Introduction
Cancer causes serious problem which leads to morbidity and mortality and creates worldwide problem in public health. Effective anti-cancer compounds are not well known. Plant based compounds are better curative compounds than synthetic compounds. However, the majority of cancer cases are still treated by chemotherapy, especially for advanced stage cancer (1). The major problems of chemotherapeutic treatment in cancer are non-specific cytotoxicity and drug resistance (2). Therefore, investigations to discover new and effective anti-cancer agents have become a serious issue (1,3). With this aim, many attentions have been paid to natural compounds in plants, marine organism and microorganisms. Regarding the low side effects of plants and other natural compounds, scientists are interested in working on them to find new medications.
Plant-derived products, known as natural therapies, have been used for thousands of years in cancer treatment with a very low number of side effects (4). Furthermore, it has been reported that plant constituents are able to directly inhibit the proliferation of cancer cells. The ability of medicinal plants to show a broad range of activity has been attributed to their ability to affect multiple targets. However, the mechanism of action is still largely unknown (5). A number of anti-cancer drugs have been shown to induce apoptosis in susceptible cancer cells (6-9). Apoptosis is a genetically controlled response by which cells commit suicide and has become a major focus in the study of cancer biology and tumor progression (10-12). Despite tremendous efforts to explore the components involved in the process of apoptotic cell death, the molecular linkages of these components and their mechanisms of action are still largely unknown.
Member of Bcl-2 family have been shown to play an important regulatory role in apoptosis, either as activators (e.g. Bax, Bid, Bim, or Bak) or inhibitors (e.g. Bcl-2, Bcl-w, or Bcl-XL) (13). Among the Bcl-2 family members, Bcl-2 itself is particularly cell death triggered by multiple stimuli in a variety of cell types (10,11). In addition, Bcl-2 has been shown to act upstream of apoptotic proteases such as caspase-3, preventing their proteolytic activation and thereby interfering with signaling pathways that lead to apoptosis (11,14,15).
Dendropanax morbifera Léveille (Araliaceae) is an endemic species growing in the south-western part of South Korea and has been used in folk medicine as a health functional food for the treatment of headache, infectious diseases, and skin diseases. However, the underlying mechanisms for the varying activities observed with D. morbifera (DP) and its components are largely unclear. Several studies have indicated that extract of DP has cytotoxic activities on a number of human cancers, such as, breast cancer, lung cancer, hepatoma, and chorioepithelioma (16-21). Active ingredients such as rutin, polyacetylenes, or oleifoliosides (triterpenoids) purified from DP have been reported. Rutin, a flavonoid, protects against thromboembolism and neuronal injury (22,23). Petylenes ((3S)-falcarinol, (3S,8S)-falcarindiol, and (3S)-diynene) were reported to inhibit complement activity (24). Oleifoliosides attenuate macrophage-mediated inflammation and show anticancer effect through inducing cell death (19,20). Recently, a new polyacetylens and triterpene oxide compounds were isolated from the DP by conventional chromatographic techniques and showed to have cytotoxic activities (24-28). In in vitro, a cycloartane-type glycoside, oleifolioside A, inhibited the metastasis of human cervical carcinoma (20). The dendropanoxide induced autophagy through ERK1/2 activation in human osteosarcoma cells and autophagy inhibition enhanced dendropanoxide-induced apoptosis (18). In addition, (9Z, 16S)-16-hydroxy-9,17-octadecadiene-12,14-diynoic acid was reported to anti-complement activity against the classical pathway of the complement system (24). However, the previous studies were focused on the crude extract of DP and limited phytochemical tests, and none on its bioactive compounds.
More recently, we have also isolated a new compound, 1-tetradecanol, a straight-chain saturated fatty alcohol, from DP, which has skin whitening and moisturizing effects (29), potential preventive or therapeutic agent for Helicobacter pylori‑induced gastric inflammation (30), and a potent T cell growth factor (31). However, the inhibitory effects of single compounds of DP on cancer cells and its underlying molecular mechanisms have been studied in very few. Therefore, we have attempted to elucidate the possible biological mechanisms controlling the anticancer activity of DP.
In this study, two new triterpenoids and four know triterpenoids were isolated from the n-hexane fraction of DP. Based on chemical and spectral analysis, the structures of the six triterpenoids were determined to be β-amyrin (1), α-amyrin (2), olean-12-en-3,24 β-diol (3), dendropanoxide (4), β-sitosterol (5), and stigmasterol (6). β-Amyrin and β-sitosterol from the Prunus africana are moderately cytotoxic against colorectal carcinoma, Caco-2 cells, while low cytotoxicity is observed on hepatocellular carcinoma, HepG2 cells (32). Stigmasterol from the Azadirachta indica has been chemopreventive effect on 7,12-dimethylbenz[a]anthracene (DMBA)-induced skin cancer (33). Dendropanoxide from the DP has anti-cancer activities in MG-63 human osteosarcoma cells (18). Given the potential of triterpmoids and the benefits of DP, there should be further investigation of this bioactive compounds derived from DP toward effective and safe anticancer agent. We evaluated the cytotoxicity of isolated six compounds 1-6 from DP against two cancer cell lines A-549 human lung cancer and MCF-7 human breast cancer cells. Furthermore, the mechanisms of triterpenoids anti-cancer activities were determined. The result of this study are expected to contribute and the development of DP as effective therapeutics against breast and lung cancer.
Materials and methods
Optical rotations were determined on a Jasco DIP-370 automatic polarimeter (Jasco, Kyoto, Japan). The fourier transform infrared spectrophotometer (FT-IR) spectra were obtained from a Jasco Reports-100 infrared spectrometer using KBr plates (Jasco). The nuclear magnetic resonance (NMR) spectra were recorded on a Agilents 400 MHz (Agilent Technologies®, Palo Alto, CA, USA) for 1H-NMR (400 MHz), 13C-(100 MHz)1D-NMR, heteronuclear multiple-bond correlation (HMBC), heteronuclear single-quantum correlation spectroscopy (HSQC) 2D-NMR. Column chromatography was carried out silica gel (Kieselgel 60F254, 70-230 mesh, Merck, Darmstadt, Germany), Lichroprep RP-18 (40-63 μM, Merck), and TLC was performed on a pre-coated silica gel 60F254 (0.25 mm, Merck). Spot were detected under UV light or after spraying with 10% H2SO4 reagent, followed by heating.
The dried leaves of DP were collected from Jangheung, Jeollanamdo, Korea, in October 2017 and authenticated by Dr. Kim at the B&Tech Co., Ltd., R&D Center, Gwangju, Korea. Extracts were obtained from leaves (1.3 kg) using (v/w) of 100% methanol (MeOH) at 60℃ for 4 h. Extracted solutions were filtered and concentrated using an evaporator under vacuum and 125 g of crude extract was obtained. The residue (90 g) was dissolved in water (1,000 mL) and partitioned with solvents to give n-hexane (35.2 g), CHCl3(8.1 g), EtOAc (810 mg), n-BuOH (17.44 g) and H2O (30.5 g) soluble portions. The n-hexane fraction (6 g) was separated over a silica gel column with hexane-acetone (100:1-1:1) solvent system as the eluent to give ten fractions (H1-H10). Fraction H-1 (146.0 mg) re-chromatographed on a silica gel column with hexane-acetone (100:1-80:1) as the eluent to give the known compounds 1 (10.0 mg), compounds 3 (3.0 mg). Fraction H-4 (80.7 mg) was purified by column chromatography over silica gel using a hexane:acetone gradient (100:1-1:1) and further separated with silica gel column chromatography to afford compound 2. Fraction H-6 (98.6 mg) was purified by chromatography over silica gel using a hexane-acetone gradient (100:1-1:1) and further purified with a silica gel column by hexane-acetone (10:1) as the eluent to give compounds 5, 6 (10 mg and 4.3 mg). Fraction H-7 (654 mg) was purified by column chromatography over silica gel using a hexane-acetone gradient (100:1-1:1) and further separated with LiChroprep RP-18 column chromatography to afford compound 4.
Two human cancer cell lines and normal African green monkey kidney cells, Vero, were used. The A-549 (Human lung cancer) and MCF-7 (human breast cancer) cell lines were obtained from the Korea Cell Line Bank (KCLB, Seoul, Korea) and Vero cell line was ATCC (CCL-81, Manassas, VA, USA) and were grow in Dulbecco’s Modified Eagle medium/Nutrient Mixture F-12 (DMEM/F-12, Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific Inc.) and penicillin/streptomycin (100 U/mL and 100 μg/mL, respectively) at 37℃ in a humidified 5% CO2 atmosphere. The experiments were done at 70-80% confluence. Upon reaching confluence, cells were detached using 0.25% trypsin-ethylene diamine tetra acetic acid (EDTA) solution.
The inhibitory effects of compounds (1-6) on the growth of human cancer cells were determined by measuring metabolic activity using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-terazolium bromide (MTT) assay according to Denizot et al. (34). Briefly, the collected cells were seeded into a 24-well culture plate (Nunc, Rochester, NY, USA) at a density of 4×105 cells per well and incubated for 24 h. Cells, replaced by FBS-free culture medium, were treated with 10, 30, 50, 100, and 200 μM of with six compounds (1-6). After incubation, 0.1 mg (50 μL of a 2 mg/mL solution) MTT solution was added to each well and the cells were then incubated at 37℃ for 4 h. The plated were centrifuged at 1,000 rpm for 10 min at room temperature and the media was then carefully aspirated. Dimethylsulfoxide (DMSO, 200 μL) was then added to each well to dissolve the formazan crystals. The plates were read immediately at 570 nm on an ELISA plate reader (Epoch 2; BioTek Instruments, Inc., Winooski, VT, USA). All the experiments were performed three times and the mean absorbance values were calculated. The results are expressed as the percentage of inhibition that produced a reduction in the absorbance by the treatment of the compounds compared to the untreated controls. The sample concentration which inhibited 50% cell line (IC50) was determined using relationship curve between the concentration of sample (χ) and the percentage inhibition (у).
The cells were plated at a density of 5×104 in six-well plates. They were allowed to grow at 37℃ in a humidified CO2 incubator until they were 70-80% confluent. Then cells were treated with IC50 concentration of compounds for 24 h. Then cells were lysed in a buffer (50 mM Tris-HCl, pH 7.4 containing 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, and 10% glycerol) that contained the protease inhibitors leupeptin (10 μg/mL) and phenyl methane sulfonyl fluoride (PMSF) (2 mM). The protein concentration was determined according to the method described by the Bio-Rad protein assay manual. Western blot was conducted to analyse the effects of compound (1-6) on the expressions of apoptosis regulators Bcl-2 and Bax in the MCF-7 and A-549 cells. Equal amounts of proteins (30 μg/lane) were diluted by loading dye and subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel was then transferred to nitrocellulose membranes (Amersham, Berkshire, UK). The membranes were blocked with 5% skim milk in TBS containing 0.1% Tween-20, and then incubated at room temperature for 2 h with primary antibodies against Bax, Bcl-2, and β-actin (1:2,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted according to the manufacturer's instructions. Following incubation with antibodies, the membranes were washed three times for 15 minutes in TBS-T and incubated for another 1 h with specific horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as the secondary antibody. Then the membranes were washed and developed with enhanced chemiluminescence (ECL) western blotting detection kit (Amersham, Arlington Heights, UK). The relative protein levels were determined using a ChemiDoc XRS+ System (Bio-Rad, Hercules, CA, USA) using Image Lab software version 5.2.1 (Bio-Rad).
The cells were plated at a density of 5×104 in six-well plates. They were allowed to grow at 37℃ in a humidified CO2 incubator until they were 70-80% confluent. Then, cells were treated with IC50 concentration of compounds for 24 h. Then cells were fixed by adding 2 mL of ice-cold 70% ethanol in a drop-wise manner while gently vortexing, after which they were stored at -20℃ until analysis. The fixed cells were rinsed with phosphate buffered saline and then incubated in 500 μL propidium iodine (PI) solution (10 mM Tris, pH 8, containing 1 mM NaCl, 0.1% Nonidet P-40, 0.7 μg/mL RNase A, and 0.05 μg/mL PI) for 15 min at 37℃. The analyses of cell apoptosis and cell cycle were subsequently analyzed using flow cytometry (FACSCaliburTM, Beckton Dickinson, GmbH, Heidelberg, Germany) equipped with an argon ion laser. CellQuest Pro software was used to record and analyze the data from 10,000 nuclei.
Results and discussion
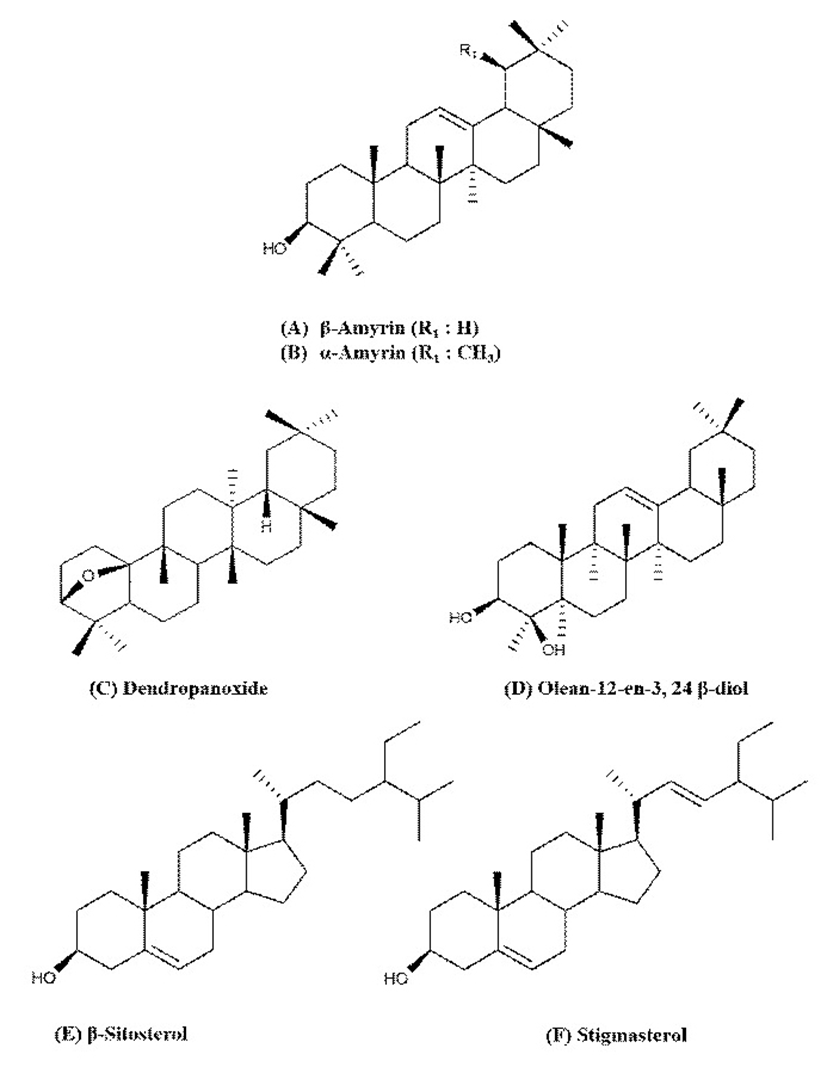
Six compounds (1-6) were isolated from the methanol extract of DP (Fig. 1). Olean-12-en-3,24 β-diol (compound 3) and stigmasterol (compound 6) were isolated for the first time from DP. Compound 3 was obtained as colorless needle. Its identified by its FT-IR spectrum, with peaks at 3,600-3,100, 1,630, 1,038, 1,020, 810 cm-1. 1H-NMR peaks at 3.34 and 4.21 δppm showed hydroxymethyl group, and 3.44 δppm indicated methine group. EI-MS spectrum, the molecular ion peak was m/z 442 [M+]. The FT-IR, 1H-NMR, EI-MS data led to propose the molecular formula as C30H50O2, which closely resembles to Olean-12-en-3,24 β-diol, reported in the literature for the first time. Compound 6 was obtained as colourless crystals. Compound 6 identified by its FT-IR spectrum, with peaks at 3,494 (OH), 1,053, and 1,020 (OH) cm-1. 1H-NMR peaks at 1.59 and 1.61 δppm showed the presence of a cyclopentane. EI-MS spectrum, the molecular ion peak was m/z 412 [M+]. The FT-IR, 1H-NMR, EI-MS data led to propose the molecular formula as C29H48O, which closely similar to those reported for stigmasterol in the published in the literature (33). The structures of the other triterpenoid compounds 1, 2, 4, and 5 were also established based on their spectral data and by comparison with the literature (24). Concerning compounds 1-6, they were obtained from the leaves extract by the identical procedure described above.
-
β-amyrin (1) : White amorphous powder; [α]D+88.3º (c=0.01, CHCl3); IR (KBr) Vmax: 3,300, 2,950 cm-1; FAB-MS: m/z 427 [M+H]+
-
α-amyrin (2) : White amorphous powder; [α]D+92.0º (c=0.005, CHCl3); IR(KBr) Vmax: 3,300, 2,950 cm-1; FAB-MS: m/z 427 [M+H]+
-
Olean-12-en-3,24 β-diol (3) : Colorless needles; [α]D+84.7º (c=0.46, CHCl3); IR(KBr) Vmax: 3,600-3,100, 1,630, 1,038, 1,020, 810 cm-1; EI-MS: m/z 442 [M]+
-
Dendropanoxide (4) : Colorless needles; [α]D+45.7º (c=0.01, CHCl3); IR(KBr) Vmax: 2,950 cm-1; FAB-MS: m/z 427 [M+H]+, 44[M+Na]+
-
β-sitosterol (5) : White amorphous powder; [α]D-40.7º (c=0.05, CHCl3); IR(KBr) Vmax: 3,400, 2,930 cm-1; FAB-MS: m/z 297 [M+H]+
-
Stigmasterol (6) : Colourless crystals; [α]D-51º (c=0.1, CHCl3); IR(KBr) Vmax: 3,494 (OH), 1,053, 1,020 (OH) cm-1; EI-MS: m/z 412 [M]+
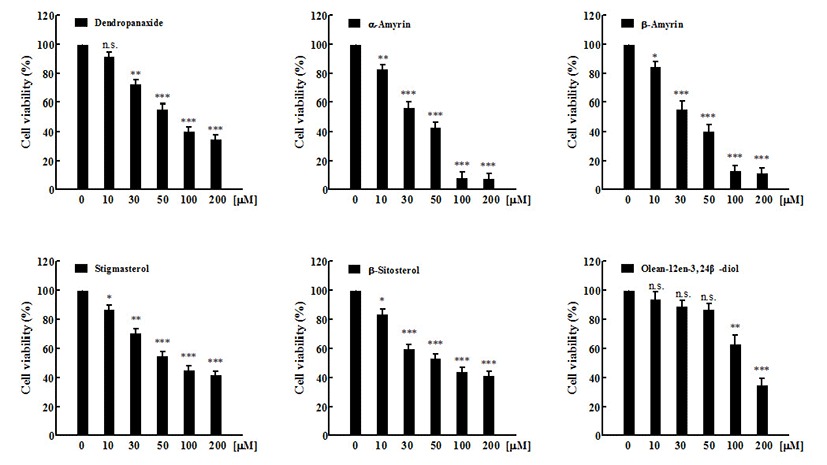
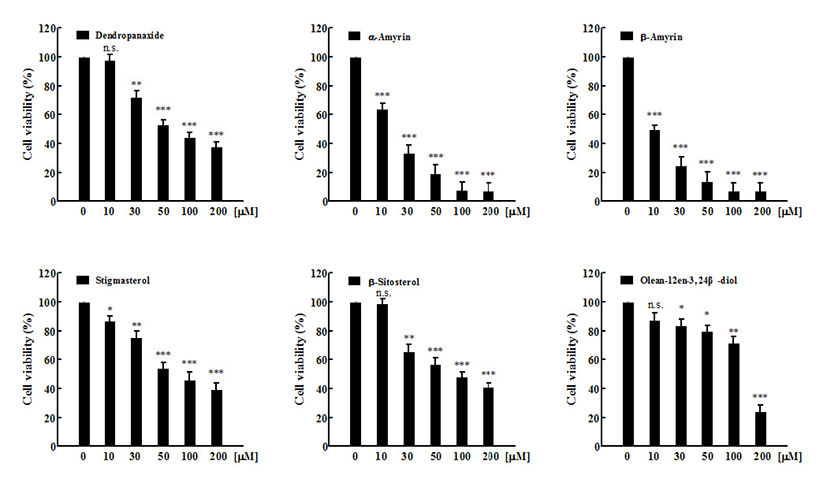
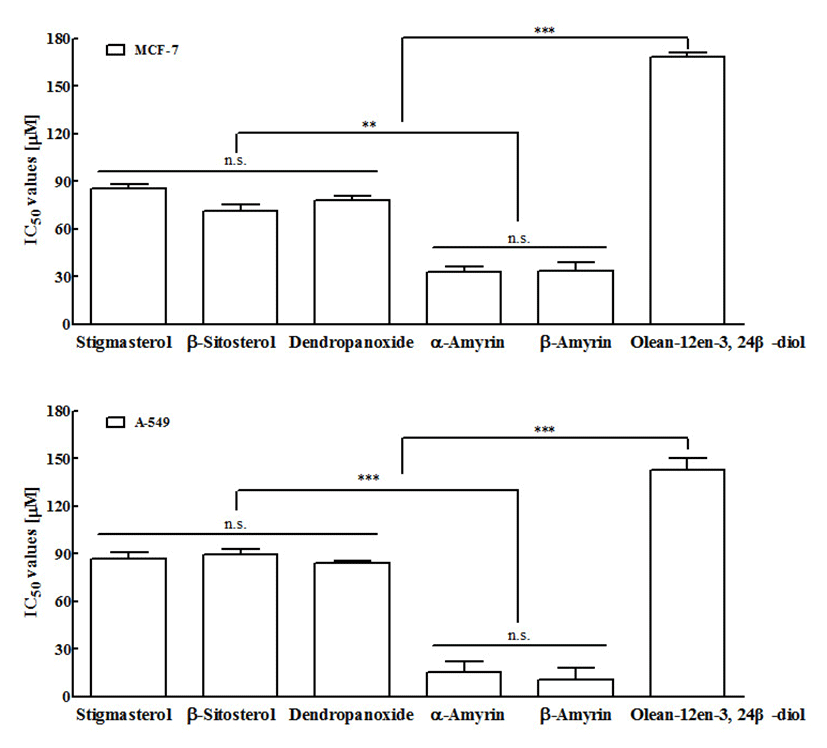
In vitro cytotoxic activity of six compounds at various concentration against MCF-7 and A549 cancer cell lines, and Vero normal cell lines were studied using MTT assay. Anti-cancer activity of six compounds at various concentrations against MCF-7 cancer cell lines was represented in Figure 2. Under the experimental conditions used in 24 h treatment, all six compounds exhibited a marked growth inhibitory effect on MCF-7 cells in a dose-dependent manner. The result of all compounds (1-6) against MCF-7 cells with IC50 values were 33.60±5.46, 32.70±3.60, 168.0±2.84, 78.27±2.20, 71.49±3.56, and 85.19±2.88 μM, respectively (Fig 4A). Compound 1 (β-amyrin) and compound 2 (α-amyrin) exhibited the most potent cytotoxicity against MCF-7 cells. The other three compounds (stigmasterol, β-sitosterol, and dendropanoxide) showed moderate inhibition of cell growth at the same concentrations. Unlike the other compounds tested, compound 3 (Olean-12-en-3,24 β-diol) was inactive with an IC50 higher than 100 μM. These different sensitivities to six compounds-induced cytotoxicity were also evident from the results of the dose-course experiment shown in Figure 3. Nearly 90% of MCF-7 cells died within 24 h of treatment with 100 μM of compound 1 and compound 2, also 24 h were required to reach similar level of cell death with A549 cells (p<0.001). At 24 h, IC50 values of compound 1 and compound 2-treated A549 cells were 10.33±7.4 μM and 15.45±6.82 μM, respectively (Fig 4B). In addition, compound 4 (dendropanoxide; IC50=83.71±1.74 μM), compound 5 (β-sitosterol; IC50=89.50±3.58 μM), and compound 6 (stigmasterol; IC50=86.58±4.28 μM) exhibited a dose-dependent inhibitory effect on the growth of A549 cells. However, compound 3 (Olean-12-en-3,24 β-diol) was inactive with an IC50 higher than 100 μM. Moreover, A549 cells were more sensitive to the α-amyrin and β-amyrin-mediated cytotoxicity than MCF-7 cells (Fig. 4) Hence, further assay were carried out with each IC50 concentrations. To the best of our knowledge, this is the first time that the activity of these compounds is reported. Moreover, no cytotoxic effect was observed in non-cancer cells, Vero, by using six compounds (up to 200 μM) which demonstrated all six compounds treatments exhibit cytoselectivity towards normal Vero cells (data not shown). The Vero cells were used to evaluate the toxicity to normal cells to determine the safe level of administration of the sample as a medicine from some natural materials. Higher concentrations that inhibit above 50% of the normal cell growth should not be used for testing against cancer cells (35).
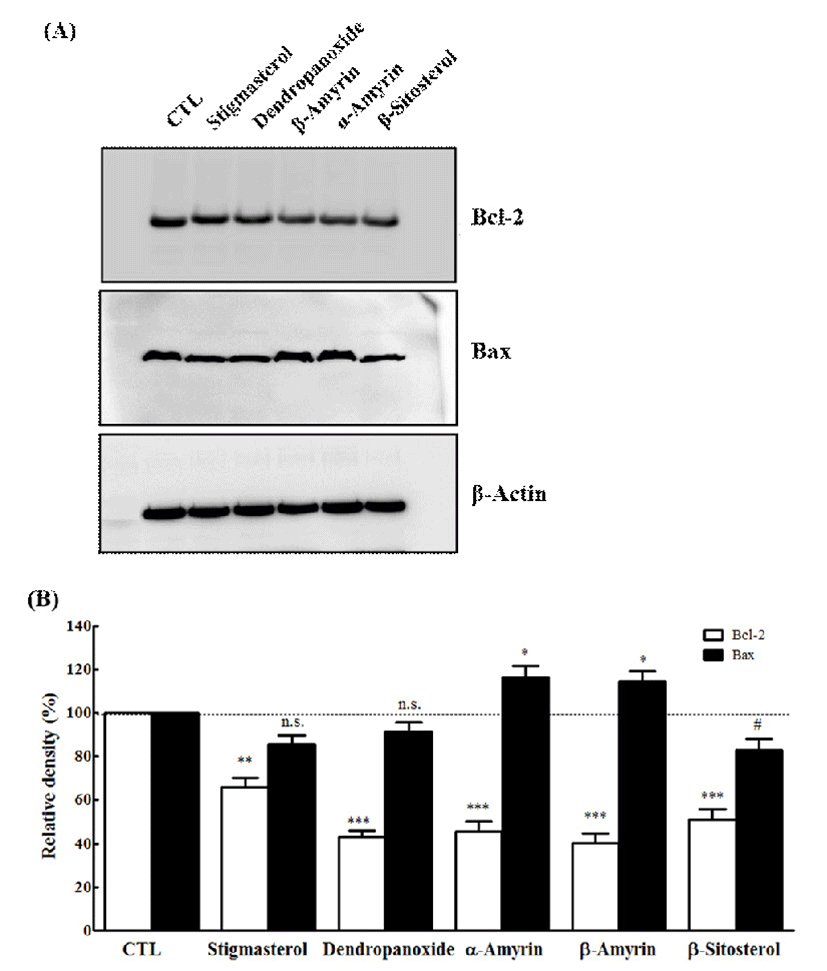
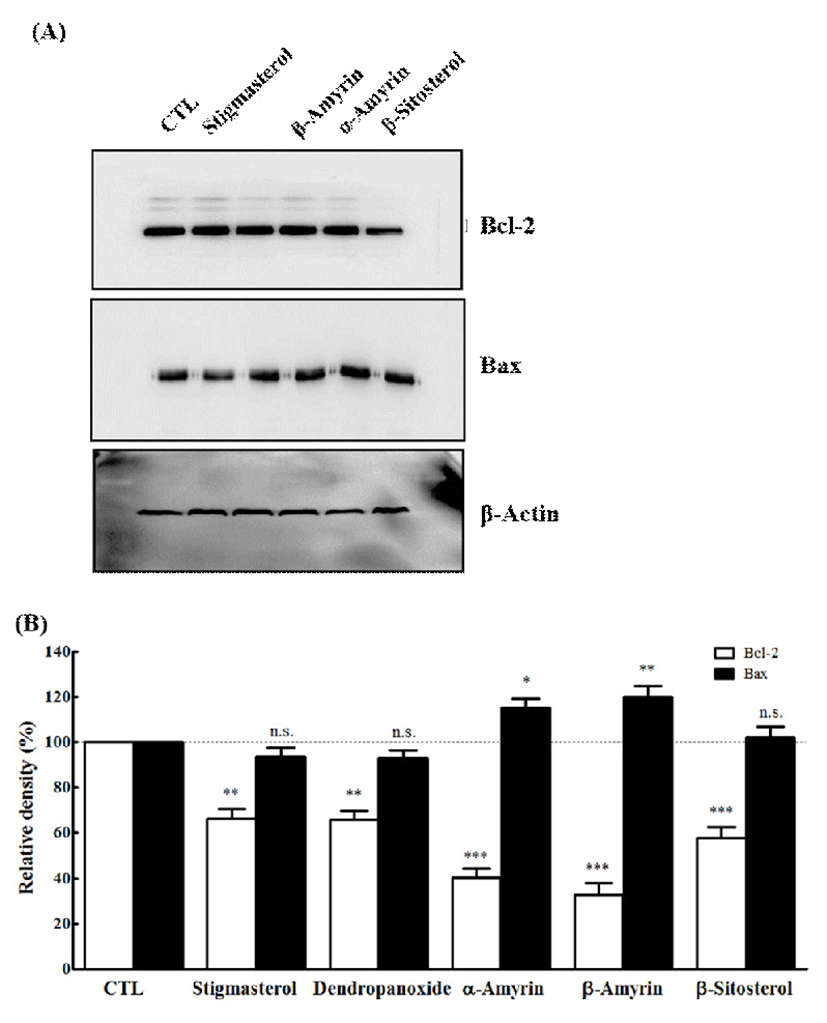
The apoptotic process is controlled by several gene products. There are group of genes involved in the regulation of the apoptosis which includes Bcl-2 and Bax (36). Bcl-2 is an anti-apoptotic gene and protects cells against the apoptosis in a variety of experimental systems (37). Various types of stimuli and anti-cancer drugs suppress the expression of Bcl-2 to promote apoptosis (36). Bax exhibits their apoptotic effect by binding to Bcl-2 protein family and also prevents the activation of cytochrome c (37). To understand the mechanism of action of five active compounds (1, 2, 4, 5, and 6), we studied the involvement of Bcl-2 and Bax in compounds mediated apoptosis and the effects in MCF-7 and A549 cells. Western blotting was performed to determine their expression level in cells treated with IC50 concentrations of compound 1, 2, 4, 5, and 6. As demonstrated in Fig. 5, all compounds caused Bcl-2 protein levels were markedly decreased in MCF-7 cells. On the other hand, only two compounds (α-amyrin and β-amyrin) resulted in significantly increased expression of Bax. As demonstrated in Fig. 6, Bcl-2 protein levels were markedly decreased by all compounds in A549 cells. However, the treatment of three compounds (stigmasterol, β-sitosterol, and dendropanoxide) failed to modify the level of Bax. The bcl-2 gene family is composed of a group related genes that either promote or prevent apoptosis (38,39). The family members include anti-apoptotic genes, such as bcl-2 or bcl-xL, and pro-apoptotic ones such as Bax. The expression ratio of pro-apoptotic and anti-apoptotic genes may determine whether a cell lives or dies following an insult (40). Overexpression of Bcl-2 protein protects cells from apoptosis following exposure to a number of different pro-apoptotic stimuli (41,42), whereas overexpression of Bax renders cells more sensitive to pro-apoptotic stimuli (43-45). Similarly, we also found that α-amyrin or β-amyrin treatment causes a decreased Bcl-2 expression and increased level of Bax, which may be responsible for the induction of apoptosis in MCF-7 and A549 cells. In our study, the levels of the anti-apoptotic Bcl-2 protein expression were decreased by compound 1 and compound 2, whereas protein expression of pro-apoptotic gene Bax was up regulated. Although we could not show that the induction of apoptosis was accompanied by the down-regulation of Bcl-2 expression and up-regulation of Bax expression on all compounds, suggesting that alteration of Bcl-2 and Bax is directly or indirectly involved in the apoptotic effect of five compounds in MCF-7 and A549 cells. Thus, α-amyrin and β-amyrin may be a potential lead compound for further structural modification for anti-cancer drug development.
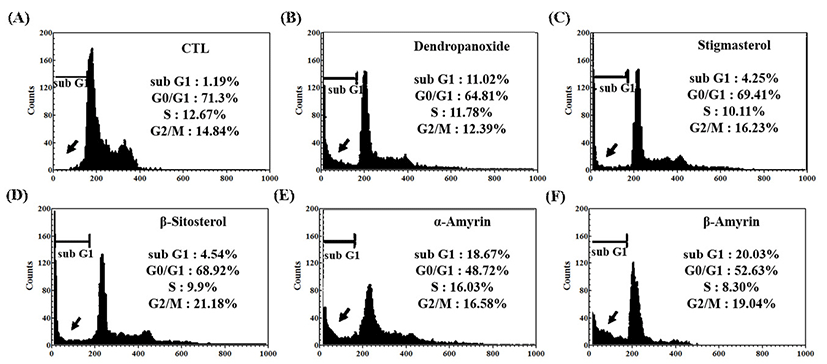
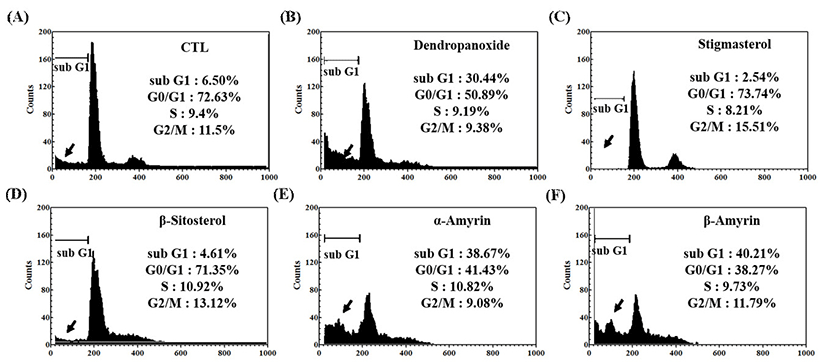
MCF-7 and A549 cells were exposed to IC50 concentrations of five compounds (1, 2, 4, 5, and 6) for 24 h, and the cell cycle distribution was analyzed by flow cytometry (Fig. 7, Fig. 8). As shown in Fig. 7, in the absence of compounds (control, CTL), the cell populations at sub-G1, G0/G1, S, and G2/M phases were found to be 1.19%, 71.3%, 12.67%, and 14.84%, respectively. Cells with sub-G1 DNA contents were scored as apoptosis based on the previous study (46). At IC50 concentration, five compounds (1, 2, 4, 5, and 6) showed an increased cell population at sub-G1, an apoptotic phase, with 20.03%, 18.67%, 11.02%, 4.54%, and 4.25%, respectively, and concomitant decrease in the other phases of cell cycle. From the results it is confirmed that treatment of α-amyrin and β-amyrin induced MCF-7 cell apoptosis maximally. As demonstrated in Fig. 8, in the control cells, we could detect only a minor cell population (6.5%) that underwent apoptosis in A549 cells. At IC50 concentration, five compounds (1, 2, 4, 5, and 6) showed an increased cell population at sub-G1 with 40.21%, 38.67%, 30.44%, 4.61%, and 2.54%, respectively, and concomitant decrease in the other phases of cell cycle. These results support the suggestion that α-amyrin and β-amyrin actively arrested progression of the cell cycle and induces apoptosis in MCF-7 and A-549 cells.
Summary
Inhibition of cancer growth has been a continuous effort in cancer treatment. The in vitro cytotoxicity of the isolated compounds showed its significant anti-cancer activity against MCF-7 and A549 cells. In the present study, we have demonstrated for the first time that Olean-12-en-3,24 β-diol and stigmasterol, a new compound isolated from DP, although fail to induce apoptosis in human breast and lung cancer cells. Therefore, the study in the future is to be extended to other cancer cell lines and there is a need to carry out in vivo studied to further authenticate the anti-cancer potentials of these compounds. However, with a very low IC50 value, the isolated triterpenoids, especially at α-amyrin and β-amyrin, are again confirming their potential to become an alternative material to inhibit breast and lung cancer cells. The more precise signaling pathway by which isolated triterpenoids from DP triggers caspase-3 activation, cytochrome c release, and the other apoptotic phenomena described here remain to be identified. Nevertheless, we concluded that triterpenoids, derived from DP extract, demonstrated cytotoxic activity with a very low IC50 value. Thus, triterpenoids have potential to become an alternative medicinal to inhibit breast and lung cancer in the future.










