Introduction
The increasing prevalence of diabetes mellitus and diabetes complications reinforces concerns in public health. The major complications of diabetes are macrovascular disease, nephropathy, retinopathy, and neuropathy (1). Diabetic complications are developed during the progression of diabetes due to hyperglycemia. Hyperglycemia observed in patients with diabetes causes oxidative stress and protein glycation, thus leading diabetes complications (2).
Oxidative stress, the status of disturbance in the balance of antioxidants and pro-oxidants, damages healthy cells through oxidation of DNA, protein, and other macromolecules, implicating in the pathogenesis of various chronic diseases (3). Oxidative stress produced by hyperglycemia accelerates the formation of advanced glycation end products (AGEs) (4).
The accumulation of AGEs from non-enzymatic glycation of protein has been implicated in pathophysiology associated with diabetes mellitus (5). In the initial stage of the non-enzymatic glycation of proteins, carbonyl group of the reducing sugar reacts with free amino groups of protein to form Schiff base, sequentially producing Amadori products. In the final stage, α-dicarbonyl compounds again react with free amino groups to form yellowish-brown, often fluorescent, irreversible compounds, usually called AGEs. AGEs cause various types of protein modifications, resulting in structural and functional impairments of proteins (6).
Meanwhile, hypoadiponectinemia appears to play an important role in insulin resistance and diabetes mellitus (7). Adiponectin, an adipokine expressed in adipose tissue, is known to sensitize the body to insulin (8). Thus, upregulation of adiponectin is a potential therapeutic target to alleviate insulin resistance and diabetes mellitus (9).
In order to prevent and/or ameliorate diabetic complications, it is important to control hyperglycemia or hyperglycemiainduced cellular events such as the formation of AGEs and oxidative stress. It is well known that antioxidants help reduce deterioration of hyperglycemia (10,11). Curcumin (CCM), a yellow pigment in turmeric, is known to possess various biological activities including antioxidant, anti-inflammatory, anti-cancer, and anti-obese activities (12-14). The phenolic hydroxyl groups in CCM is responsible for the antioxidant properties while suppression of the nuclear factor kappa B signaling transduction pathways by CCM alleviates inflammation (12-13). Moreover, CCM exhibits anti-obesity activity through reducing adipocyte differentiation, one of the important target to prevent the development of obesity (14). Among natural compounds with antioxidant activity, CCM is one of the strongest antioxidant compounds, and thus CCM could have beneficial effects on hyperglycemia. Here inhibitory effects of CCM on high glucose-induced damages were investigated.
Materials and Methods
Bovine serum albumin (BSA) was obtained from Amresco (Solon, OH, USA). Curcumin (CCM), D-glucose, sodium azide, nitro blue tetrozolium (NBT), sodium formate, Girad-T solution, glyoxal, insulin, dexamethasone, isobutylmethylxanthine, and 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s medium (DMEM), 0.25% trypsin-EDTA, sodium pyruvate, and penicillin/streptomycin were obtained from Fisher Scientific (Pittsburgh, PA, USA). Fetal calf serum (FCS) and fetal bovine serum (FBS) were obtained from PAA Laboratories (Pasching, Austria).
A physiological plasma glucose condition was mimicked for setting in vitro BSA glycation system. Mean blood glucose concentrations at 5, 11, and 25 mM are considered as healthy fasting, healthy postprandial, and diabetic feeding concentration, respectively. Incubation time of 21 days was determined based on circulating half-life of plasma albumin. In order the examine the role of CCM on BSA glycation, BSA (42 g/L) was incubated with 25 mM of glucose and 0.02% sodium azide in 0.2 M phosphate buffer (pH 6.8) with or without 1 mM CCM. Reaction mixture was incubated at 37℃ for 21 days. Samples were collected every week and stored frozen at -70℃ (Ultra-Low Temperature Freezer, Sanyo Electric Co., Ltd., Osaka, Japan) until analyzed.
A colorimetric fructosamine assay using NBT reagents was used to estimate Amidori products, the early stage of the non-enzymatic glycation of proteins (15). Samples (40 μL) were mixed with 160 μL of 300 μM NBT reagent in 100 mM sodium carbonate buffer (pH 10.35) and incubated for 30 min at room temperature. Absorbance of the reaction mixture was measured at 530 nm using a microplate spectrophotometer (Epoch, Bio-Tek Instruments, Inc. Winooski, VT, USA). The data was expressed as % of control.
Girard-T assay was used to detect α-dicarbonyl compound, the intermediate of glycation of protein (16). Samples (200 μL) were incubated with 100 μL of Girad-T solution and 1,700 μL of 100 mM sodium carbonate buffer (pH 10.35) for 1 h at 25℃. Absorbance of the reaction mixture was measured at 290 nm using an Ultraviolet-visible spectrophotometer (Hitachi, Ltd., Tokyo, Japan). A standard curve was prepared using glyoxal (GO) standards treated in the same way. The values of samples were calculated based on the equation of standard curve and data was expressed as mg GO equivalent/mL.
For detection of AGE formation, plate reader (VICTOR X3, PerkinElmer Singapore Pte Ltd., Turku, Finland) was employed (15). Fluorescence intensity from the glycated BSA was read at 460 nm after excitation at 355 nm. Data was expressed as arbitrary units (AU).
The 3T3-L1 preadipocytes were cultured in DMEM containing 10% (v/v) FCS, 100 U/mL penicillin, 100 μg/mL streptomycin and 0.11 g/L sodium pyruvate. Adipogenesis of 3T3-L1 preadipocytes were initiated in 10% (v/v) FBS with hormonal cocktail described in the paper (14). Day 10 of differentiated 3T3-L1 cells were tested for measurement of intracellular reactive oxygen species (ROS) and adiponectin.
The 3T3-L1 preadipocytes pre-treated with various concentrations of CCM for 30 min at 37℃. After removal of CCM from the medium, 125 μM of DCFDA was added directly to the medium for 1 h at 37℃ (17). Intracellular ROS generated by high glucose was detected by plate reader (VICTORX3, PerkinElmer, Turku, Singapore).
Total RNA was prepared from mature 3T3-L1 cells using TriZol according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, US). cDNA was synthesized using Superscript II reverse transcriptase system (Invitrogen) and subjected to real-time RT-PCR using Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, US) (18). The specific primers used in quantitative RT-PCR are as follows: adiponectin (forward, 5′-GAT GCA GGT CTC TTG GTC CTA A-3′; reverse, 5′-GGC CCT TCA GCT CCT GTC A-3′) and β-actin (forward, 5-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′; reverse, 5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3′).
Results were presented as mean±SE obtained at least three independent experiments with triplicate. Statistical analysis was performed using SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA). Data were evaluated statistically using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison method (Bonferroni’s methods) to estimate significant difference between control and treatment groups. The statistical test is significant at the present level of p<0.05.
Results and Discussion
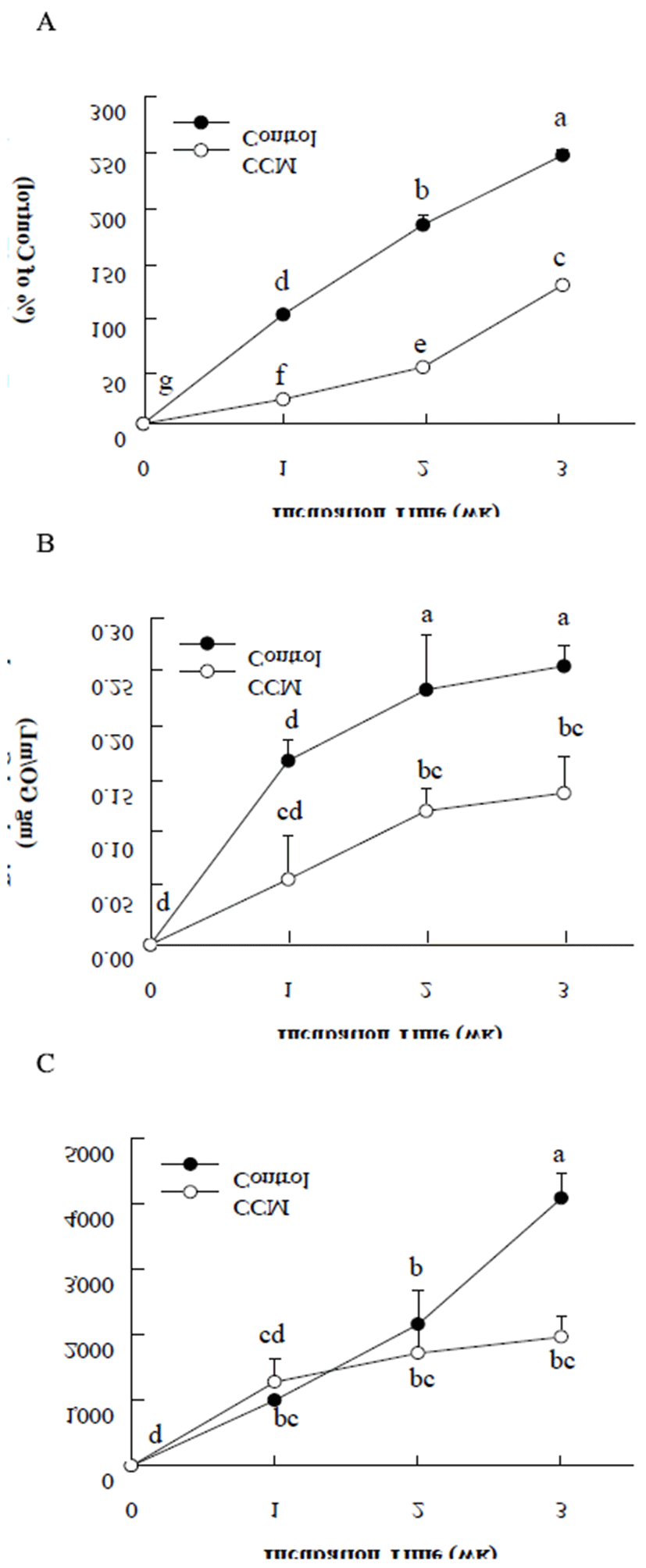
In order to evaluate inhibitory role of curcumin (CCM) on protein glycation, albumin, the most abundant protein in the blood, was chosen as a protein for the in vitro glycation reaction with high concentration of glucose. During 3 weeks of incubation of bovine serum albumin (BSA) and 25 mM of glucose in the absence or presence of 1 mM CCM, formation of advanced glycation end products (AGEs) was determined by a fructosamine assay, α-dicarbonyl compound measurement, and fluorescence analysis. CCM exhibited a potent inhibitory effect on AGEs formation in BSA glycation system (Fig. 1). The fructosamine assay for determining Amadori products generated in the early stage of the protein glycation suggested that CCM significantly suppressed Amadori product formation by approximately 50%. Also, CCM significantly mitigated the generation of α-dicarbonyl compounds, which are formed in the intermediate stage of glycation. As AGE formed, fluorescent signal produced from BSA glycation with glucose was gradually increased for 3 weeks of incubation. The results of 3 weeks of incubation showed that CCM treatment significantly reduced AGE formation by 52%.
Since the level of blood glucose fluctuates throughout the day depending on meals and physical activity. In order to understand the effect of glucose concentration on generation of α-dicarbonyl compounds, increasing doses of glucose (5.6, 11, and 25 mM) was incubated with BSA in the absence or presence of 1 mM CCM. At 3 weeks of incubation, formation of α-dicarbonyl compounds was significantly elevated as a glucose dose increased (Fig. 2). However, CCM significantly diminished the production of α-dicarbonyl compounds due to high level of glucose.
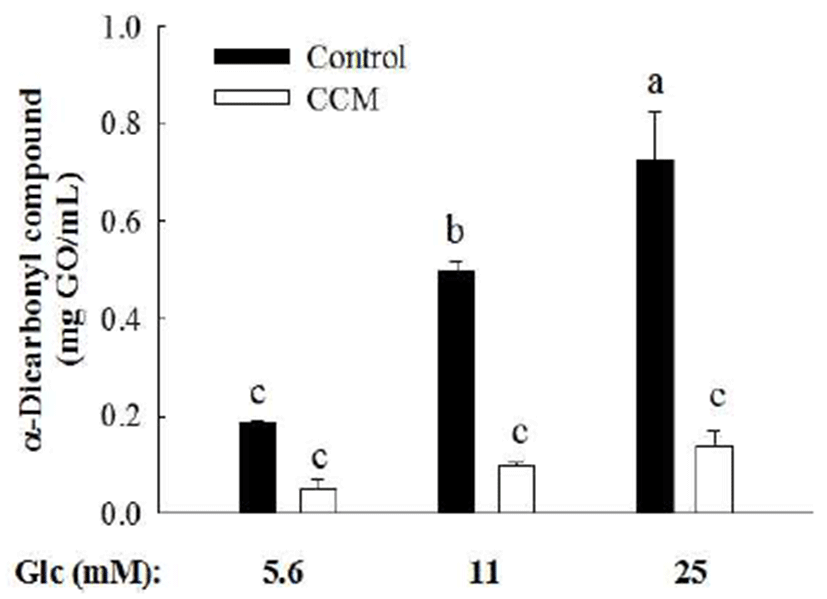
It is well known that AGE formation stimulates diabetic complication and thus inhibition of AGE generation is one of the key targets to prevent the progression of diabetic complication. However, the development of anti-glycation agent is a challenge. Aminoguanidine, an AGE inhibitor, is no longer available as a drug due to multiple safety issue (19). Therefore, there has been an increasing interest in the use of natural compounds as anti-glycative agents. It has been reported that inhibition of AGE formation by guava leaf extract (15), cyaniding-3-rutinoside (20), feruloyl oligosaccharides (21), and vitamins and minerals including ascorbic acid, tocopherol, pyridoxal, and selenium (11). Similar with these compounds, CCM has a strong inhibitory activity in protein glycation under high concentration of glucose. Considering the fact that the formation of AGEs due to hyperglycemia is a major contributor to lead diabetic complication in patient with diabetes, CCM can be used as a glycation inhibitor to prevent diabetic complications.
Hyperglycemia is known to induce intracellular oxidative stress, thus contributing the development of diabetic complications (2). In order to determine inhibitory effect of CCM treatment on high glucose-induced oxidative stress, intracellular reactive oxygen species (ROS) were estimated by DCFDA assay. Compared to low glucose (5.6 mM) treatment, high glucose (25 mM) slightly elevated intracellular oxidative stress but CCM treatment alleviated high glucose-induced ROS production (Fig. 3). Inhibitory effect of CCM on high glucose-stimulated ROS production was found to be in a dose-dependent manner. Intracellular ROS generation was completely abolished by CCM at a concentration of 30 μM.
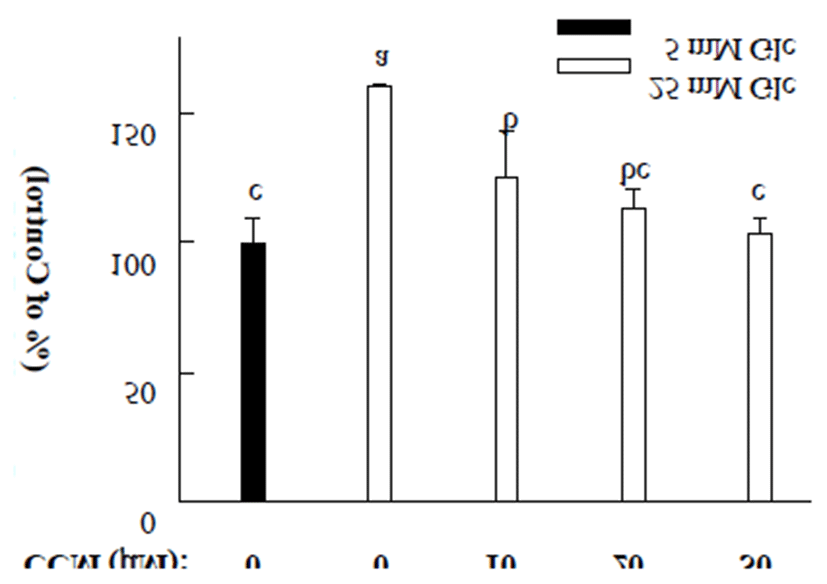
It is recommended for people with diabetes to take daily requirement of vitamin and mineral which have a strong antioxidant activity (22). Moreover, several studies suggested that compounds with combined antiglycative and antioxidant activities showed synergistic effects on not only preventing AGE production but also suppressing oxidative stress-induced toxicity. Based on our data that CCM has abilities to inhibit oxidative stress and protein glycation, it is believed that CCM could be a potent compound to prevent diabetic complications. Among natural compounds, green tea is known to help diabetic complications because it has protective activity against protein oxidation and glycation (10).
Since adiponectin, a protein hormone secreted from adipocytes, improves glucose regulation, upregulation of adiponectin expression elicits protective function against type 2 diabetes (23). In order to test whether CCM treatment in adipocytes stimulates adiponectin expression, the adiponectin gene expression in 3T3-L1 adipocytes was detected by quantitative RT-PCR. The results reveal that 20 μM of CCM treatment in mature adipocytes significantly increased adiponectin gene expression in a time-dependent manner (Fig. 4). Results showed that CCM treatment for 24 h and 48 h significantly increased the level of adiponectin gene by approximately 1.2 folds and 1.5 folds, respectively.
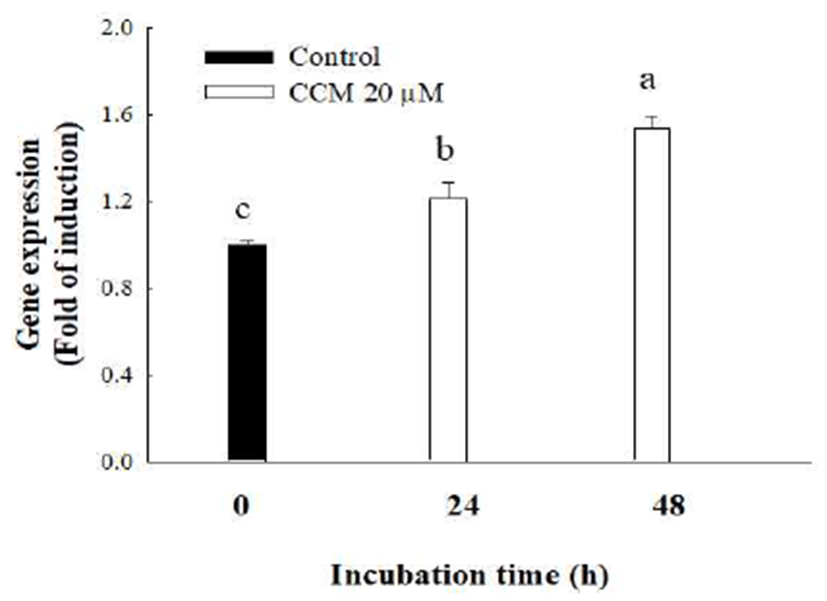
Many studies suggested that natural compounds are able to increase adiponectin expression. Phloretin (50 μM), a flavonoids found in apples and strawberries, increased adiponectin mRNA expression in mature 3T3-L1 adipocytes by 2.3 folds (24). In addition, naringin, a flavanone glycoside found in grapes and citrus fruits, is also known to increase serum adiponectin and ameliorate insulin resistance in a rat model of type 2 diabetes (25). Since the protein level of adiponectin in plasma and mRNA expression of adiponectin in adipocytes are reversely correlated with obesity and insulin resistance (9), administration or upregulation of adiponectin by dietary compounds may reverse insulin resistance and diabetes. Therefore, it is possible that CCM alleviates the symptoms of diabetes and diabetic complications. Even though our data show that CCM stimulated adiponectin mRNA expression in 3T3-L1 adipocytes, further study is necessary to confirm the physiological relevance of this result.
Summary
Preventive activities of CCM on high glucose-induced damages were determined by the level of BSA glycation with glucose, intracellular ROS production, and adiponectin gene expression in mature adipocytes. CCM inhibited formations of fructosamine, α-dicarbonyl compounds, AGEs in BSA/glucose system during the 3 weeks of incubation. Regardless of glucose concentration, CCM significantly reduced the level of α-dicarbonyl compounds. High concentration (25 mM) of glucose-induced intracellular ROS production, whereas CCM suppressed high glucose-induced intracellular ROS production in a dose-dependent manner. Moreover, CCM upregulated the gene expression of adiponectin, an adipocyte-specific secretory protein. The results of this study suggested that CCM could be beneficial for preventing the development of diabetic complication.










