Introduction
Salmonella has been one of the most prevalent foodborne pathogens, ranking higher in incidence than Campylobacter, E. coli O157:H7, Listeria monocytogenes, Shigella, Vibrio, Yersinia, Cryptosporidium, and Cyclospora (1,2,3). Approximately 1.4 million cases of Salmonella infections have been reported in the United States annually, and the annual cost for medical treatment and lost productivity have been estimated to be 0.5 to 2.3 billion dollars (1). The major serotypes causing Salmonella infections are S. enterica serovar Typhimurium (20%), Enteritidis (15%), Newport (10%), Javiana (7%), and Heidelberg (5%), which combined account for 56% of all human cases (3,4).
Salmonella outbreaks has been frequently associated with meats, poultry, eggs, milk, and dairy products. However, outbreaks associated with poultry products have drawn the most attention because poultry consumption has consistently increased whereas beef consumption has consistently decreased (56.8 lbs of poultry per capita vs 64.5 lbs of beef per capita annually; poultry per capita consumption 2002). In addition, although several prevention strategies have been employed in the poultry industry, it is hard to prevent contamination during processes (5). Rose et al. (6) found that 14.4% of poultry products randomly collected from a federally inspected processing plant were contaminated with Salmonella. In addition, another study (7) also found that 35% of ground poultry products were contaminated with Salmonella. Therefore, on-site applicable rapid detection methods need to be performed in poultry plants for the inspection of poultry products (8,9).
A considerable number of studies have been focused on the development of on-site applicable rapid detection methods in order to overcome the limitations of existing rapid detection methods (10,11,12). Recently, our research group has developed a gold biosensor combined with a light microscope imaging system (LMIS) for foodborne pathogen detection. Previous studies demonstrated that the gold biosensor combined with LMIS was successfully able to detect Listeria monocytogenes and Escherichia coil O157:H7 in food (5,12,13). Using the same principle and methodologies, this system will be expanded to detect Salmonella in poultry as on-site applicable rapid method.
Following regulations in the US, poultry carcasses should be chilled to 4.4℃ or lower for a certain period of time to ensure a high quality and safe product (8). Therefore, all the processes in poultry plants are performed in cold temperatures (approximately 4~6℃) in order to inhibit or minimize the microbial growth during processes (14). Cold temperatures delay or injure Salmonella growth because of the extension of its lag phase (15). The sluggish growing and/or viable-but-non-culturable state under unfavorable temperatures might provide false-negative results, so Salmonella needs to be enriched or rejuvenated prior to employing a gold biosensor combined with LMIS. Therefore, the objectives of this study was to determine the best enrichment medium for rejuvenating and increasing the number of Salmonella injured from cold temperatures for the employment of a gold biosensor combined with LMIS.
Method and Materials
Two strains of nalidixic-resistant Salmonella enterica serovar Typhimurium (ATCC13311), and Enteritidis were obtained from Auburn University (Auburn, AL, USA). Each strain of Salmonella was cultivated in 20 mL of tryptic soy broth (TSB, Difco Laboratories, Sparks, MD, USA) for 16 hr at 37℃ with agitation. After incubation, the cultures were washed three times with phosphate buffered saline (PBS, pH 7.2, Sigma-Aldrich Co., St. Louis, MO, USA) by centrifugation at 5,000×g for 5 min. The collected bacterial cells were re-suspended in PBS and the bacterial concentration was adjusted to 109 CFU/mL using a pre-constructed standard curve determined by the optical density at 640 nm. Salmonella mixture was prepared by mixing equal amounts of the adjusted bacterial suspension prior to serial dilution.
Buffered peptone water broth (BPW, EMD Science, Darmstadt, Germany), lactose broth (LB, EMD Science), brain heart infusion broth (BHI, EMD Science), universal pre-enrichment broth (UPB, Difco Laboratories), nutrient broth (NB, EMD Science), and TSB were prepared as pre-enrichment media following the manufacturers’ recommendation. Brilliant green broth (BG, Difco Laboratories), rappaport-vassiliadis R10 broth (RV, Difco Laboratories), selenite cystine broth (SC, Difco Laboratories), selenite broth (SB, Difco Laboratories), and tetrathionate brilliant green broth (TBG, Difco Laboratories) were prepared as selective enrichment media following the manufactures’ recommendation.
Chicken skins were randomly collected from Koch Food Company (Montgomery, AL, USA) and cut into approximately 25 g portions. The chicken skin was washed with 200 ppm chlorine solution (Sigma-Aldrich Co.) and washed 10 times with sterilized deionized water to eliminate existing microorganisms in chicken. Two hundred μL of Salmonella mixture (1,000 CFU/200 μL) were spread onto chicken skins for inoculation. An equal amount of PBS buffer was applied to chicken as a negative control. After attachment of Salmonella for 30 min under a safety cabinet, each inoculated chicken skin was put into a sterile stomach bag containing 100 mL of non-selective or selective media with 100 ppm nalidixic acid then placed at 4℃ for 24 hr and blended in a stomacher (Seward 400, Seward Inc., Bohemia, NY, USA) at 260 rpm for 2 min. The blended suspension was transferred to an Erlenmeyer flask for incubating at 37℃ in an orbital shaker at 250 rpm. The bacterial number was determined by spread-plate method using tryptic soy agar (TSA, Difco Laboratories) plates containing 100 ppm nalidixic acid at 2, 4, and 6 hr. The medium that promoted the most growth of Salmonella was chosen as the bacterial enrichment medium for further study.
For the selection of the best media, various concentrations of Salmonella mixture were inoculated onto chicken and enriched in BHI and BG media. Two hundred μL of Salmonella mixture containing 10, 50, 100, 500, and 1,000 CFU were inoculated on a chicken sample, respectively, followed by the same procedures described in the section above. The medium that promoted the most growth of Salmonella was chosen as the best enrichment medium for the employment of a gold biosensor combined with LMIS.
Results and Discussion
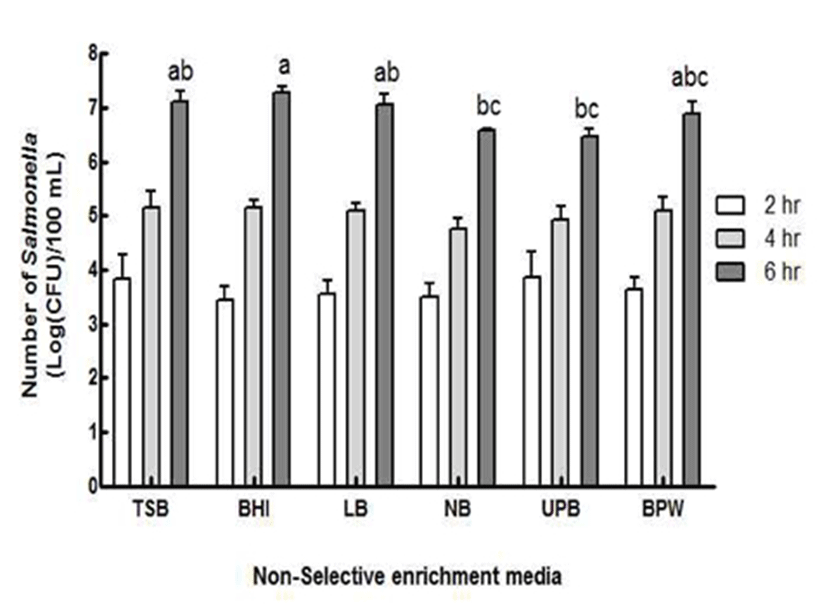
Poultry plants should be chilled to 4.4℃ or lower for a certain period of time, because the minimum growth temperature of Salmonella in poultry was reported as 5℃ (14). Therefore, a similar temperature condition was introduced in this study by placing chicken at 4℃ for 24 hr after the inoculation of Salmonella. The media’s effect on Salmonella growth after storing was then investigated using a series of non-selective enrichment media including TSB, BHI, LB, NB, UPB, and BPW (Fig. 1). As time increased, the concentration of Salmonella increased approximately 3~4 log CFU/chicken, 5 log CFU/chicken, and 6~7 log CFU/chicken at 2, 4, and 6 hr, respectively. However, there were no significant differences among non-selective media both 2 and 4 hr (p<0.05). Significant differences in the growth number of Salmonella was obviously noticed at 6 hr. The number of Salmonella enriched in BHI at 6 hr was significantly greater than the number of Salmonella enriched in NB and UPB at 6 hr (p<0.05). Although Salmonella enriched in TSB, BHI, BPW and LB did not demonstrate any significant differences among the media, BHI was selected as the best non-selective media because it caused the highest increase in the number of Salmonella. The number of Salmonella increased up to (7.3±0.2) log CFU/chicken, followed by TSB media [(7.1±0.4) log CFU/chicken]. The result of this study was in good agreement with the result of Boer (17), which demonstrated that BHI and TSB exhibited significantly greater increases than other non-selective media. Since TSB and BHI contain similar nutrients such as casein, glucose, disodium phosphate and sodium chloride, except the meat substrate in BHI and soybean in TSB, the bacterial number enriched in both media did not show significant differences. Although UPB also possesses a similar nutrient composition to TSB and BHI, except magnesium sulfate, the bacterial numbers enriched in UPB were significantly lower than those enriched in TSB or BHI, presumably due to the relatively lower pH of UPB (pH 6.3) when compared to TSB or BHI (pH 7.3). Therefore, among the six non-selective media, BHI was selected as the most efficient non-selective media for further study.
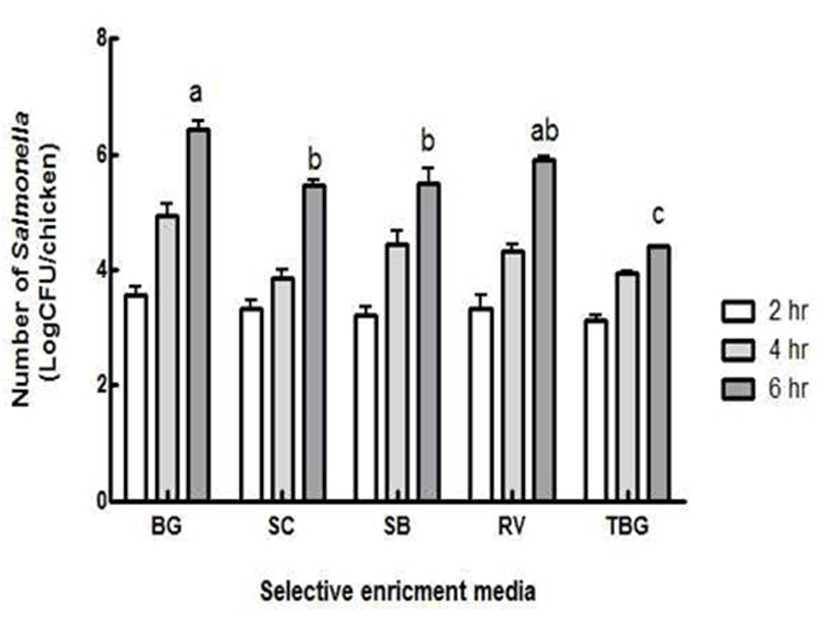
Since Salmonella co-exists with other competitive foodborne pathogens, it needs to be enriched using selective media only by multiplying the target pathogens while inhibiting other foodborne pathogens (17). Salmonella was enriched in BG, SC, SB, RV and TBG to determine the best efficient medium for Salmonella recovery injured in a cold environment (Fig. 2). As time increased, the number of Salmonella increased proportionally. However, there were no significant differences among the media until 4 hr (p<0.05). Unlike other media, the number of Salmonella enriched in SC did not increase obviously between 2 hr and 4 hr. Other media provided similar increase patterns in the number of Salmonella, which was proportional to the increase in time. At 6 h-enrichment period, it was noticed that there were significant differences among the selective media. The number of Salmonella in BG [(6.4±0.3) log CFU/chicken] was significantly greater than the number of Salmonella in SC [(5.5±0.2) log CFU/chicken], SB [(5.5±0.6) log CFU/chicken] or TBG [(4.4±0.1) log CFU/chicken] (p<0.05). Although Salmonella in RV [(5.9±0.1) log CFU/chicken] was not significantly different from that in BG, BG media was demonstrated to be the most efficient selective media for Salmonella growth after cold injury. This result was in agreement with other studies (18,19,20,21,22).
Selected enrichment media (BHI and BG) were compared further for the determination of the best enrichment medium for Salmonella injured from cold temperatures. Various concentrations of Salmonella inoculated on chicken were enriched up to 6 hr and compared its detected number at each concentration (Fig. 3). During 2 hr incubation, the detected number of Salmonella increased as the inoculated concentration of Salmonella increased. Although, Salmonella was detected starting from the inoculated concentration at 50 CFU/chicken, however, no obvious increases in the number of Salmonella were found. Presumably, Salmonella showed a relatively longer lag phase due to injury from the cold temperature for 24 hr. In addition, there were no significant differences in the number of Salmonella enriched in BHI and BG media.
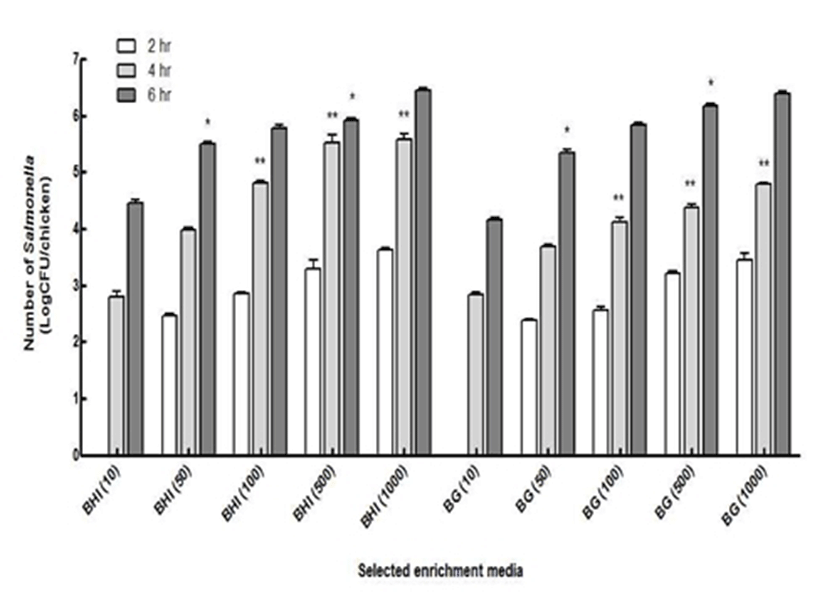
From the 4 hr incubation, the significant differences were observed starting from the inoculated concentration of 100 CFU/chicken between two media (p<0.05). The number of Salmonella enriched in BHI was significantly greater than the number of Salmonella enriched in BG. The maximum growth of Salmonella was found in BHI media with (5.6±0.2) log CFU/chicken from an initial concentration of 3 log CFU/chicken. Furthermore, 4 hr incubation in both BHI and BG enriched the number of Salmonella up to the detectable levels (3 log CFU/chicken) of the gold biosensor combined with LMIS method except for 10 CFU/chicken (5). During 6 hr incubation period, the number of Salmonella enriched in BHI starting with 50 cells was significantly greater than the number of Salmonella enriched in BG (p<0.05). In the meantime, the number of Salmonella enriched in BHI starting with 500 cells was significantly lower than the number of Salmonella enriched in BG (p<0.05). Except for those instances, there were no significant differences between BHI and BG media among various concentrations of Salmonella. Even at the smallest inoculation (10 CFU), the Salmonella concentration was reached up to approximately 4 log CFU/chicken, which was a detectable level for the developed biosensor method. In conclusion, BHI medium was selected as the most efficient enrichment medium for Salmonella growth injured from cold temperatures during processing or storage.
요 약
본 연구의 목적은 가금류에서 문제가 되고 있는 살모넬라 신속검출을 위해 광학현미경 기반 이미징 시스템 적용에 앞서서 냉장온도로부터 손상된 살모넬라를 증균시키기 위한 최적의 배지를 선정하는 것이다. 대표적인 식중독 유발균주인 S. Typhimurium과 S. Enteritidis를 닭에 도말 하였고 4℃에서 24시간 동안 저장한 후 BPW, LB, BHI, UPB, NB, 그리고 TSB 배지와 BG, RV, SC, SB 그리고 TBS 선택배지에 각각 6시간 동안 배양하였다. 배양 후, 2시간마다 살모넬 라 균수를 측정하였고 그 결과 BHI와 BG를 선택되었다. 최종적으로 최적의 배지 선택을 위해 다양한 농도의 살모넬라를 닭에 각각 도말 후 6시간 동안 배양하면서 균수를 변화를 측정하였다. 그 결과 BHI가 냉해로부터 손상된 살모넬라를 증균시키기 위한 가장 최적의 배지로 선정되었으며 본 연구의 결과는 광학현미경 기반 이미징 시스템을 활용한 신속검출법 적용을 위한 증균배지로 이용될 것이다.










