Introduction
Zizyphus jujuba Miller belonging to the family Rhamnaceae, is found in many countries especially in the subtropical and tropical regions of Australia, southern and eastern Asia, and Europe (1). Jujube fruit is commonly used as a crude drug in traditional Oriental medicine for many years, and is known to effectively prevent some diseases, such as tumors and cardiovascular diseases (2). Fresh fruit contains abundant amounts of nutrients, especially vitamin C: 10-fold as much as that in other fruits. It is increasingly consumed as fresh or used as food additives and flavoring agents (3). The fruits also have been reported in several food processing products such as compotes, alcoholic beverages, cakes, and bread (4).
The water vapor barrier properties of fruit-based powders, like vacuum-dried jujube powder, are significantly influenced by the presence of moisture. Therefore, an understanding and evaluation of water adsorption behavior of the jujube powder are essential for their application in the development of various types of stable functional food additives or ingredients. To understand the whole adsorption process, the studies may be done by measuring either the equilibrium condition or the rate of approach to equilibrium. The former can be done through determining the water adsorption isotherms; however, isotherms only explain the final equilibrium condition. The latter is done by plotting adsorption curves (weight vs. time at constant relative humidity and temperature). To the best of our knowledge, no information is available on the water vapor adsorption kinetics of jujube powder in any forms.
The objective of the present study was to provide reliable experimental data regarding initial water vapor adsorption behavior of vacuum-dried jujube powder and to identify and test simple relationships to characterize the initial water vapor adsorption kinetics at different temperatures and water activity levels.
Materials and Methods
Fresh jujube (Ziziphus jujuba Mill.), harvested in Gyeongsan, Gyeongbuk, Korea at October in 2010, was purchased from a local market and stored at room temperature before use. Mean moisture content of the sample was 75.29% (wet basis), measured in triplicate by using a hot-air oven (DMC-122SP, Daeil Engineering Co., Seoul, Korea) at 105℃ for 24 h.
After sliced to 3-4 mm in thickness, fresh jujube slices were spread in a single layer on a tray and dried by a vacuum dry oven (VOS-301SD, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) at 60℃ and milled into particle size of 250 μm by a 60-mesh sieve (D-55743, Fritsch GmbH, Idar-Oberstein, Germany). Approximately 1.0 g of jujube powder was placed on an aluminum dish and stored at the selected relative humidities ranging from 31-77% and temperatures ranging from 10-40℃. The relative humidities (RH) were prepared by using saturated-salt aqueous solutions of MgCl2, NaBr, and NaCl, respectively. The increase in weight of the jujube powder was recorded in duplicate every 30 min during the water vapor adsorption process. The water vapor adsorption rates of jujube powder were determined from the weight gain of jujube.
Water activity inside each desiccator after attaining equilibrium with each saturated salt solution at each temperature was determined using a Thermo Recorder (TR-72U, T&D Corp., Nagano-ken, Japan). Temperature dependency of water activity was tested using the Clausius- Clapeyron equation as follows:
where aw is the water activity, T is the absolute temperature (K), ΔH is net isosteric heat of sorption (kJ/mol), and R is the universal gas constant (8.314 J/mol·K).
Water vapor adsorption curves of the jujube powder were obtained by plotting increase in weight (g) due to water vapor sorption expressed in g H2O/g dry solid against adsorption time (h). The rate of constant of water sorption process was determined by a linear form as follows:
where me and m0 are the saturation moisture constant and the initial moisture content, respectively, and k is the rate constant of the sorption process. The inverse of m-m0 vs. inverse of t to get a straight line. The reaction rate constant was determined by taking inverse of the slope value.
The temperature dependency of k value was determined by an Arrhenius-type equation as below:
where k0 is a pre-exponential factor, Ea is the activation energy for hydration process, R is the universal gas constant (8.314 J/mol·K), and T is the absolute temperature (K).
Results and Discussion
Water activity values and net isosteric heat of sorptions for the saturated salt solutions are summarized in Table 1. The water activity values of saturated salt solutions generally decreased with an increase in temperature. The temperature dependency of water activity followed the Clausius-Clapeyron equation. The net isosteric heat of sorption increased with an increase in water activity. The values for saturated salt solutions of MgCl2, NaBr, and NaCl were 3.65, 4.82, and 5.59 kJ/mol, respectively.
| Salt | Water activity | Δ H1) (kJ/mol) | |||
|---|---|---|---|---|---|
| 10℃ | 20℃ | 30℃ | 40℃ | ||
| MgCl2 | 0.35 | 0.34 | 0.31 | 0.31 | 3.65 |
| NaBr | 0.61 | 0.6 | 0.56 | 0.56 | 4.82 |
| NaCl | 0.77 | 0.75 | 0.73 | 0.73 | 5.59 |
Water vapor adsorption behaviors of vacuum-dried jujube powder at different relative humidities and temperatures are shown in Fig. 1. The jujube powder took longer time to reach the plateau and stable moisture contents at higher RH, and it obtained the highest equilibrium moisture contents stored at 40℃ and 75% RH. The sorption curves also indicated that the amount of water vapor adsorbed increased with temperature in general. Higher the temperature, sharper the slope. Even though there were some overlaps of the moisture contents for jujube powder stored at higher temperatures (30 and 40℃) with 75% RH and lower temperatures (10 and 20℃) with 32 or 58% RH, the slope of the water vapor adsorption curves generally increased with temperature. Under each RH condition, the rapid initial moisture sorption followed by a slower adsorption at later stages due to the filling capillaries of the surface of jujube powder (5,6). During the process of moisture sorption preceded, the adsorption rate decreased probably due to the filling of free capillary with water vapor (7). The sorption curves also indicated that the amount of water vapor adsorbed increased with the increases in temperature and water activity in general. Similar findings were reported for soybeans (5) and Inonotus obliquus powders (6).
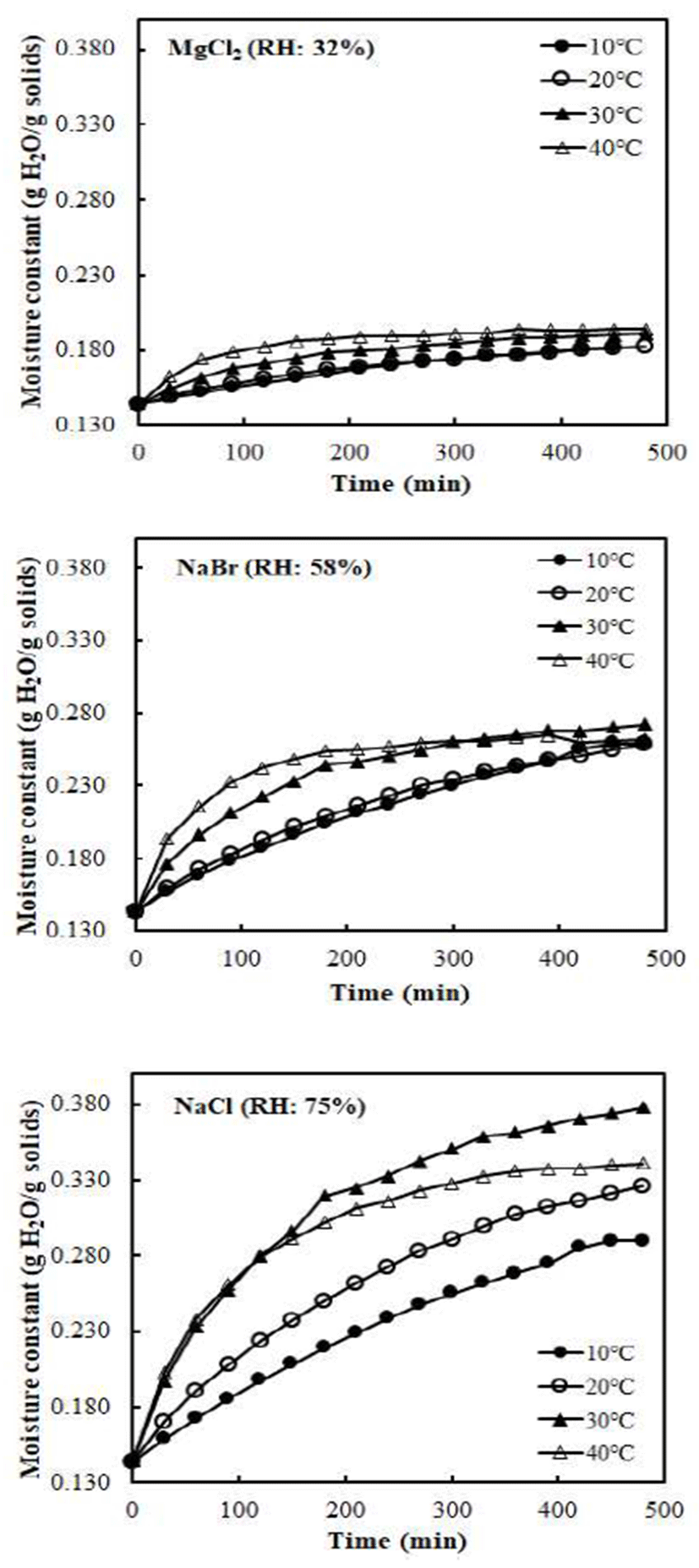
The inverse of m-m0 was plotted against inverse of t following the Eq. B to get a straight line. The reaction rate constant was determined by taking inverse of the slope value. From water vapor adsorption curves, it was found that water vapor adsorption rate varied depending on temperature. Figure 2 shows kinetic plot lines for water vapor adsorption of vacuum-dried jujube powder at different temperatures and RHs. Good linear relationships were observed with high R2-values, indicating that the Arrhenius model is appropriate to describe the temperature dependency of the water vapor adsorption rate of vacuum-dried jujube powders. Regardless of RH conditions, slope of fitted lines was higher at lower temperatures. From the slope value of the lines, water vapor adsorption rate constants (k) were calculated and results are summarized in Table 2. The k values increased as the temperature increased from 10 to 40℃ at each relative humidity condition. The water vapor adsorption rate for all samples increased with temperature, which is in good agreement with the finding of other researchers (5,6). The range of R2-values was from 0.977 to 0.999, indicating a good linear relationship. Finally, the temperature dependency of k values could be described using the Arrhenius model.
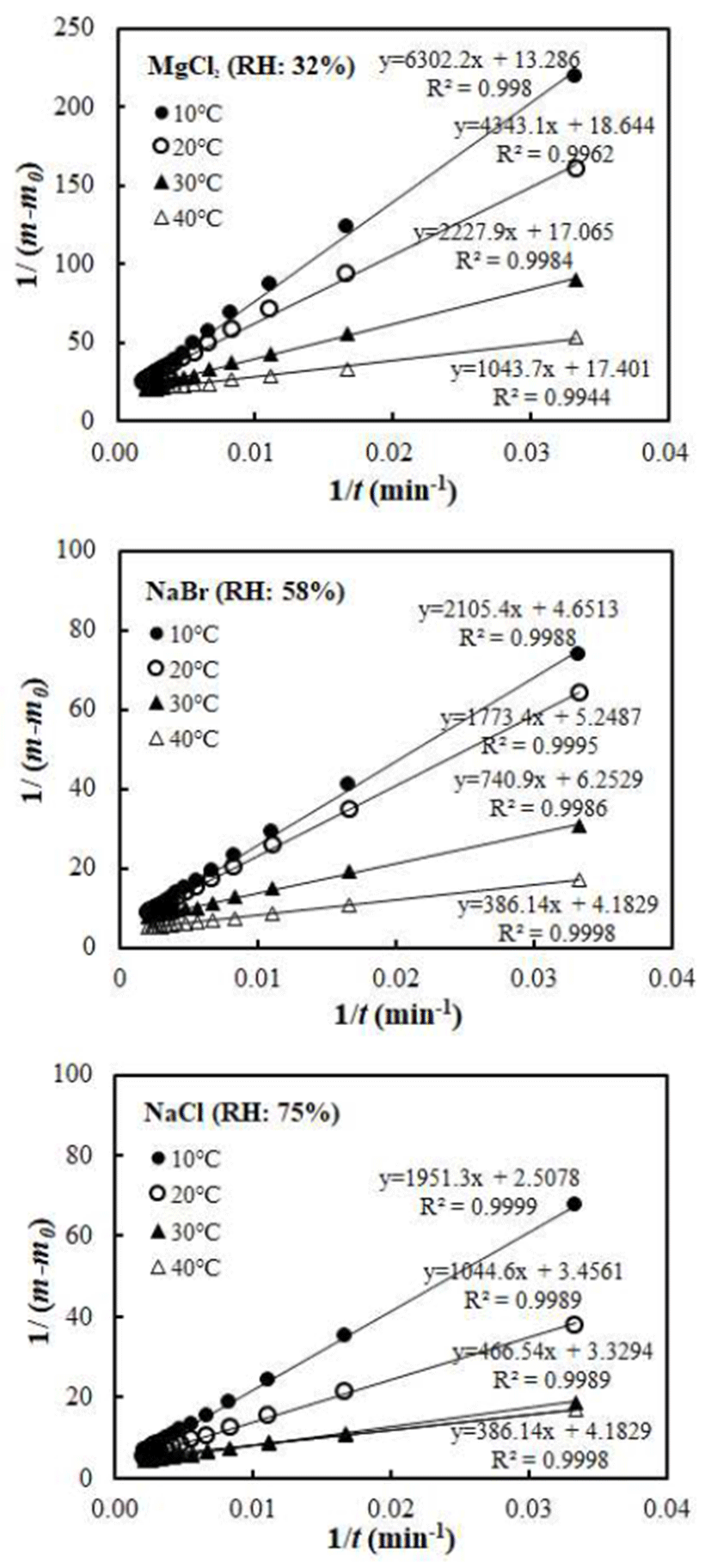
By using the Arrhenius-type equation in Eq. C, temperature dependency of the water vapor adsorption rate of vacuumdried jujube powder was determined. The temperature dependency of water vapor adsorption rate is shown in Fig. 3. The good linear relationship with high R2-values of 0.995, 0.961, and 0.978, respectively for 32, 58, and 75% RH conditions was obtained. This result indicated that Arrhenius model is appropriate to describe the temperature dependency of the water adsorption rate of jujube powder. The Arrhenius kinetic parameters, activation energy (Ea), and pre-exponential factor (k0) for the water vapor adsorption by the jujube power were determined by linear regression method and results were summarized in Table 3. The activation energy ranged from 50.90 to 56.00 kJ/mol depending on the RH. The values are within the range of those reported for the hydration of food stuffs (5,8,9). The activation energy in the middle range of water activity (NaBr) was higher than that of lower and higher ranges of water activity. This indicates that the rate of water vapor sorption by the vacuum-dried jujube powder is more temperature sensitive at the middle range of water activity. Similar observation was made in Inonotus obliquus mushroom powders (6).
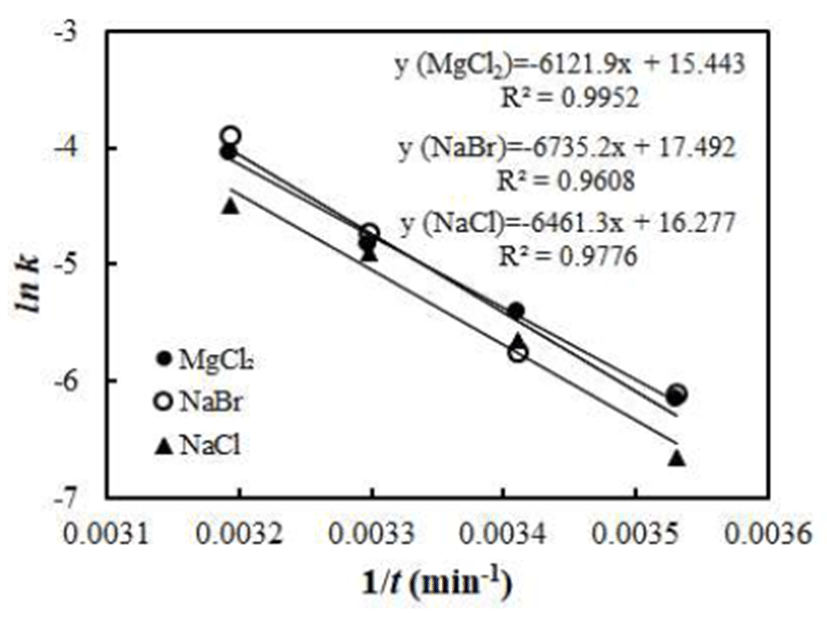
| Saturated salt solutions | k0 (g H2O/g dry solid/min) | Ea (kJ/mol) | R2 |
|---|---|---|---|
| MgCl2 | 5.093×106 | 50.9 | 0.995 |
| NaBr | 3.951×107 | 56 | 0.961 |
| NaCl | 1.173×107 | 53.72 | 0.978 |
Numerical values of kinetic parameters are influenced by the reaction conditions such as reactant concentration, pH, water activity, and so on; however, the Arrhenius kinetic parameters (Ea and k0) for water vapor adsorption rate usually show linear relationship between ln k0 and Ea, which is known as kinetic compensation effect (6,10). The relationship between Ea and k0 for water vapor adsorption rate of vacuum-dried jujube powder is shown in Fig. 4. This result shows a highly linear relationship between the kinetic parameters, which indicates a systematic variation of one parameter depending on others. This is known as a kinetic compensation effect (11), which is usually observed in a family of reactions with same reaction mechanism. This suggests that Ea and k0 are not independent of one another and any change in the Ea is compensated by changes in k0.
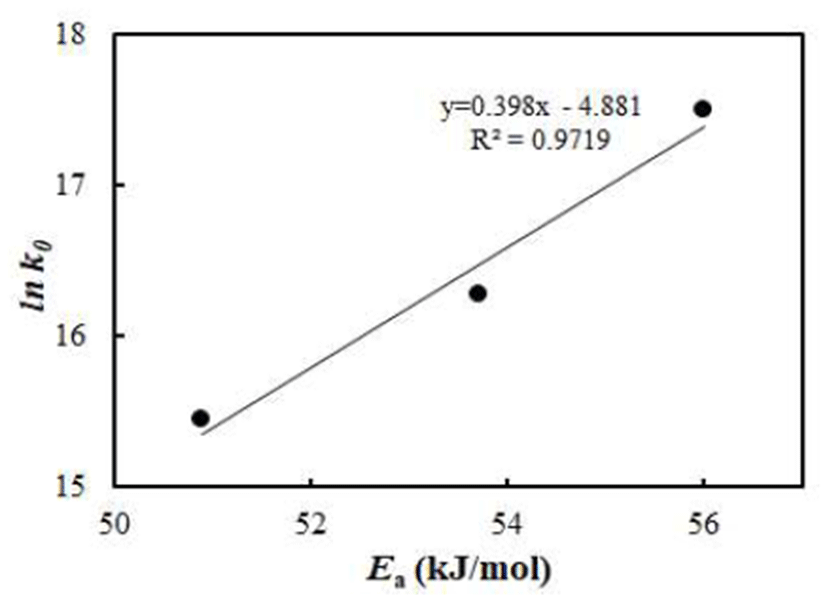
This unique relationship can be used for the prediction of reaction rates or other kinetic parameters (12). This linear relationship implies the existence of a unique temperature called isokinetic temperature (Tc) (11), where the rate constants of all reactions in the series have the same value (7), mathematically reflecting the dependence of activation energy on the temperature (13,14). The isokinetic temperature is usually calculated based on the slope from the linear relationship shown in Fig. 4, using the relationship of slope=1(R·Tc). From the slope, the isokinetic temperature of water vapor adsorption for vacuum-dried jujube powder was determined and found to be 302.51 K, which is in good agreement with for some selected proteins and starch foods (15) with a high degree of linear correlation.










