Introduction
Production of high quality wines requires the cultivation of wine-exclusive grape varieties with excellent wine-specific properties such as high sugar content, appropriate acid content and good flavor. In particular, wine aroma is one of the most important factors to determine the character and quality of wine. Wine flavors dependent on the presence of aromatic compounds originating from grapes, the fermentation process, ageing, and storage. Wine volatiles include as many as 800 different compounds detected thus far, represented mainly by alcohols, esters, aldehydes and ketones (1). Grape volatiles include a great number of compounds, among which monoterpenes, C13-norisoprenoids, alcohols, esters and carbonyls represent some of the main groups (2). The profile of volatile compounds in wine depends on many factors such as geographical origin, grape variety, vintage, as well as growing conditions (3-8). The great variety of volatile compounds, all with different polarities, volatilities and a wide range of concentrations, are responsible for the complexity of wine bouquet and ensure the specific character of different varietals (9). The role of these volatile compounds is related to their odor perception threshold, i.e. the concentration / threshold ratio, known as the odor activity value (OAV) that enables us to estimate the contribution of volatile compounds found in grapes and wines (10).
Cultivars of Vitis vinifera form the basis of most wines produced around the world. The V. vinifera cultivar Chardonnay is renowned for the production of high-quality French white wines and champagne (11). Riesling is the second most widely planted aromatic variety of white wine grapes, and is used to produce varietal wines with aroma attributes that include stone fruits, flowers and wet stones (12). The majority of wine-only varieties introduced in Korea are V. vinifera species that usually winter kill at around -2 to -3℃ (13). Additionally, V. vinifera is not suitable for hot and humid summer environment, which results in poor quality grapes. However, rainfall in the Republic of Korea exceeds 1,000 mm a year, with almost half of this quantity falling during June and July. Further, the lowest temperature in Korea during the period 2000-2016 was -21.6℃ (14). As a consequence, the V. vinifera cultivars have to be cultivated in greenhouses in order to avoid winterkill in Korea. Therefore, new wine cultivars are in high demand in Korea.
Cheongsoo grape variety was produced as a plant cross between Seibel 9110 and Himrod Seedless grape varieties at the National Institute of Horticultural and Herbal Science (NIHHS) in Korea in 1993. Cheongsoo is a highly appreciated grape variety with a golden color and a pleasant fruit flavor. In addition, Cheongsoo is resistant to winter cold and mildew (15). In a previous study, the changes in volatile compounds in Cheongsoo wine were analysed at different stages of ripening (16). Cheongsoo wine was found to possess a high content of ester compounds that give rise to strong sweet flavors such as pineapple, banana and pear. However, the differences between Cheongsoo and other white wine varietals produced from grapes grown in Korea have not been analysed to date. Therefore, in order to understand the volatile characteristics of Cheongsoo grapes, it is necessary to compare it with renowned white grape varieties such as Chardonnay and Riesling. Therefore, the objectives of the present study were to identify the volatile compounds present in grapes and wine produced from three white wine grape varietals Cheongsoo, Chardonnay and Riesling using headspace-solid phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS).
Materials and Methods
Sodium chloride (NaCl) and 4-nonanol were purchased from Sigma-Aldrich Korea Ltd. (St. Louis, MO, USA). Calcium chloride (CaCl2) was purchased from Kanto Chemical Co., INC. (Tokyo, Japan). All reagents used were of analytical grade.
Three grape cultivars, Cheongsoo, Chardonnay, and Riesling were cultivated in the orchards at the National Institute of Horticultural and Herbal Science in Korea. Cheongsoo Chardonnay and Riesling Grapes were harvested on 23 August, 25 August and 31 August, 2016, respectively. All samples were frozen at -20℃ prior to analysis. Before isolating the volatile compounds, the grape samples were defrosted at 5℃ under nitrogen atmosphere.
The wine making process followed a modified Chang’s method (16) and micro-vinification method. Grapes were removed from grape bunches and potassium metabisulfite (K2S2O5) was added at a concentration of 200 mg/kg. Before fermentation, grape (500 g) must was adjusted to 22 °Brix using table sugar. Five hours after the addition of potassium metabisulfite, active yeasts were inoculated into grape must at a ratio of 0.02% (w/w). The yeast strain, Saccharomyces bayanus (EC-1118;Canada) was used for fermentation in all winemaking processes. The grapes were first fermented for two weeks at a constant temperature of 15℃ in a 750 mL DURAN® laboratory bottle equipped with an airlock. After the initial fermentation, the fermented residual sugar and sediment were isolated from wine. The isolated wine was subjected to a second round of fermentation at a constant temperature of 15℃ for 1 week, after which the prepared wine samples were analysed.
A SPME fiber coated with divinylbenzene/carboxen/ polydimethylsiloxane (50/30 μm, DVB/CAR/PDMS) (Supelco, Bellefonte, PA, USA) was used for the analysis owing to its high sensitivity for odorants and good reproducibility in grapes (17). Samples of grape berries (50 g) with CaCl2 (1 g) were defrosted and homogenised using a commercial blender. CaCl2 was added to inhibit enzyme activity prior to crushing (18). The homogenized samples were clarified by centrifugation at 2,700 ×g for 10 min at 4℃. A sample of the supernatant (25 mL) or fermentation-finished wine (25 mL) was transferred into a capped 50 mL solid-phase microextraction vial, and 3 g of NaCl and 20 μL of internal standard (4-nonanol in EtOH, 1 mg/mL) were added. Samples were heated at 70℃ in an automated heating block (Wise Therm®, HB-48P). After 20 min of equilibration, the SPME fiber was manually inserted into the sample vial headspace. After 20 min, the fiber was withdrawn and introduced into the GC injection port for desorption at 250℃ and maintained for 10 min in splitless mode. Samples of three different batches of grapes were examined in triplicate. All samples were examined in triplicate.
An Agilent gas chromatograph model 6890N coupled to an Agilent 5975 series mass selective detector (Agilent technologies, Santa Clara, CA, USA) was used to perform the analysis. Volatile compounds were separated on an HP-INNOWAX capillary column (30 m×0.32 mm×0.25 μm; Agilent technologies), with purified helium as the carrier gas, at a constant flow rate of 2 mL/min. Desorption of the DVB/CAR/PDMS fiber was carried out for 10 min in the GC injection port at 250℃. The oven temperature was held at 40℃ for 5 min, increased to 220℃ at a rate of 3℃/min and finally held at 220℃ for 5 min. The injector and source temperature were set at 250℃ and 230℃, respectively. The mass detector was operated in electron impact ionization mode at a voltage of 70 eV, with the range set at 50-700 m/z. Selected GC-MS peaks were identified based on the comparison of mass spectra with the NIST11 (Agilent, Gaithersburg, MD, USA) mass spectral database. Content of all compounds was quantified relative to the known concentration of 4-nonanol internal standard.
A one-way analysis of variance (ANOVA) was performed to identify statistically significant differences between samples. A principal components analysis (PCA) was performed to evaluate and perform a reduction in the number of attributes to a smaller set of underlying variables based on patterns of correlations between original variables. The R Commander (version 2.13.0, A Basic-Statistics GUI for R statistical software package; McMaster University, Ontario, Canada) was employed for this analysis where statistical significance was defined as p<0.05.
Results and Discussion
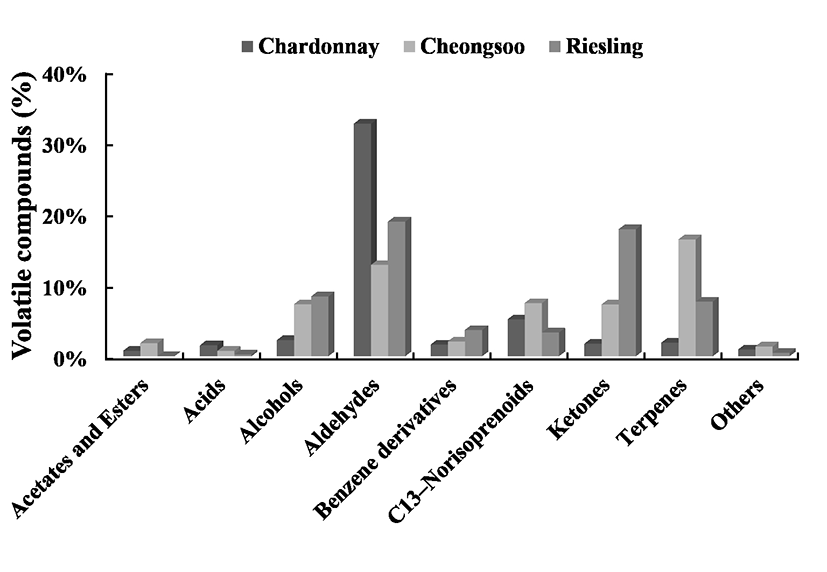
The major volatile compounds found in the juice extracted from the three grape berries were terpenes, C13-norisoprenoids, ketones, alcohols and aldehydes (Fig. 1). However, the % content of the various volatile compounds varied as a function of grape cultivar. The most abundant volatiles in Cheongsoo grapes were terpene compounds, while aldehydes predominated in Chardonnay and Riesling grapes. Terpenes are known to play an important role in grape berries as they are responsible for the floral notes of aromatic grape varieties such as Gewürztraminer, Sauvignon Blanc and the Muscat family (19-21). Currently, about 50 monoterpene compounds that occur in grapes and wines most commonly have been identified (22). Free and bound glycoside terpenes can be found in grapes. Specifically, odorless precursors (bound fraction) present in grapes are transformed into odor-active forms (free fraction) during the winemaking process (23). Therefore, quantification of bound glycosides can be used as a useful index by winemakers for the determination of wine aroma and character (22). Recently, it has been reported that diendiol II (3,7-dimethylocta-1,7-dien-3,6-diol) is generated enzymatically from linalool in grape berries (24). However, the biosynthesis and oxidative transformation of monoterpenes in grapes remain largely unexplored in spite of their importance as a volatile wine compounds. Taking into consideration of the high content of terpene, Cheongsoo wine possesses quite a strong characteristic aroma compared to Chardonnay and Riesling wines.
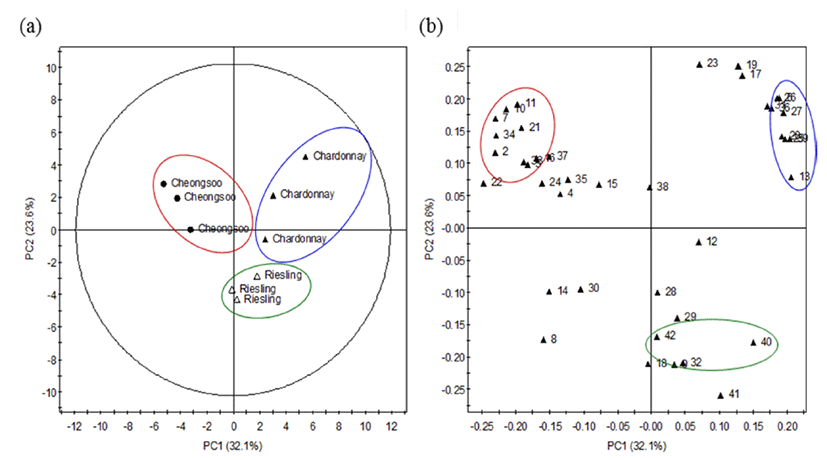
PCA based on GC-MS data was performed to compare the content of volatile compounds as a function of grape cultivar (Fig. 2). PCA is a powerful tool for the interpretation of multivariate statistical analysis. The three white wines examined in this study were found to adopt three overlapping, yet distinct zones. Cheongsoo wine was identified by the presence of isoamyl acetate, ethyl hexanoate, ethyl octanoate and ethyl decanoate. In contrast, Chardonnay wine was distinguished by the ethyl heptanoate, methyl-2-hydroxybenxoate, 3-hexen-1-ol and 1-heptanol content. Finally, Riesling wine was categorised by 2-methyl-hexanoic acid, trans-(2-chlorovinyl)methyldiethoxysilane and 1,1,6,-trimethyl-1,2-dihydronapthalene (TDN).
In the present study, 16 ester volatile compounds were detected in three white wine varietals (Table 1). The concentration of esters was about four times higher in Cheongsoo than in Chardonnay and Riesling wines. Five esters found in Cheongsoo wine, namely isoamyl acetate, ethyl butanoate, ethyl hexanoate, ethyl octanoate and ethyl decanoate, exhibited a high OAV of >1. In contrast, only four and two esters displayed a high OAV (>1) in Chardonnay and Riesling wines, respectively. Additionally, the isoamyl acetate (OAV=16.28) odorant associated with banana and sweet aroma, was detected only in Cheongsoo wine. In several studies, isoamyl acetate was one of the esters that contribute most significantly to the aroma profile of white and red wines. This compound was also considered to be one of the most powerful odorants and key fermentation volatiles of Zalema white wine, 52 young red wines and Vitis vinifera cv. Pinotage and Sake (10,25-27). The esters produced enzymatically during yeast fermentation are the most important group of aromatic compounds in wines. In particular, ethyl esters formed from medium-chain fatty acids are responsible for the fruity character of fermented beverages, and thus constitute a vital group of aromatic compounds in both wine and beer (28,29). In our results, ethyl hexanoate and ethyl octanoate exhibited a high OAV of >1 in all three wines (Table 1). However, the concentration of ethyl hexanoate in Cheongsoo wine was about six and three times higher than its content in Chardonnay and Riesling wines, respectively. Furthermore, the concentration of ethyl octanoate was about five and eight times higher in Cheongsoo wine than in Chardonnay and Riesling wines. While ethyl decanoate was detected in all white wines examined, it exhibited a high OAV of >1 only in Cheongsoo wine. In contrast, ethyl butanoate, which displayed an OAV of above 1 was detected in both Cheongsoo and Chardonnay wines. Finally, ethyl heptanoate was detected with a high OAV (>1) only in Chardonnay wine. In summary, five esters were found to be the predominant fruity and sweet volatile odorant compounds in Cheongsoo wine.
Fourteen alcohol volatile compounds were detected in three white wine varietals (Table 2). At a concentration of 6.0-10.5 mg/L, alcohols represented the largest group of volatile compounds in the three examined white wine varietals. Nevertheless, the influence of alcohols as odorants in wine was lower than those of esters as a result of their high odor threshold value (OTV). In fact, with the exception of 1-hexanol, the alcohols were detected lower than their OTVs in quantities. In addition, alcohols with six carbon atoms, which supply vegetal and herbaceous nuances to wine, usually exert a negative effect on wine quality when present at concentration above their OTV (30). In our work, 3-hexan -1-ol with an OAV <1 was detected in Chardonnay wine only, and can be thus used to distinguish Chardonnay wine from others.
Five acids and 7 other volatile compounds were detected in three white wine varietals (Table 3). C6-C10 fatty acids at a concentration of 4 to 10 mg/L impart mild and pleasant aroma to wine. At levels beyond 20 mg/L, however, their impact on wine becomes negative (31). In this study, fatty acids were found to be present in the wine samples only in trace amounts. C13-norisoprenoid components such as α-ionene were detected with high OAV (>1) only in Cheongsoo wine. In contrast, 2-methyl-hexanoic acid and TDN were detected in Riesling wine only. These volatile compounds may contribute to the distinction of Riesling wine from non-Riesling wines (12).
This is the first study comparing the volatile compounds found in white grape varieties grown in Korea. The obtained analytical results allowed us to determine the content of major volatile components in Cheongsoo, Chardonnay and Riesling grape cultivars. The differences detected in the content of volatile components were successfully used to distinguish grapes and wines associated with the three varietals. The concentration of terpenes, which are known to determine the wine aroma after fermentation, was found to be about eight and two times higher in Cheongsoo grapes than in Chardonnay and Riesling grapes, respectively. Furthermore, the quantity of odorants in Cheongsoo wine exceeded those found in Chardonnay and Riesling wines. In particular, esters such as isoamyl acetate, ethyl butanoate, ethyl hexanoate, ethyl octanoate and ethyl decanoate were detected in Cheongsoo wine with an OAV of >1. These ester components are key odorants in wine associated with fruity and sweet aroma. Therefore, we anticipate that Cheongsoo grape variety can be employed in Korea as a new wine grape for winemaking owing to its high content of sweet and fruity odorants.










