Introduction
Red pepper (Capsicum annuum L.) are widely cultivated in many countries and is widely used in processed foods, such as spices and oleoresin, as well as consumed as a fresh fruit. In South Korea, red pepper is a highly important agricultural product with a market value of about one billion dollars a year. It is widely used as the main ingredient in traditional Korean foods such as Kimchi and Gochujang. Furthermore, about 60% of Korean red pepper is consumed in powder form, and its annual consumption is 2.5~3.5 kg red pepper powder per person in South Korea.
The major factors involved in the international quality control of red pepper include hot taste (heat, pungency), color, moisture content and sanitation. The quality properties of the Korean red pepper are its unique sweet taste, as well as its pungency (1,2). For many years capsaicin (vanillylamide of 8-methylnontrans-6-enoic acid) was thought to be the only compound responsible for the pungency of peppers. Five closely related pungent compounds have also been reported: capsaicin, nor-dihydrocapsaicin, dihydrocapsaicin, homocapsaicin and homo-dihydrocapsaicin. These compounds are structurally similar to capsaicin; and this family of compounds, including capsaicin, is termed "capsaicinoids" (3). The capsaicin is the primary contributor to the heat of capsicums and capsaicin content in the fresh red pepper is two to three times greater than in green pepper (4). And ASTA value has been used as a unit of objective red pepper color internationally. ASTA value was recognized as an index to evaluate the red pepper color in the American Spice Trade Association. It was reported that the higher ASTA value of red pepper generally contained higher red color content (5-7). Free sugars play an important role in the flavor characteristics of fruit. Especially, Korean red pepper varieties have a sweet with hot taste and contain a free sugar content of 15-30%, which includes glucose, fructose and sucrose.
In general, consumers purchase, grind and directly use whole red pepper in large quantities at red pepper harvest season (8). Many consumers purchase sun-dried whole red pepper and air-dried whole pepper directly from farmers. Because of skepticism about commercial red pepper powder, consumers directly purchase whole red pepper from market or farmer and prepare red pepper powder as food ingredients at home. However, recently, as a result women's participation in society and the adoption of a more western diet, consumer purchase pattern of red pepper is changed a small amounts of packaged products including other spices, garlic, green onion, etc. In South Korea, the packaged red pepper powder is sold mainly large supermarkets; and most consumers that purchase small packaged-red pepper want information such as cultivation area, nutrients and heat intensity, etc.
The aim of this study was provide useful information in making guide of quality index on the physicochemical and microbiological quality of packaged red pepper in South Korea. We examined the moisture, free sugar, ascorbic acid, capsaicinoids content and microorganism distribution in the sun-dried and air-dried packaged red pepper marketable in South Korea.
Materials and Methods
A total of 40 samples of air-dried packaged red pepper (n=20) and sun-dried packaged red pepper (n=20), were collected from different retail market and farms in South Korea. The red pepper samples were collected various provinces on the basis of red pepper cultivation area. The whole pod was: 11-13 g , 12-15 cm pod length, and 2.5-3.5 cm pod diameter. All the red pepper fruits (Capsicum annuum L.,) samples were collected between September and November in 2012 from different provinces according to the proportion of the total production: Kangwon (n=2), Gyeongsang (n=15), Junra (n=15), Chungchung (n=6), Gyunggi province (n=2). Air-drying methods were applied using bulk dryer (conventional drying method). For conventional air-dried method, whole red pepper pods were washed with water, de-stemmed and dried at 80℃ for 5~10 hr followed by 60℃ for 18~35 hr according to sample contents. Sun drying was made by spreading red pepper on a ground with net in a single layer and exposed directly to sunlight for 3-6 days. The moisture content of samples was determined using the AOAC method (9) and dried about 14~15% moisture before analyses. The particle size of each sample was kept below 1.0 mm using a To-Tap sieve shaker (Cheonggesa CG-213, Seoul, Korea). Fructose, glucose, sucrose, capsaicin and dihydrocapsaicin were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). Solvents used for analyses were of HPLC grade and purchased from Fisher Scientific Korea (Seoul, Korea).
The ASTA (American Spice Trade Association) color value was determined ASTA-20.1 method (5). Red pepper powder (about 100 mg) was added to 100 mL acetone, and the mixture was stored at room temperature in the dark with intermittent stirring. An aliquot of the transparent extract was taken and the absorbance at 465nm was measured using a UV/Vis spectrophotometer (V-550, Jasco, Tokyo, Japan). The ASTA color value was then calculated using the following equation.
ASTA value = Absorbance of acetone extract × 16.4 × If ÷ Sample weight (g)
To measure the color value, the red pepper was put in a plastic petridish (5 cm diameter, 5 mm height) and placed on 5 sheets of white paper. The L, a, b values were calculated using a colorimeter (CE-400, Macbeth, Minolta, Koyto, Japan). The tests were repeated more than 3 times to obtain a mean value.
Red pepper powder (2 g) and 9 mL of 100% methanol were placed in a round-bottom glass flask and several glass beads were added to aid boiling. The samples were then heated gently for one hour by a Dri-Block Heater (FDB03DD, Bibby scientific Ltd, UK). After filtering through a 0.2 μm filter Millex-HN (Millipore, Bedford, MA, USA), the samples were used for high performance liquid chromatography analysis. The filtered extracts were analyzed on an HPLC system (Agillant Co.). Chromatographic analysis was performed on a XTerra TMRP18 (5 μm, 150 × 4.6 mm id., Waters, Milford, MA, USA) column with isocractic mobile phase. The mobile phase for analysis was water containing methanol at a methanol:water ratio of 70:30. The HPLC operating parameters were as follows: injection volume, 20 μL; column flow rate, 0.8 mL/min; chromatographic run time, 10 min; UV 280 nm.
Scoville heat unit (SHU) = ppm capsaicinoids × 15 (10,11)
Red pepper powder (2 g) was mixed with 40 mL of 80% ethanol and blended with a vortex mixer for 2 min. The extract was then filtered through a 0.2 μm filter ((Millex-HN, Millipore). Total free sugar content corresponded to the sum of fructose, glucose and sucrose contents analyzed by HPLC. Chromatographic analysis was performed on a Supelcogel Ag2(5μm, 300 × 7.8 mm id., Supelco, Milford, MA, USA) column using an isocractic mobile phase. The mobile phase for analysis was 100% water. The HPLC operating parameters were as follows: injection volume, 20 μL; column flow rate 0.5 mL/min; chromatographic run time, 20 min: model 830-RI detector.
In the ascorbic acid, red pepper powder (1 g) was mixed with 12 mL of metaphosphoric acid (1%, v/v), and it was extracted by shaking for 5 min, and then diluted with 5% metaphosphoric acid. The supernatant was filtered using a 0.2 μm PVDF syringe filter (Whatman, Clifton, NJ, USA), and the filtered samples were analyzed on an HPLC system. Chromatographic analysis was performed on an XTerraTMRP18(5 μm, 150×4.6 mm id., Waters) column with isocractic mobile phase. The mobile phase for analysis was water containing methanol at a methanol:water ratio of 70:30. The mobile phase for analysis was water containing methanol at a 0.05M KH2PO4:methanol of ratio of 60:40. The HPLC operating parameters were as follows : injection volume, 20 μL; column flow rate, 0.8 mL/min; chromatographic run time, 12 min; UV 254 nm.
The red pepper powder (25 g) and whole red pepper (25 g) were added to 225 mL of Butterfield’s phosphate buffered dilution water and homogenized using a BagMixer Blender (Interscience, St. Nom, France) for 2 min. Coliforms and Escherichia coli counts were determined with the three-tube most probable number (MPN) system using three 10-fold dilutions in 5 mL brilliant green bile lactose broth (BGLB) tubes, each containing a Durham’s tube (12). The BGLB tubes were incubated for 24 hr and 48 hr at 37℃ for selective enrichment of coliforms. Broth cultures from the tubes showing both growth and gas production were streaked on coliform agar plates. The coliform agar plates were incubated for 24 hr at 37℃ and then examined for the dark-blue to violet characteristics E. coli colonies. Selected colonies were identified using the Vitek-2 compact system. Fungi (yeast and mold) and Aspergillus flavus were enumerated in Potato dextrose agar and AFPA Base incubated at 25℃ and 30℃ for 5~7 days and 24~48 hr, respectively. The results were expressed as CFU/g.
Bacillus cereus and Salmonella spp. were detected according to the Korea Food additives code standard (12). For B. cereus detection, 25 g of the sample was added to 225 mL of Butterfield’s phosphate buffered dilution water and homogenized using a Bag Mixer Blender for 2 min. After decimal dilution, 100 μL samples were placed on the surface of mannitol egg yolk polymyxin agar including egg yolk emulsion, and B. cereus selective supplement in triplicate. Following incubation at 30℃ for 24 hr, pink colonies surrounded by a zone of precipitation were counted, and B. cereus was identified using the Vitek-2 compact system. For Salmonella spp. detection, 25 g samples were mixed with 225 mL of buffered peptone water and enriched for 18~24 hr at 37℃. 100 μL of mixture was inoculated into the rapapport-vassiliadis broth and incubated at 42℃ for 24 hr. Selective growth was conducted in Xylose-Lisine- Deoxycholate agar and Rambach agar at 37℃ for 24 hr, respectively. Identification of red colonies showing a black center and typical red colonies were conducted using the Vitek-2 compact system. For Staphylococcus aureus detection, 25 g samples were added to 225 mL of tryptic soy broth containing 10% NaCl and incubated at 37℃ for 18~24 hr. The enriched samples were inoculated on Baired-Parker agar including egg yolk tellurite emulsion and incubated at 37℃ for 24 hr. Convex black and shiny colonies with a narrow white entire margin and surrounded by clear zone into opaque medium were selected. Detected S. aureus was identified using the Vitek-2 compact system
For Clostridium perfringens detection, 25 g of samples was added to 225 mL of 0.1% pepton water and homogenized using a stomacher for 2 min. The mixtures were incubated at 35℃ for 24hr in anaerobic jar. After incubation, 1 mL of mixture was inoculated into 10 ml of the cooked meat medium (Becton Dickinson, Le Pont de Claix, France) and incubated at 35℃ for 24hr in anaerobic condition. The enriched samples were streaked in tryptose sulfite cycloserine (TSC, Oxoid, England) with egg yolk and incubated anaerobically for 24 hr at 35 ℃. Colonies displaying a black centre with opaque white zone surrounding the colony were identified as positive isolates of C. perfringens. Detection of C. perfringens was conducted using PCR method. Positive colonies were grown on TSA at 35℃ for 24 hr. Template DNA were extracted by the boiling method.
Chemical analysis of the red pepper powder and whole red pepper samples were conducted in triplicate and the results are presented as the means +/- SD. Principal component analysis (PCA) was conducted to summarize and verify the relationships between the mean values of the chemical quality characteristics and total viable cell number of the red pepper samples. The PCA method used was covariance matrix extraction with varimax rotation. All of the statistical analyses were conducted using Xlstat version 2010 for Windows software.
Results and discussion
The capsaicinoids content and ASTA of air-dried and sun-dried red pepper samples is shown in Table 1. The moisture of the red pepper affects its chemical components and causes sanitary problems in the red pepper powder. The moisture content is a very important quality factor in red peppers; Korean Industrial Standard for the quality of red pepper powder (13) requires the moisture content to be below 13% (12). International Organization for Standardization (10) of red pepper is moisture content of less than 11%. The moisture content of air-dried samples and sun-dried samples was 10.38~15.60% and 9.46~17.22%, respectively. The capsaicinoids content of air-dried and sun-dried samples showed 10.85~126.39 mg% (1,627~18,958 SHU) and 0.43~164.09 mg% (64.5~24.613 SHU), respectively. Todd et al. (14) reported that capsaicin, dihydrocapsaicin and nodihydrocapsaicin produce rapid pungent sensations, whereas homocapsaicin and homodihydrocapsaicin tend to produce longer, low intensity sensations in the mid mouth and midpalate regions. In addition, Suzuki et al. (15) reported that the hot taste components of red pepper was: capsaicin: 46~77%, average 70%; dihydrocapsaicin: 21~40%; norhidyrocapsaicin: 2~12%; homocapsaicin: 1~2%; and norhydrocapsaicin: 0.5% depending on the red pepper species. In addition Ku et al. (15) reported that it evaluated about 11 score of hot taste intensity of 15 line scale sensory evaluation in the red pepper samples with capsacinoids content more than 80 mg% (12,000 SHU). And also, the samples less than 26.08 mg% were evaluated 5 score and samples of 36.82~67.97 mg% were evaluated 6.63~8.70 score in the sensory evaluation (15). In this study, capasicinoids content showed widely range from mild to hot taste intensity in the air-dried and sun-dried red pepper samples.
In the ASTA value, air-dried and sun-dried red pepper samples ranged 49.12~154.69, 70.08~182.13, respectively. The air-dried samples were L (lightness) of 34.32~43.59, a (redness) of 14.62~28.7, b (yellowness) of 10.04~19.14 and the sun-dried samples were L (lightness) of 35.43~42.47, a (redness) of 15.21~22.80, b (yellowness) of 10.82~ 17.60. The red color of red pepper is very important quality factor in the dried red pepper. The ASTA value of red pepper is affected by the quality of raw fruit, drying condition, ratio of seed, packaging, storage temperature and cultivation area (1,17-20). In the 47 varieties of Korean red pepper powder, the reported range of capsaicinoids was 10.54~250.87 mg% and ASTA 64.55~124.07 (21). In this study similar ranges of ASTA value and capsacinoids were found as other reports (1,17-21).
On the other hand, the free sugar related sweetness of red pepper is a very important quality factor that affects consumer acceptability (22). Generally, the periscarp of red pepper has mainly free sugar and previous studies reported that the Korean red pepper had a free sugar content of about 20% (23). In this study, the total free sugar of air-dried and sun-dried samples was a wide range of 10.82~20.77%, 9.26~23.10%, respectively. In the free sugar components of red pepper samples, the fructose content was approximately 2 times more than glucose content. A wide range and component ration of free sugar content in red pepper samples, it seems to be due to several factors including varieties, cultivation region, red pepper seed content ratio (24).
The ascorbic acid of air-dried and sun-dried red pepper samples was found to be 0~1,010 mg% and 620~1,050 mg%, respectively. In this study, group of sun-dried samples were higher content range than group of air-dried samples. Choi (25) reported that ascorbic acid content was different according to part of red pepper. Periscarp has a high content of ascorbic acid, and seeds have a low content. And also, Kim et al. (19) reported that ascorbic acid content decreased with storage periods. Howard et al. (26) reported that 75% of ascorbic acid in red pepper was lost during drying, and the ascorbic content of red pepper was differed depending on harvesting time. In this experiment samples, generally, sun-dried red pepper samples showed higher content range than air-dried red pepper samples.
The microbiological contamination level and prevalence number in red pepper samples is shown in Table 2. For total viable cell, the air-dried red pepper samples was ranged from 2~6.67 log CFU/g and sun-dried samples ranged from 1.74~5.77 log CFU/g. The contamination level of yeast in the air-dried and sun-dried samples ranged from 1~6 log CFU/g. This results showed wide range data that contamination levels of fungi have been reported as 3.1~3.7 log CFU/g in red pepper powders (27,28). Coliforms and E. coli microogranism is an important hygiene indicator, especially for fecal contamination. Coliforms ranged N.D~7.3 log CFU/g in the air-dried samples and N.D~5.24 log CFU/g in the sun-dried samples. This results showed wide range data that contamination levels of coliform reported as 2~5 log CFU/g in the packaged red pepper powder (29).
The foodborne pathogens, Salmonella, Salmonella spp, S. aureus were not detected in the red pepper samples. However B. cereus was detected in 60% of air-dried samples and 40% of sun-dried samples. C. perfringens was detected in 10% of samples. It was reported that food borne diseases can develop when B. cereus exists in food at levels greater than 3~6 log CFU/g. And B. cereus forms heat stable spores that can survive heating at temperatures of 135 ℃ up to 4hr, and thus these spores do pose a health risk (30). In this study, the level of B. cereus in air-dried sample was similar level to the reference level mentioned above, while sun-dried sample was lower than the reference level. These results indicate that there was a probability of food borne disease originating from some red pepper itself. If red pepper samples contaminated B. cereus and C. perfingens put in some food and stored improperly, the B. cereus and C. perfringens can multiply in the food and then that food become a potential risk (30,31). This microbiological result suggested that more consideration need to be given to control microbiological contamination during red pepper processing and distribution.
The correlation between the principal quality characteristics of the forty-red pepper sample is shown in Table 3. The correlation of ASTA and redness (a) was significant at 0.417 (R2). And there was negative correlation between ‘redness (a)’ and moisture; the correlation of ascorbic acid and total viable cell number was negative. But other quality characteristics of red pepper powder did not show any significant correlations. It was reported that pepper has high ascorbic acid content is correlated with a high sugar concentration (32,33); however, in this study, the correlation between sugar content and ascorbic acid was not significant value.
To determine overall distribution pattern of the air-dried and sun-dried red peppers, principal component analysis (PCA) performed (Fig. 1). Principal component analysis (PCA) is a statistical technique used to identify the smallest number of latent variables called “principal components” that explain the greatest amount of observed variability. PCA is a way of identifying patterns in data, and expressing the data in such a way as to highlight their similarities and differences. The first and second principal components (PC1 and PC2) accounted for 56.78 % of the total variances (38.47% and 18.31%, respectively). Ascorbic acid, ASTA, color value (L, a, b) strongly correlated with the PC1, Quality characteristics such as moisture, microorganism, sample (drying method) showed a negative correlation with the PC1.
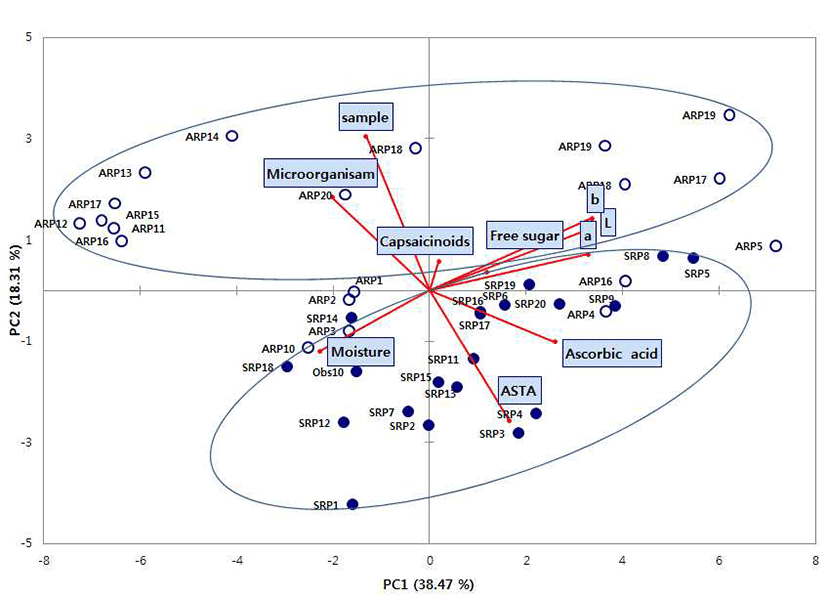
In the red pepper samples distributions, air-dried red pepper samples (ARP1~ARP20) was located in above of figure and sun-dried red pepper (SRP1~SRP20) was located a wide distribution in below of figure. The air-dried and sun-dried red peppers were divided into two parts in the PC2, it was not divided into two parts and mixed red pepper samples in the PC1 of principle component analysis. As a results of this study, it was not showed exactly the differences between the air-dried and sun-dried red pepper samples in south Korea. The results obtained information of physicochemical properties and microbiological contamination of domestic red pepper samples. It is hoped that this study will be useful in making guide further quality index for consumer’s favorite red pepper.
요 약
본 연구는 국내에서 유통되고 있는 국내산 건고추의 품 질 기준을 제시하기 위하여 농가에서 가장 많이 사용하고 있는 열풍 건조한 고추와 소비자가 선호하는 태양 건조한 고추를 지역별로 수집하여 물리화학적 및 미생물학적 품질 비교를 하였다. 열풍건조한 고추와 태양 건조 고추의 건물 량으로 환산한 캡사이신노이드 함량의 경우 열풍건조 시료 는 10.85~126.39 mg%, 태양 건조 시료는 0.43~164.09 mg% 로 넓은 범위의 분포도를 나타내었고, 총 유리당 함량은 각각 10.82~20.77%, 9.26~23.10%로 건조 방법에 따른 뚜렷 한 차이를 보이지 않았다. 그러나 ASTA 값과 비타민 C 함량의 경우, 열풍건조 시료는 49.12~154.69, 0.0~1,010 mg%, 태양건조 시료는 70.08~182.13, 620~1,050 mg%로 태양건조 시료가 열풍건조 시료보다 높은 범위의 값을 보였 다. 총균수와 효모의 경우, 열풍건조 시료군은 2.01~6.67 log CFU/g, 1.03~4.12 log CFU/g이었고, 태양 건조 시료군은 1.74~5.77 log CFU/g, 1.05~6.10 log CFU/g이었다. 또 병원 성 미생물인 Clostridium perfringens와 Bacillus cereus도 건 조 방법에 상관없이 검출되었다. 한편 이들 품질 특성들을 이용한 주성분 분석 결과 제1요인은 38.47%, 제2요인은 18.31%였고, 총 설명력은 56.78%였다. PC1은 비타민 C, ASTA값, 색도와 관련이 있었고, PC 2는 수분함량, 미생물 수, 건조 방법과 관련이 있었다. 본 연구결과는 국내에서 유통되고 있는 고춧가루의 건조방법에 따른 품질 차이와 미생물의 오염도가 확인되었고, 소비자가 원하는 고추 품 질 지표에 유용한 정보로 활용될 수 있을 것으로 기대된다.










