was used to analyze sub-G1 populations.
Characteristic apoptotic morphological changes were assessed by fluorescent microscopy using bis-benzimide (Hoechst 33258, Sigma-Aldrich) staining(18). The cells were plated at density of 5×105 cells/well in 6 well plates, and then treated with PE and PEEF for 24 h. After harvesting, the cells were washed twice with PBS and stained with 200 μL bis-benzimide (1 μg/mL) for 10 min at room temperature. Furthermore, 10 μL of this suspension was placed on a glass slide and covered with a cover slip. The cells were examined with a fluorescence microscope (Olympus Optical Co. Ltd., Tokyo, Japan) to determine nuclei fragmentation and chromatin condensation.
The cells were plated at a density of 2×106 cells in a 100 mm dish containing DMEM, and cultured for 24 h. The cells were treated with the indicated PE and PEEF concentrations for 24 h, and later collected by centrifugation. The pellets were lysed by DNA lysis buffer (10 mM Tris-HCl (pH 7.5), 10 mM EDTA (pH 8.0), 0.5% Triton X-100, 20% sodium dodecyl sulfate (SDS), 10 mg/mL proteinase K) and then centrifuged. The supernatant was fixed in ice-cold 70% ethanol and stored at 4℃ overnight. The supernatant was extracted with phenol buffer (phenol-chloroform and phenol-chloroform-isoamylalcohol) and the pellets were incubated in TE buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, pH 8.0) and RNase (2 mg/mL) for 1 h at 37℃. Gel electrophoresis was performed on 2% agarose containing ethidium bromide (GIBCOⓇ/ InvitrogenTM, GrandIsland, NY, USA). The DNA bands were visualized using a UV Transilluminator Imaging System (UVP Laboratory Products, Upland, CA, USA).
The cells were plated at a density of 5×105 cells/well in a 6 well plates containing DMEM, and cultured for 24 h. The cells were preincubated with pan-caspase inhibitor z-VAD-fmk for 2 h, followed by treatment with various PEEF concentrations for 24 h. Determination of growth inhibition and SRB assay measurement was carried out by fixing the cells with 50% trichloroacetic acid to terminate these actions following which 0.4% SRB in 1% acetic acid was added to each well. The plates were washed following 1 h incubation and the dyes were dissolved with 10 mM Tris buffer. Following this, the 96-well plates were read using a micro-plate reader (540 nm) to obtain the absorbance.
Western blot analyses were performed as described previously(19). The cells were plated at a density of 2×106 cells in a 100 mm dish and cultured for 24 h in medium. Following this, the cells were treated with various PEEF concentrations for 24 h, followed by centrifugation. The resulting pellets were lysed with lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 30 mM Na4P2O7, 1 mM PMSF, 2 μg/mL of aprotinin) for 30 min on ice. The protein content of the supernatant was measured using a BCA protein assay kit. Briefly, 10 μg of protein samples were loaded per lane and separated by 12% SDS-polyacrylamide gel electrophoresis (PAGE) at 100V of constant voltage/slab for 1.5 h. Furthermore, the proteins were transferred on to nitrocellulose membranes and blocked with 2.5 and 5% BSA for 1 h at 37°C. The membranes were then incubated with primary antibody at 4℃ overnight. Primary antibodies anti-Bax (Santa Cruz sc-493), anti-Bcl 2 (Santa Cruz sc-492), anti-PARP (Santa Cruz sc-7150), anti-caspase-3 (Santa Cruz sc-7148), anti-caspase-8 (Santa Cruz sc-7890), anti-caspase-9 (Santa Cruz sc-7885) and anti-ß actin (Santa Cruz sc-47778) were used at 1:1,000 dilution. Finally, the membranes were treated with secondary antibodies for 1 h at 4℃. Secondary antibodies, goat anti-rabbit IgG (AP132P) and goat anti-IgG (AP124P) (Millipore, MA, USA) were used at 1:10,000 dilution. The membranes were washed with T-TBS after each antibody binding reaction and, each protein was detected with an ECL reagents kit (Santa Cruz Biotechnology, Inc.).
Results
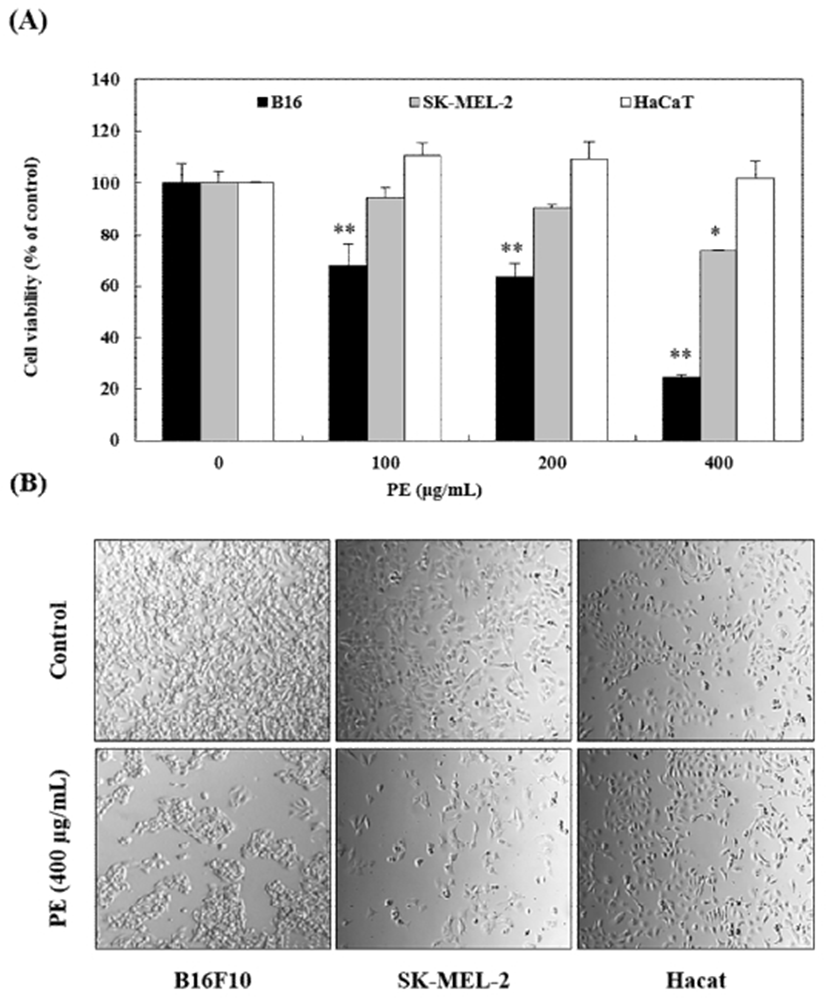
Initially, the ability of PE to inhibit B16 and SK-MEL-2 cell growth was investigated. The cells were treated with 400 μg/mL PE for 24 h. The PE effect on cell proliferation was evaluated by assessing morphological changes and sulforhodamine B (SRB) assay. The results revealed that the viability of B16 and SK-MEL-2 melanoma cells decreased with PE treatment (Fig. 1A). Whereas, HaCaT human keratinocytes were undamaged by high PE concentration. Phase-contrast microscopy revealed significant morphological changes in B16 and SK-MEL-2 melanoma cells due to PE treatment. The control cells, on the contrary, maintained a regular shape and size. Following treatment with 400 μg/mL PE, decreased cell number, irregular cell shape, and weakened cell adhesion was observed (Fig. 1B). These results indicated that PE inhibited B16 and SK-MEL-2 cell proliferation without significant toxicity in HaCaT human keratinocytes.
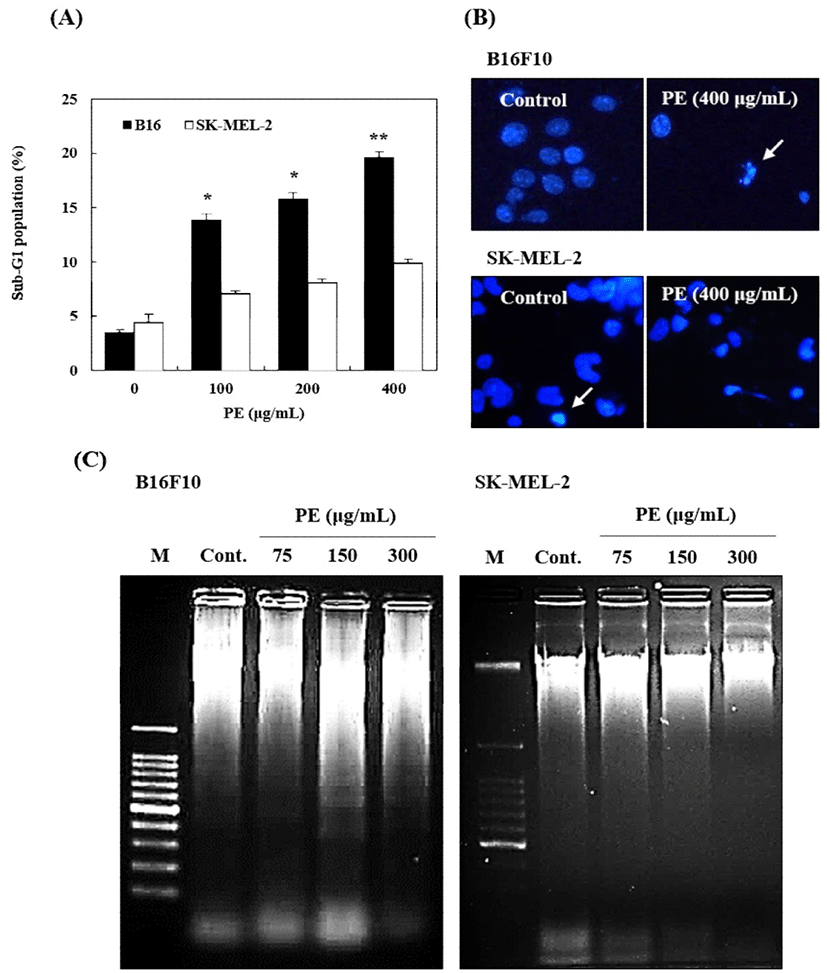
PE-induced growth inhibition of B16 and SK-MEL-2 cells via apoptosis was confirmed by flow cytometry, Hoechst 33258 staining, and DNA fragmentation assay. Cell proliferation in sub-G1 peak was noted as dramatically increased against B16 mouse melanoma cells (Fig. 2A). This proportion was also increased in SK-MEL-2 human melanoma cells, however, the rate of peak increase was lower than that of B16 cells. Furthermore, PE treatment (400 μg/mL) not only significantly increased chromatin condensation and apoptotic body formation, but also DNA fragmentation in only B16 melanoma cells (Fig. 2B, 2C). The data suggested that cell proliferation inhibitory effect of PE in B16, is related to apoptosis.
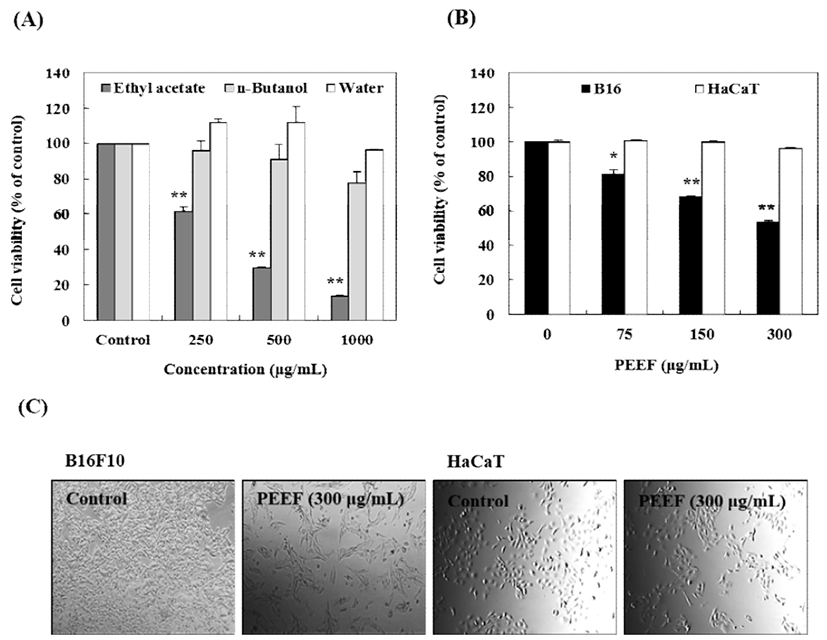
To identify more specific cancer inhibitory effect, PE was partitioned using ethyl acetate (PEEF), butanol (PEBF), and water (PEWF), and the anticancer activity of each fraction was compared. The melanoma cells and keratinocytes were treated with 0, 250, 500, and 1,000 μg/mL of different PE fractions for 24 h, and the cell viability was analyzed by SRB and morphological assays. B16 cell viability was noted to significantly decrease with PEEF treatment (in concentrations 75-300 μg/mL), whereas, PEBF and PEWF did not affect significant cytotoxicity in B16 cells (Fig. 3A). However, HaCaT human keratinocytes incubated with PEEF, were undamaged in concentrations of 75-300 μg/mL (Fig. 3B). Cell morphology was assessed using a phase-contrast microscope which revealed significant morphological changes in B16 melanoma cells due to PEEF treatment. In contrast, HaCaT cells maintained a regular shape and size (Fig. 3C). The results indicated that PEEF selectively inhibits the growth of B16 melanoma cells without significant toxicity in HaCaT keratinocytes.
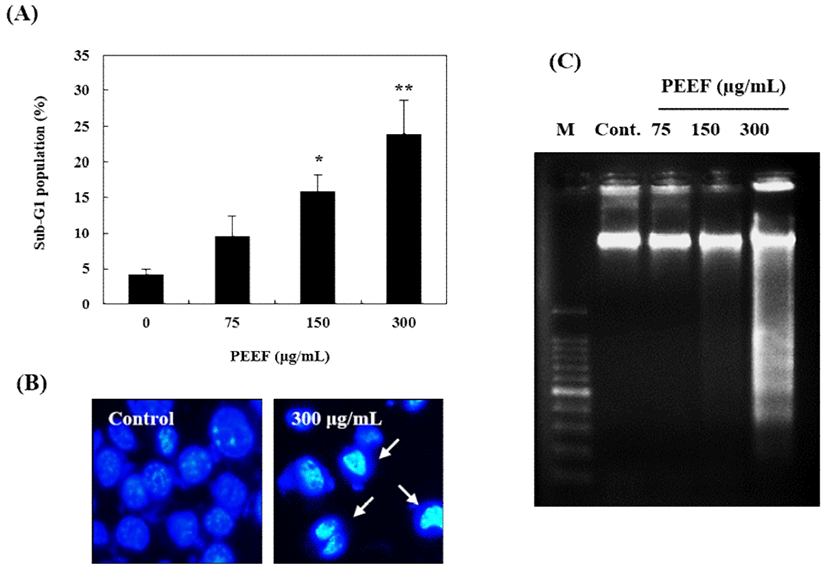
PEEF-induced apoptosis in B16 mouse melanoma cells was confirmed by flow cytometry, Hoechst 33,258 staining, and DNA fragmentation assessment. As shown in Fig. 4A, the sub-G1 peak was significantly increased in B16 mouse melanoma cells. Furthermore, 300 μg/mL PEEF induced morphological changes such as nucleus shrinkage, chromatin condensation, and apoptotic body formation (Fig. 4B). Moreover, DNA fragmentation (a distinct feature of apoptosis) was identified using 2D agarose gel electrophoresis. The DNA ladder pattern in PEEF-treated cells was indicative of typical internucleosomal fragmentation, particularly after treatment with 300 μg/mL PEEF (Fig. 4C). These findings demonstrated that PEEF-induced B16 cell growth inhibition is associated with apoptosis.
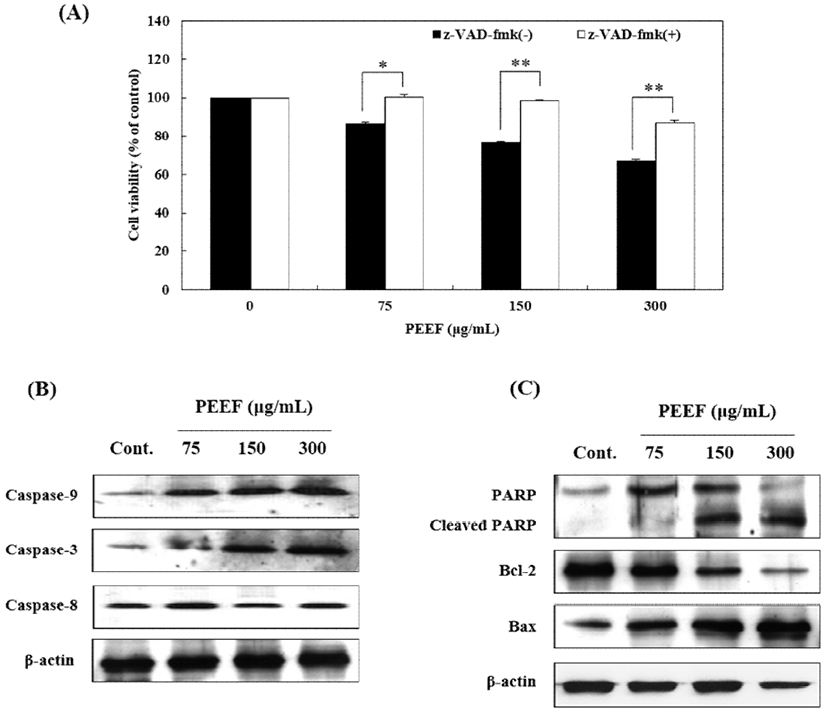
To confirm the involvement of caspase proteins in PEEF-induced apoptosis, B16 cells were pretreated with 10 μM pan-caspase inhibitor, z-VAD-fmk; before PEEF treatment. As presented in Fig. 5A, z-VAD-fmk pre-treatment significantly prevented PEEF-induced cell death as compared to the cells exposed to PEEF alone. Moreover, caspases-3, -8, and –9 activations were monitored by western blotting to elucidate the mechanism underlying PEEF-induced apoptosis in B16 cells. Furthermore, PEEF increased the protein expression of caspase-3 and -9 except for caspase-8 (Fig. 5B). It also down-regulated anti-apoptotic Bcl-2 proteins, and up-regulated pro-apoptotic Bax and cleaved-PARP protein expression (Fig. 5C). The results suggested that caspases-3 and -9 activation along with the regulation of Bcl-2 family proteins were involved in PEEF-induced apoptosis of B16 cells.
The total phenolic contents of the PEEF, PEBF, and PEWF were 44.27, 15.4, and 8.20 mg/GAE (gallic acid equivalent) g, respectively (Table 1). Amongst PE fractions, PEEF displayed the highest total phenolic contents. The results indicated that the phenolic compounds of PEEF may be involved in PEEF-induced apoptotic death of B16 mouse melanoma cells.
| Total polyphenol content (mg/GAE g)* | ||
|---|---|---|
| PEEF1) | PEBF2) | PEWF3) |
| 44.27±1.17 | 15.4±0.92 | 8.20±0.14 |
Discussion
Nowadays, the number of studies reporting the detection of compounds from natural materials to replace the existing chemotherapeutic agents is steadily increasing. These natural materials derived reagents have been observed to induce apoptosis in various cancer cell lines. Several studies have reported that P. frutescens and its derived compounds, possess various bioactive activities. However, only few studies displaying that PEEF exerts anticancer effects against melanoma cells, have been published. Therefore, the anticancer activities of P. frutescens extract and its fractions in melanoma cells were investigated in the present study.
There are 2 major cell death pathways: apoptosis and necrosis. Necrosis is a form of traumatic cell death that results from acute cellular injury(20). In contrast to apoptosis (programmed cell death) it results from internal cellular signal pathway. Unlike necrosis, apoptosis confers advantages during an organism's life cycle(21). Apoptosis produces cell fragments called apoptotic bodies that are engulfed by phagocytic cells and are quickly removed before the contents spill out onto the surrounding cells and cause damage. Generally, apoptosis causes distinct cell morphology changes such as blebbing, cell shrinkage, sub-G1 population accumulation, chromatin condensation, and DNA fragmentation(22). Previous investigations have revealed that P. frutescens leaf extract increases apoptosis in human hepatocarcinoma HepG2 cells(23), and markedly reduces tumorigenesis in a murine model(24). In the present study, 200-400 μg/mL PE inhibited B16 mouse melanoma cells growth and significantly increased cell number in sub-G1 phase in a dose-dependent manner, indicating the presence of apoptotic cells. Hoechst staining disclosed that PE-treated B16 cells acquired apoptotic bodies and underwent nuclei condensation. It was also demonstrated that PE induced apoptosis by promoting DNA fragmentation. Interestingly, PE did not display significant increase of sub-G1 population, nuclei condensation, and DNA fragmentation in SK-MEL-2 human melanoma cells. Kwak et al. (25) reported that ethanol extract of P. frutescens leaf (500-1,000 μg/mL) induced DNA fragmentation and cell cycle arrest in HL-60 human leukemia cells. Although further studies are required to identify the precise cancer inhibition mechanism of PE in SK-MEL-2 cells, these results indicate that PE suppresses the proliferation of mouse and human melanoma cells through different pathways.
Apoptosis commonly promotes caspase activation through a mitochondria-dependent pathway(26). During apoptosis, caspases act as important apoptotic signaling factors activated by Bcl-2 family protein expression(27). Recently, studies have reported that compounds derived from natural materials can activate pro-apoptotic signaling pathways (15,19,28). Previous reports have indicated that P. frutescens leaf extract induced apoptosis in human leukemia(25) and human hepatoma cells(29) through caspase- and mitochondria-dependent pathways at 100-1,000 μg/mL concentrations. However, results from the present study displayed that PEEF induced apoptosis through a caspase-dependent pathway.
PEEF activated caspase-3 and -9, but not caspase-8. Moreover, it down-regulated pro-apoptotic Bcl-2 protein expression levels, while anti-apoptotic Bax and cleaved PARP proteins were up-regulated. Typically, initiator caspase activation leads to the proteolytic activation of downstream effector caspases. The intrinsic apoptotic pathway is initiated by mitochondria membrane permeabilization and release of cytochrome c into the cytoplasm. Cytochrome c then forms apoptosomes and activates the caspase cascade through caspase-9. The extrinsic apoptotic pathway-activated caspase-8 then directly cleaves procaspase-3/7 or other executioner caspases, eventually leading to apoptosis. Caspase-8 can also cleave the BH-3-only protein Bid, resulting in truncated Bid (t-Bid) that then moves to the mitochondria and induces cytochrome c release, leading to the activation of caspase-9 and -3(26). These data suggested PEEF-induced apoptotic cell death to be triggered by intrinsic apoptosis signals, including caspase activation.
A previous study has demonstrated that P. frutescens is a rich source of phenolic compounds such as apigenin, ascorbic acid, beta carotene, caffeic acid, citral, dillapiol, elemicin, limonene, luteolin, myristicin, perillaldehyde, protocatechuic acid, quercetin, rosmarinic acid, perilla ketone, elsholzia ketone, isoegomaketone, naginata ketone, and safrole(3). The phytochemical constituents of P. frutescens are mainly present in the essential oil(30), and the major aroma compounds are egomaketone and isoegomaketone(31). In present study, PEEF possessed higher total phenolics than the other fractions. Similar relationships were observed in suppressive effect of various phenolics on human cancer cells(13,32), and other anticancer activity of P. frutescens extract on human cancer cells was also associated with rich polyphenolics and flavonoid contents(25,29). Therefore, effective cancer suppressive activity of PEEF was supported by the presence of phenolic compounds rich content.
In conclusion, this finding suggested that P. frutescens could serve as a potential cancer-preventive natural medicine. However, further studies are required to investigate the active compounds in PEEF and the underlying mechanisms of the anticancer effects in melanoma cells.










