Introduction
Prostate cancer is the most malignant cancer and 70% of prostate cancer patients develop bone lesions, suggesting that metastases are a significant event contributing to prostate cancer morbidity and mortality (1,2). The process of metastasis has been described as a series of events. The initial step involves detachment of tumor cells from the primary tumor mass. Detached tumor cells adhere to the extracellular matrix (ECM), most likely through cell surface receptors. ECM proteins are degraded by tumor cell produced proteolytic enzymes. Tumor cells can migrate through proteolytically modified ECM and penetrate into the blood or lymph (3,4). Tumor cells that survive in the blood are carried to distant sites where they attach to basement membrane. Motility and invasion into the new target tissue results in the formation of a secondary tumor (4). This event occurs repeatedly during tumor invasion and acquisition of increased adhesiveness, and enhanced motility and invasiveness are likely to contribute to cancer progression (3,5,6). These stages appear to be determined both by characteristics inherent to the tumor cell such as hormone receptor status, production of proteolytic enzymes, expression of cell adhesion molecules and increase in motility (7,8) as well as a number of growth factors, matrix molecules and cytokines in the metastatic microenvironment (9).
Epidemiological studies have shown that the incidence of prostate cancer is lower in humans who eat large amount of cruciferous vegetables, such as broccoli, cauliflower, cabbage and Brussels sprouts (10,11). Cruciferous vegetables contain indole 3-carbinol (I3C) which is chemically unstable in acid environments and rapidly converted to a variety of polymeric products in the stomach (12-14). Among them, 3,3’-diindolylmethane (DIM) is a major acid catalyzed metabolite. Since I3C readily converted to DIM under a variety of biological conditions, the biological effects of I3C may be attributable to both I3C and DIM (12). I3C has been suggested to reduce of breast tumor growth (15,16) and incidence of lung metastasis in experimental animals (17). DIM has an effect on the inhibition of angiogenesis and the growth of transplantable human breast carcinoma in athymic nude mice (18). DIM was also reported to induce apoptosis and inhibit growth of prostate cancer cells (12,13). However there is a little information if DIM inhibits the metastasis of prostate cancer.
In present study, we examined the inhibitory effects of DIM on the adhesion, migration and invasion of highly invasive PC3 and DU145 human prostate cancer cell lines. These data suggest that DIM may prove to be a therapeutic intervention for prostate cancer progression.
Materials and Methods
The PC3 and DU145 human prostate cancer cell lines were originally derived from bone and brain metastases, respectively, and obtained from ATCC (Rockville,MD). All cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Gaithersburg), supplemented with 3.0 g/L glucose, 3.7 g/L sodium bicarbonate and 10% fetal bovine and incubated in a humidified incubator at 37°C and 5% CO2. Cultures used in subsequent experiments were at <20 passages.
3,3’-Diindolylmethane (DIM, Sigma Chemical Co., St. Louis, MO, USA) was dissolved in DMSO to prepare DIM stock solution. The final concentration of DIM stock solution was 0.1 M. The final concentration of DMSO in the culture medium was 0.1% (v/v).
Plates (12-wells) were coated with 30 μg of Matrigel (Becton Dickinson, Bedford, MA). To block nonspecific binding sites, Matrigel coated culture plates were rehydrated with serum free DMEM containing 0.1% BSA for 90 min at 37°C and then washed with same medium. PC3 and DU145 cells are trypsinized and resuspended in serum free DMEM containing 0.1% BSA. PC3 cells were seeded at 2×105/well and DU145 cells were seeded at 1×105/well in the presence of DMSO. 1, 10, or 50 μM DIM and incubated for 90 min at 37°C and 5% CO2. At the end of 90 min, the medium was removed and the cells were washed gently two times with PBS to remove unattached cells. The attached cells were harvested by trypsinization, resuspended and counted. In some experiment, cells were seeded into Matrigel coated wells in the presence of DMSO or 50 μM of DIM and incubated for 15, 30, 60 or 90 min at 37°C and 5% CO2. To examine the effect of pretreatment of DIM, PC3 cells were treated with DMSO, 1, 10 or 50 μM of DIM and incubated for 24 hr at 37°C and 5% CO2 before the adhesion assay. The pretreated cells were used for adhesion assay without DMSO or DIM during the assay. Each assay was performed in triplicates and repeated in three independent experiments. Values were expressed as the average of triplicate experiment.
Motility was assessed using a scratch wound assay (19). Cells were seeded into 6 well cell culture plates at a concentration of 2×105 cells and cultured in 10% FBS DMEM to near confluence. Confluent monolayer was carefully wounded using a sterile pipette tip and washed cellular debris gently with PBS. The wounded monolayer was incubated in 10% FBS DMEM containing 16 μg/mL fibronectin and DMSO, 1, 10 or 50 μM of DIM for 24 or 48 hr. Migrating cells were examined under 10X by phase contrast microscope and photographed.
The ability of cells to migrating across Matrigel barrier (invasion) was determined by the modified Boyden chamber. Briefly, Boyden chambers were assembled using 8 μm Falcon transwell inserts (Becton Dikinson, Bedford, MA) as the upper chamber and the 12 well plates as the lower chamber. Matrigel was applied to the insert at 25 μg and left overnight to dry in a laminar flow fume hood. The insert was re-hydrated with serum free DMEM for 90 min at 37°C and 5% CO2. Following rehydration, Matrigel coated inserts were washed with the same medium. Exponentially growing prostate tumor cells were harvested and resuspended in 0.1% BSA DMEM. PC3 cells were seeded at 2×105 and DU145 cells were seeded at 1×105. DMSO, 1, 10 or 50 μM of DIM was carefully added to the upper chamber. Twenty microgram of fibronectin (for PC3 cells) or 10% FBS (For DU145 cells) was added as a chemoattractant in the lower chamber. The Boyden chamber was incubated for 24 hr at 37°C and 5% CO2. At the end of incubation the cells in the upper chamber were removed gently. Cells that had transverse the Matrigel and attached to the lower surface of the insert were fixed with 10% formalin and stained with 0.5% crystal violet. Inserts were examined under 10X or 20X by phase contrast field microscopy and photographed. To examine the effect of pretreatment of DIM, PC3 cells were treated with DMSO, 1, 10 or 50 μM of DIM and incubated for 24 hr at 37°C and 5% CO2. The invasion assay was performed without DMSO or DIM. Values for invasion were expressed as the number of migrated cells per microscopic field. Four fields were counted. Each assay was performed in triplicates and repeated in three independent experiments. Values were expressed as the average of triplicate experiments.
Results and Discussion
The process of metastasis consists of a series of interactions between tumor cells and their environment. The ability of malignant tumor cells to invade host tissues contributes to their locally destructive nature and can play an important role in their metastatic spread (4-6). Therefore, inhibition of adhesion, motility or invasion may be therapeutic target of metastasis. We chose two human prostate cancer cell lines, PC3 and DU145, derived from bone and brain metastases, respectively, and investigate the effect of DIM on the adhesion, migration and invasion. It was reported that DIM at a concentration of 150 μM did not cause a significant increase in the LDH levels compared with the control in the LDH assay which is the most widely used for measuring cellular lysis (20). We, therefore, assume that no toxic effects were manifested at the concentration used in this study.
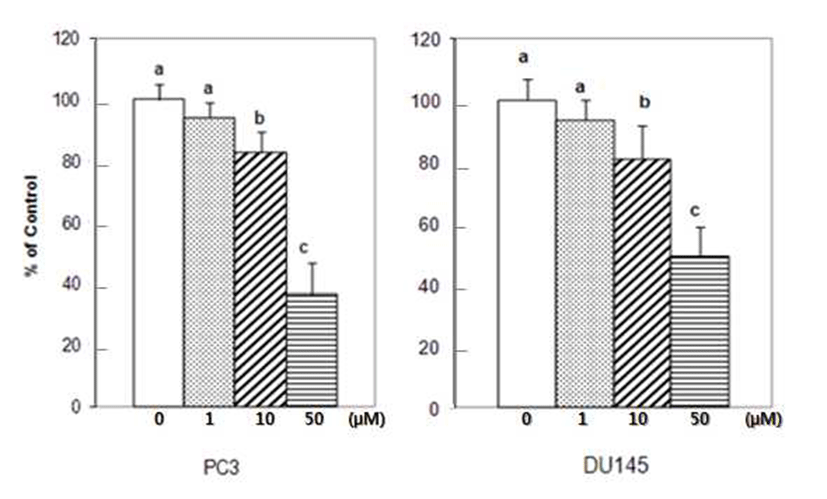
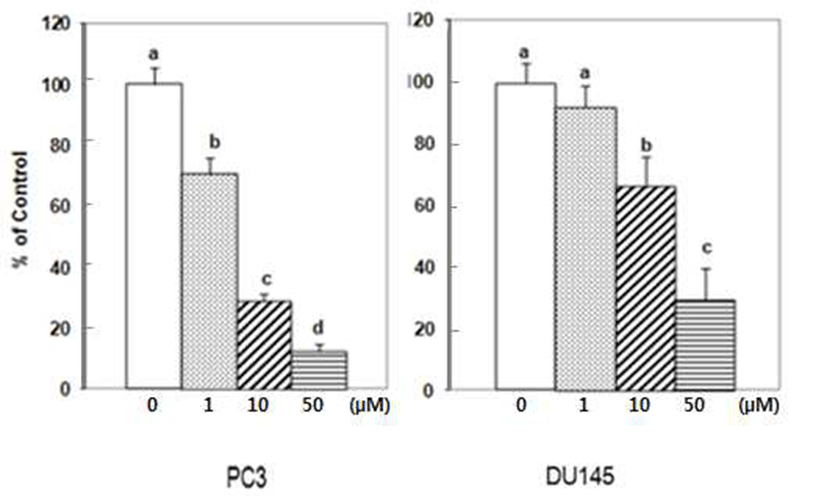
The initial invasive action of metastatic cells requires tumor cells and the ECM interaction. Adhesion assays performed for 90 min, over 85% of the tumor cells attached to Matrigel coated wells. Treatment of 1 μM of DIM reduced adhesion of PC3 cells by 6%. At the higher concentration of 10 μM and 50 μM of DIM, PC3 cell adhesion to Matrigel coated wells was reduced by 17.2 and 65.5% respectively (Fig. 1).
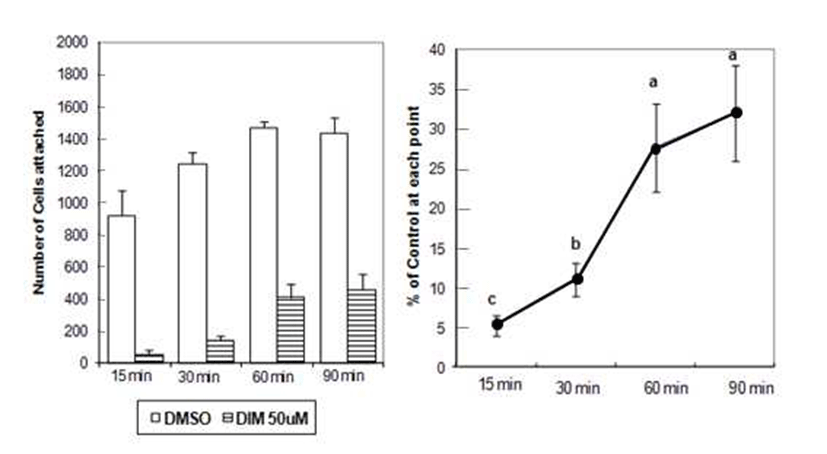
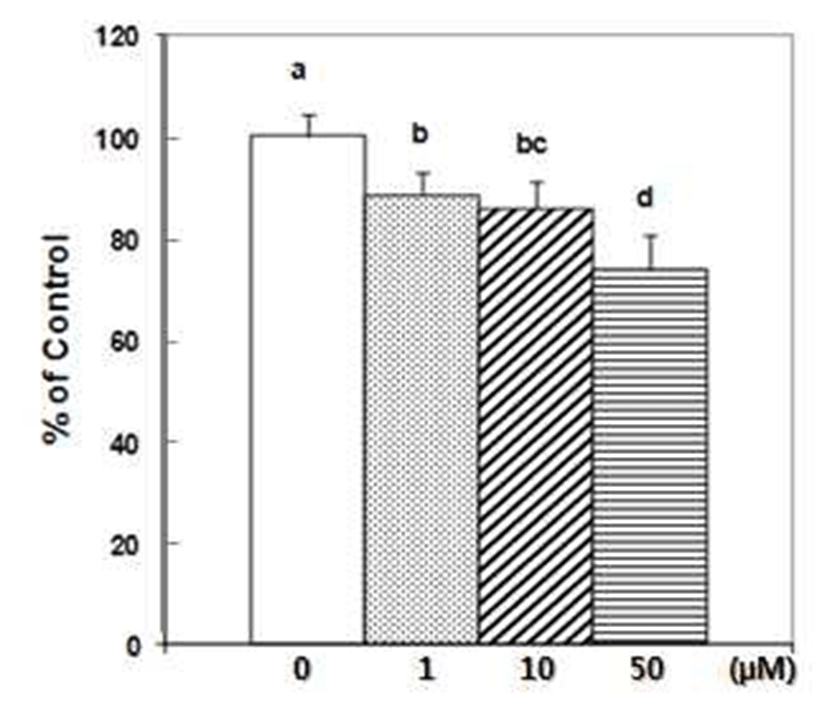
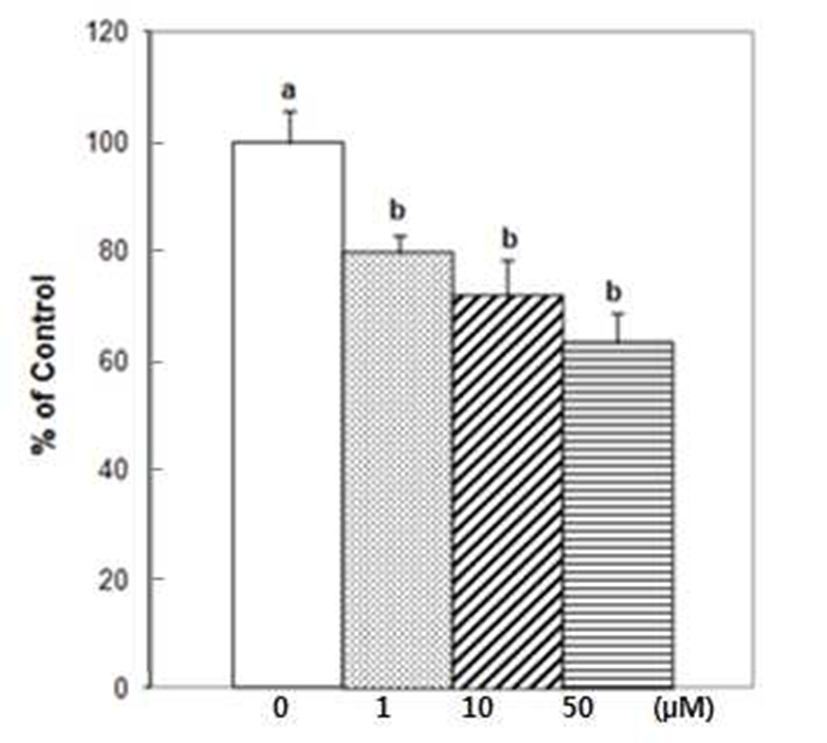
DU145 cell adhesion was also reduced by DIM. DIM at concentration of 50 μM reduced adhesion of DU145 cells by 50% (Fig. 1). Several studies have indicated that prostate cancer cell lines express different number of protein familyeg. integrins- that are known to mediate interactions between cells and ECM proteins (21-24). I3C has been reported to inhibit the adhesion, migration and invasion of breast cancer cells by modulating the expression of a series of signaling proteins associated with adhesion, invasion and migration (25). When adhesion assay was done for 15 min with DIM, 50 μM of DIM inhibited adhesion of PC3 cells by 95% (Fig. 2). PC3 cells were pretreated with DIM for 24 hr before adhesion assay and adhesion assay was performed without DIM. Pretreatment of PC3 cells with 50 μM of DIM reduced adhesion by 27%. Sung and Feldman (26) reported that pretreatment of vitamin D3 for 72 hr reduced adhesion of PC3 and DU145 cells about by 30% and this effect of vitamin D3 is through modulation of cell surface adhesion molecules. However, in the present research, inhibitory effect of DIM during the adhesion assay (for 90 min) was higher than that of pretreatment of DIM for 24 hr (Fig. 1 vs 3). This suggests that DIM may reduce adhesion of PC3 cells by inhibiting both interaction between cancer cells and Matrigel coated membrane and expression of proteins which play an important role in adhesion.
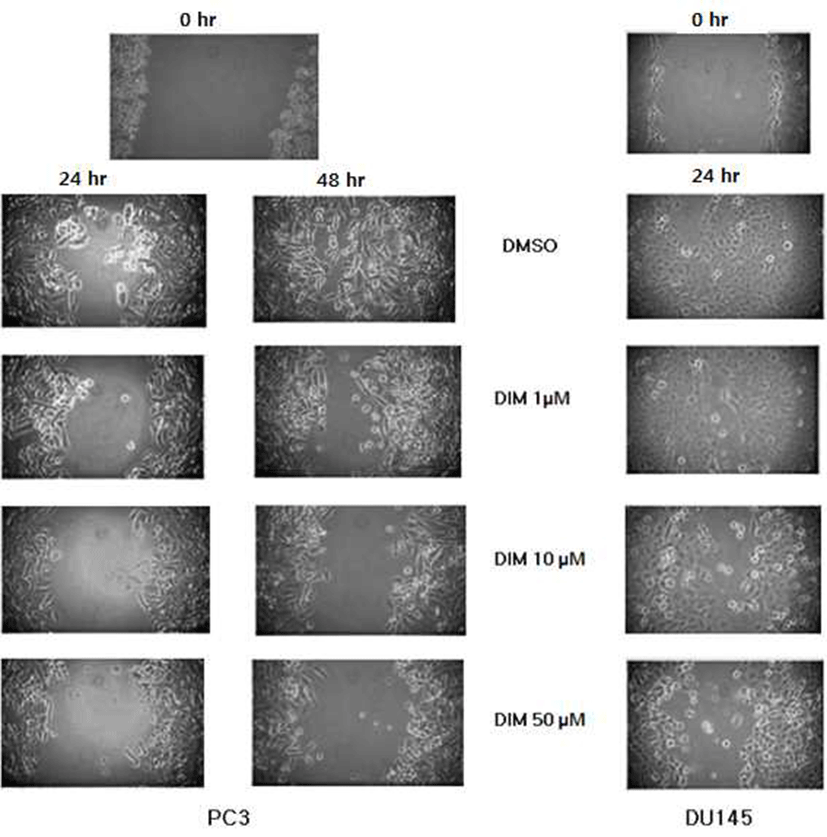
DIM reduced migration of prostate cancer cells. Wound assay with PC3 cells was performed for 48 hr, since PC3 cells treated with DMSO did not cover the whole wound area for 24 hr. In our motility model, DIM reduced migration of prostate cancer cells dramatically. Migration of PC3 cells was reduced even by 1 μM, and 50 μM of DIM inhibited migration of PC3 cells more strongly. DIM at concentration of 10 μM and 50 μM reduced migration of DU145 cells (Fig. 4). Tumor metastasis involves extensive interaction of the invasive cancer cells with host and such interactions promote degradation of the ECM by specialized proteolytic enzymes produced by cancer cells (27,28). In vitro capacity of cells to migrate correlates with their invasive behavior in vivo (29).
To successfully penetrate the Boyden chamber, cells must successfully adhere, degrade and transverse the Matrigel coated insert(30). DIM reduced invasion of PC3 cells and DU145 cells. DIM at concentration of 10 μM reduced invasion of PC3 cells by 70% and 50 μM of DIM by 90% (Fig. 5). DIM at concentration of 50 μM reduced invasion of DU145 cells by 70% (Fig. 5). Pretreatment of PC3 cell with DIM for 24 hr before assay reduced invasion of PC3 cell by 37% (Fig. 6). Similar to adhesion assay, pretreatment with DIM for 24 hr was less effective than treatment of PC3 cells during invasion assay. This suggests that DIM may affect the process of adhesion, degradation and transverse the Matrigel coated insert, in addition to expression of the molecules which need for the process such as adhesion molecules and proteolytic enzymes.
In the present study, we detected similar trends in response of PC3 and DU145 cells to DIM. But there was also small cell line difference in responses to DIM. For example, although adhesion to Matrigel of both cell lines showed similar response to DIM, migration and invasion by PC3 cells seemed more sensitive to DIM. It is interesting when we consider the response of proliferation curve of both cell lines to DIM. It was reported that the IC50 values for the cells treated with DIM were 40 μM for the PC3 cells and 20 μM for the DU145 cells (20). In summary, the current study has established that DIM can diminish prostate cancer cell adhesion, motility and invasion, and suggest that DIM may be an effective therapy in addition to traditional chemotherapy.
Examination of the in vivo effects of DIM on prostate cancer metastasis will help to determine whether administration of DIM is truly a promising treatment to decrease invasive and metastatic disease.
요 약
본 연구는 3,3’-diindolylmethane(DIM)이 인간전립선암 세포인 PC3 세포와 DU145 세포의 부착, 이동, 침윤에 미치 는 영향을 살펴보았다. DIM은 PC3와 DU145 세포의 부착 DU145 세포에 대한 억제효과보다 큰 것으로 관찰되었다. DIM은 150 μM 까지 세포 독성이 관찰되지 않은 것으로 보고되고 있으므로 본 연구결과 DIM은 세포 독성이 없는 수준에서 인간 전립선암 세포인 PC3와 DU145 세포의 부 착, 이동, 침윤을 효과적으로 억제하여 전립선암 전이 억제 제로 이용될 수 있을 것으로 사료된다.










