Introduction
Rapeseed (Brassica napus L.) is used in the production of animal feed, bio diesel, and vegetable oil for human consumption since it has a high yield per unit area and high oil content (1). Traditional rapeseed contains glucosinolate and erucic acid. Glucosinolate contributes a bitter taste and causes goiter when consumed in high amounts (2). Erucic acid constitutes about 58% of the total fatty acid content of rapeseed and causes heart disease and chronic myocardiopathy at high amounts (3). To overcome such disadvantages, Canada, the United States, and the European countries have developed rapeseed varieties with lower contents of glucosinolate and erucic acid, resulting in canola oil or low-erucic acid rapeseed oil in the market (4).
Rapeseed cake, a byproduct of oil extraction from rapeseed, is composed of 11.4% water, 2.8% crude fat, 36.1% crude protein, and 6.3% crude ash (5). Rapeseed cake also contains natural antioxidants, known as phenolic compounds, and major phenolics are sinapic acid derivatives. Sinapic acid constitutes over 73% of the free phenolic acid content of rapeseed cake, whereas sinapine (choline ester of sinapic acid), as the main phenolic compound, constitutes about 80% of the total phenolic content along with 1-Ο-β-Dglucopyranosyl sinapate (sinapoyl glucose) and vinylsyringol (a decarboxylated sinapic acid) (6,7). Thiyam et al. (8) previously reported phenolic compounds in rapeseed cake with 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity in the order of sinapic acid > sinapoyl glucose > sinapine, suggesting that structural features, such as esterification of sinapic acid with a choline or glucose moiety could reduce antioxidative activities. Sinapic acid is known to be a more effective antioxidant than sinapine during lipid oxidation. Sinapine was shown to be slightly prooxidatve or have no inhibitory effect on hydroperoxide formation while sinapic acid was shown to have a higher antioxidative capacity at higher concentrations (9).
Fish oil contains large amounts of ω-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). DHA plays a significant role in brain development due to its beneficial effects on neurogenesis, neurotransmission, and protection against oxidative stress (10). EPA has lowering effects on blood triacylglycerol and LDL-cholesterol concentrations and prevents atherosclerosis and other heart diseases. However, oils and foods containing large amounts of ω-3 fatty acids can be easily oxidized through processing, storage, and distribution, resulting in production of various radicals and peroxides. Furthermore, their decomposition products can cause off-flavors that diminish consumer preferences for these foods and their original qualities. Many foods exist as an oil-in-water emulsion. To utilize fish oil in an emulsion-based food, addition of antioxidants, such as phenolic compounds is needed to increase oxidative stability (11).
In the present study, defatted rapeseed cake was extracted with 80% ethanol and this crude extract was fractionated with solvents having different polarities. The total phenolic content and antioxidant capacity of each fraction were assessed, and three fractions with high antioxidant capacities were combined and hydrolyzed using NaOH. The antioxidant capacity of hydrolyzed extract was compared with crude extract, and then the effect of hydrolyzed extract on oxidative stability in fish oil-in-water emulsion was investigated.
Materials and Methods
Rapeseed cake from Hanla cultivar was obtained from the Rural Development Administration (RDA) (Junju, Korea) in 2011 and stored at -20℃. Fish oil was provided from Neomega Co., Ltd. (Deajeon, Korea). Sinapic acid, catechin, 6-hydroxy- 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and 1,1,3,3 –tetraethoxypropane were purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). 2,2´-azinobis-3- ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,4,6-tripyridyls- triazine (TPTZ), Folin-ciocalteu’s phenol reagent, DPPH, barium chloride, ferrous sulfate ammonium thiocyanate, sodium azide, hydrogen peroxide, and Tween 20 (Polyoxyethylene sorbitan monolaurate) were purchased from Sigma-Aldrich Co., Ltd.. All solvents were HPLC grade and obtained from Fisher Scientific Co., Ltd. (Norcross, GA, USA).
Rapeseed cake was first extracted with hexane to remove the small quantity of fat residue. Phenolic compounds were then extracted from defatted rapeseed cake with 50 mL of 80% (v/v) ethanol at 55℃ and 135 rpm for 1 h in a shaking water bath, centrifuged at 730 g for 5 min, and then filtered. Extraction was repeated two more times. Supernatants were combined, and water was removed by passing through an anhydrous sodium sulfate column. Ethanol was evaporated by a rotary vacuum evaporator at 60℃, after which residual ethanol was removed under nitrogen. The prepared crude extract was stored at -20℃ until further investigation.
Rapeseed cake crude extract (400 mg) was re-dissolved in 80% ethanol (4 mL), after which 0.5 mL was loaded onto a syringe column (5 mL) packed with 1.5 g of Diaion HP-20 (Sigma-Aldrich) and covered with sea sand. After adsorption for 1 h, fractionation was carried out using solvents with different polarities in H2O (I) and 30% (II), 50% (III), 70% (IV), and 100% ethanol (V), respectively. The elution solvent (each 6 mL) was passed through the syringe column by reduced pressure with a vacuum manifold (VisiprepTM, Supelco Inc., Bellefonte, PA, USA).
The TPC of each fraction was determined according to the method of Thiyam et al. (8) with slight modification. Fractions I-V (0.5 mL), 0.45 mL of distilled water, and 0.5 mL of Folin-Ciocalteu reagent were mixed together. After 3 min, 1 mL of 1 N Na2CO3 was added and the mixture was in the dark for 1 h, after which absorbance was measured at 725 nm. A standard curve was prepared with sinapic acid, and TPC was expressed as mg of sinapic acid equivalents (SAE)/mL fraction.
The DPPH radical scavenging activities of the fractions were determined by mixing 0.2 mL of each crude extract fraction (I-V), 1.8 mL of ethanol, and 2.5 mL of DPPH reagent (0.15 mM) (12). After 30 min in the dark, absorbance was measured at 516 nm with a spectrophotometer. DPPH radical scavenging activity (RSA) was calculated using the following equation:
Ferric reducing antioxidant potential (FRAP) assay was performed according to the method of Wong et al. (13) with slight modification. FRAP reagent was prepared by mixing 10 mM TPTZ in 40 mM HCl, 20 mM ferric chloride solution, and 300 mM acetate buffer (pH 3.6) at a 1:1:10 ratio (v/v/v). Fractions (150 μL) were added to FRAP reagent (5 mL), vortexed, and reacted for 4 min at 35℃. Absorbance was then measured at 593 nm. A standard curve was generated with ascorbic acid, and FRAP values were expressed as mg of ascorbic acid equivalent/mL of rapeseed cake extract fraction.
To determine the trolox equivalent antioxidant capacities (TEACs) of the fractions, ABTS radicals were generated by mixing 7 mM ABTS and 2.45 mM potassium persulfate (1:0.5, v/v) (14). Solution was incubated at room temperature in the dark for 12-16 h, after which ABTS radical solution was diluted with ethanol until an absorbance of 0.7±0.02 at 734 nm was achieved. Fraction (100 μL) was mixed with ABTS radical solution (5 mL) in a test tube and left for 6 min. Absorbance was then measured at 734 nm. Trolox was used as a standard, and results were expressed as mg of Trolox equivalent (TE)/mL of crude extract fraction.
Hydrolysis of fractions obtained from crude extract was performed according to the method of Vuorela et al. (15) with slight modification. The combined fractions (I, II, and III) were hydrolyzed with 4 M NaOH solution by stirring for 90 min at 300 rpm in an Erlenmeyer flask. After hydrolysis, 37% HCl solution was added until a pH level was reached to 2. Phenolic acids were released when the pH was lower than 2, and ethyl acetate:diethyl ether (1:1, v/v) was added to extract phenolic acids from the hydrolyzed reaction mixture in a separatory funnel. The ethyl acetate:diethyl ether layer was separated, dehydrated with an anhydrous sodium sulfate column, and evaporated with a rotary vacuum evaporator following N2 flushing for dryness. Finally, hydrolyzed extract containing highly concentrated phenolic acids, mostly sinapic acid, was obtained.
Quantification of sinapic acid content was performed by reversed-phase high performance liquid chromatography (RP-HPLC). The 20 μL sample was injected into an HPLC equipped with a UV detector (325 nm) and NovaPak® C18 column (4 μm, 3.9×150 mm, Waters Co., Tokyo, Japan). Gradient elution was performed using methanol as solvent A and 0.02 M phosphate buffer/methanol (75:25, v/v) as solvent B with the following gradient: hold A:B=5:95 (v/v) ratio for 15 min, 35:65 for 20 min, and 95:5 for the last 17 min. The flow rate of the mobile phase was set at 0.6 mL/min. Sinapic acid was used as a standard for quantification.
The fatty acid composition of fish oil was analyzed using a gas chromatograph (GC, Agilent 6890, Avondale, PA, USA) equipped with a flame ionization detector and SPTM-2560 column (biscyanopropyl polysiloxane, 100 m×0.25 nm×0.2 μm film thickness, Supelco Co., St. Louis, MO, USA) following the modified method of Gan et al. (11).
Tween 20 (0.3%, Polyoxyethylene sorbitan monolaurate) was dissolved in Tris-bis buffer (pH 7.0) by sonication for 1 h. The 10% (w/w) fish oil-in-water emulsion was prepared by pre-mixing 10 g of fish oil and 90 mL of buffer containing 0.02% sodium azide (as a preservative) with Silverson mixer (L4RT, Silverson Machines Ltd., Chesham, UK) for 2 min at 5,000 rpm, followed by a Microfludizer (M-110Y, Microfludics, Newton, MA, USA) at 3,000 PRI. The hydrolyzed extracts (0, 200, 500, and 1,000 ppm) were added to the fish oil-in-water emulsion. Emulsion with 200 ppm of catechin was also prepared for comparison with hydrolyzed rapeseed cake extract.
Oxidation of each emulsion was carried out at 35℃ for 30 days. To measure hydroperoxide values, 0.144 M barium chloride in 0.4 M HCl and 0.144 M ferrous sulfate (freshly made, 1:1= v/v) were mixed and centrifuged for 5 min at 1,143 ×g (11). The supernatant and 4 M ammonium thiocyanate (1:1, v/v) were mixed to prepare the reagent. In a separate test tube, 3 mL of methanol/butanol (2:1, v/v), 20 μL of emulsion, and 30 μL of reagent were mixed, after which absorbance was measured at 510 nm after 20 min. Hydroperoxide value was quantified by the obtained standard curve with hydrogen peroxide (H2O2), and PV was expressed as meq of H2O2/L emulsion
Results and Discussion
TPC of crude extract was 33.03 mg/g while DPPH radical scavenging activity was 42.64% (Table 1). According to Kim and Na (16), TPC of rapeseed cake extracts using water, hot water, methanol, and acetone were 10.0, 32.1, 49.6, and 40.1 mg/g, respectively. Teh and Birch (17) also reported that the TPC of canola cake extract with methanol: acetone:water (7:7:6, v/v/v) after ultrasonic treatment was 19.72-22.05 mg/g. The main phenolic compounds of rapeseed cake are sinapine, sinapoyl glucose, and sinapic acid. Sinapine, the choline ester of sinapic acid, is the main phenolic ester and constitutes about 80% of total phenolics while sinapoyl glucose is the glycoside of sinapic acid as a minor derivative (18). Sinapic acid is a free phenolic acid and is known to be a potent radical scavenger (6,9).
2) Sinapic acid contents (mg/g) of crude and hydrolyzed extracts after concentration with vacuum evaporator and dried under nitrogen.
Crude extract was then fractionated based on its polarities in H2O (I), 30% ethanol (II), 50% ethanol (III), 70% ethanol (IV), and 100% ethanol (V). The highest TPC was detected in fraction II (0.47 mg of SAE /mL). TPCs were in the order of fractions II > III > I > IV > V, and no significant difference was detected between fractions II and III (p>0.05) (Table 2). The antioxidant capacity of each fraction was determined based on DPPH RSA, FRAP, and TEAC, and the results are shown in Table 2. DPPH RSA was the highest in fraction II (88.34%) while fractions III and I had the second and third highest RSAs (86.33% and 85.22%, respectively). DPPH RSAs were not significantly different among fractions I, II, and III (p>0.05), whereas values for fractions IV and V were significantly lower (p<0.05). DPPH RSAs of rapeseed cake extract fractions in the present study were similar to those of Korean rapeseed extract using the same fractionation method in the order of 30% > 50% > H2O > 70% > 100% ethanol (19). The FRAP and TEAC results for each fraction were similar to the DPPH RSAs. Fractions I, II, and III appeared to have higher FRAP and TEACs than fractions IV and V. Specifically, the FRAP of fraction II was higher than that of fraction III (p<0.05), whereas no significant difference in TEAC was observed (p>0.05). These results indicate that phenolic compounds contributed to the antioxidant capacity of each fraction.
Three fractions enriched in TPC (fractions I, II, and III) were combined and hydrolyzed with NaOH. Sinapic acid and sinapine from crude extract and hydrolyzed extract were separated and quantified by HPLC, and the chromatogram shows that sinapine identified in crude extract disappeared in hydrolyzed extract (Fig. 1) since NaOH breaks the esterlinkage in sinapine and liberates sinapic acid and choline during hydrolysis, and the released sinapic acids were extracted using ethyl acetate:diethyl ether and then concentrated in the present study. Sinapic acid content was 0.07 mg/g in crude extract while the content increased up to 18.97 mg/g in hydrolyzed extract (Table 1).
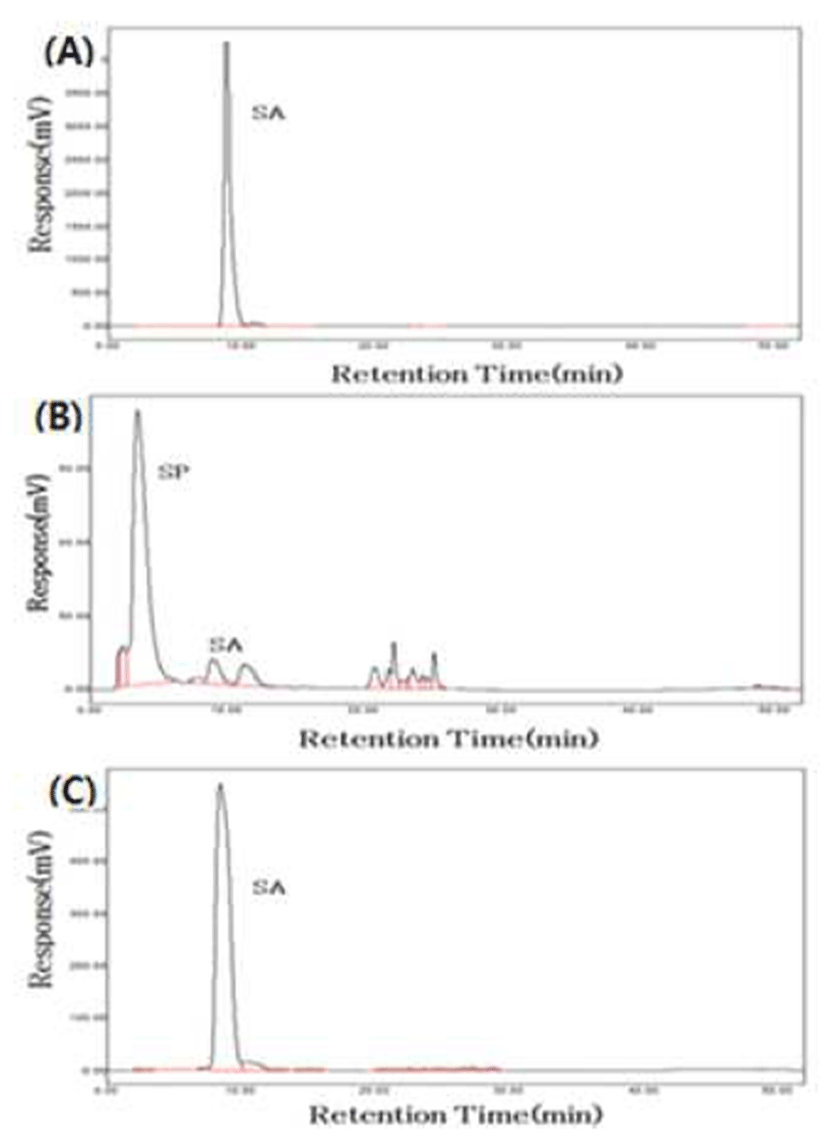
After hydrolysis, DPPH RSA and TPC were increased by 2.2-fold and 21-fold in hydrolyzed extract compared with crude extract (Table 1). Sinapine has been reported to have lower antioxidant potential than other phenolic compounds such as sinapic acid and sinapoyl glucose in rapeseed cake (8). In addition, sinapic acid was shown to be a more effective antioxidant against lipid oxidation than sinapine by inhibiting formation of hydroperoxide (9). Although sinapic acid and sinapine possess the same substitution of one hydroxyl and two methoxyl groups on their phenol rings, the side chain is structurally different having -CHCHCOOH in sinapic acid and -CH=CHCOOCH2CH2N(CH3)3 in sinapine, and the esterified side chains (choline ester) may explain their different radical scavenging activities (20). Kikuzaki et al. (21) reported that DPPH radical scavenging activity is higher in phenolic acids than in their ester derivatives.
Fish oil consisted of 32.29% ω-3 fatty acids, such as DHA and EPA (Table 3), which are highly susceptible to lipid oxidation and generate secondary oxidation products that promote serious problems with respect to food quality and consumer health (22). In this study, oxidation status of the fish oil-in-water emulsion with hydrolyzed extract was evaluated based on hydroperoxide values (PV) as the primary oxidation index. Many foods exist as an oil-in-water emulsion, and the behavior of antioxidants in emulsions depends on chemical structure. Generally, phenolic compounds are plant secondary metabolites possessing one common structural feature of an aromatic ring with at least one hydroxyl substituent. Therefore, phenols inactivate free radicals by hydrogen atom transfer and single electron transfer mechanisms as well as by a transition metal chelation mechanism (23). As a phenolic acid, the chemical structure of sinapic acid improves antioxidant efficiency due to the presence of two methoxy (-OCH3) substitutions at the ortho position, and the presence of a CH=CH-COOH group that improves stabilization of the phenol ring in the molecule (24).
In Fig. 2, PV of the control emulsion was the highest among emulsion groups during the storage period (p<0.05). As the concentration of hydrolyzed rapeseed cake extract in the emulsion increased from 200 to 1,000 ppm, PV significantly decreased (p<0.05). On day 3, PVs of the oil-in-water emulsions at any concentration (200, 500, and 1,000 ppm) of hydrolyzed extract were significantly lower (1.24-6.12 meq/L emulsion) than that of the control (11.04 meq/L emulsion). Moreover, the same concentration (200 ppm) of catechin and hydrolyzed extract cake did not result in significantly different PVs on day 3. When a high concentration (1,000 ppm) was added to the emulsion, hydrolyzed extract showed a significantly reduced PV (p<0.05) during the storage period compared to other emulsions. Patterns of hydroperoxide generation were not distinctively different until day 16 of oxidation.
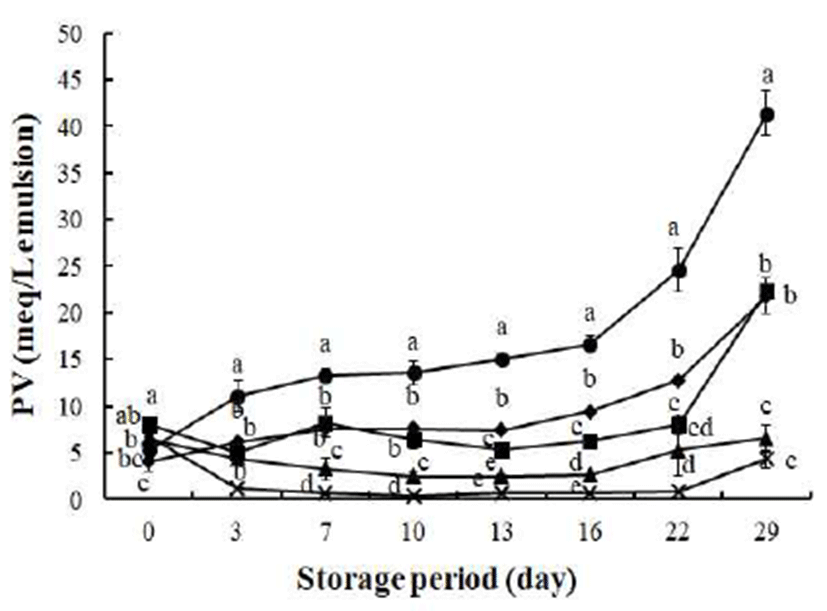
However, after 16 days of oxidation, PV of the control abruptly increased, and the highest PV (41.4 meq/L emulsion) was observed on day 29. Meanwhile, 200 ppm of catechin prolonged the lag phase of hydroperoxide formation until day 22 (7.99 meq/L emulsion), after which PV increased. Thus, the emulsion with 200 ppm of catechin had a lower PV compared to the emulsion with the same concentration (200 ppm) of hydrolyzed rapeseed cake extract up to 22 days (p<0.05). Following this, primary oxidation dramatically increased, and similar PVs were observed in the emulsions containing 200 ppm of catechin and hydrolyzed extract after 29 days. Until 22 days of oxidation, the polyphenol catechin showed slightly better antioxidative activity against hydroperoxide formation than hydrolyzed extract when the same amount (200 ppm) was applied. One possible explanation is that the antioxidative potentials of phenols are dependent on structure, and polyphenols are more efficient antioxidants than monophenol, as a second hydroxyl group in the ortho or para position increases antioxidative activity (24).
In this study, hydrolyzed rapeseed cake extract was applied to a fish oil-in-water emulsion. The extract contained a large amount of sinapic acid released from sinapine by NaOH hydrolysis, and sinapic acid was found to be a better antioxidant and relatively nonpolar compared to sinapine due to esterification of choline. Thiyam et al. (9) reported that sinapic acid exhibited considerable antioxidant activity compared to sinapine on hydroperoxide formation during lipid oxidation. Generally, an oil-in-water emulsion consisting of three parts (lipid droplets, aqueous phase, and oil-water interface) is better protected from oxidation by nonpolar antioxidants than by polar ones, since nonpolar or amphiphilic antioxidants are concentrated at the interface and form a protective membrane around the lipid droplet in the emulsion, whereas relatively polar antioxidants are dissolved in the aqueous phase. Thus, free radicals can be scavenged by lipophilic antioxidants at the interface before they contact the lipid droplet (25).










