Introduction
Polycyclic aromatic hydrocarbons (PAHs) are abundant in cooked foods, cigarette smoke and other environmental pollutions. In particular, PAHs are present in food products in large quantities due to thermal processes such as smoking, grilling and smoke drying. In addition, foods can become naturally contaminated by the accumulation of PAH in the food chain due to their lipophilic properties and tendency to accumulate in adipose tissue. PAH is classified as a carcinogen, so PAH exposure is a major concern for human health (Veyrand et al., 2013). Benzo[a]pyrene (B[a]P) is a representative compound of PAH and it can also converted to ultimate carcinogen, benzo[a]pyrene-7,8-diol-9,10-epoxide (B[a]PDE), via cytochrome P450-mediated metabolism after entering human cells (Phillips, 1983). B[a]P and its metabolite (B[a]PDE) have been shown to be complete carcinogens in many animal models, including those for smoking-associated cancers, such as skin and lung cancers (Conney, 1982). However, direct role of B[a]PDE in skin-aging and its underlying molecular mechanisms have not been fully understood.
Binding of PAHs, such as 2,3,7,8,-tetrachlorodibenzo-p-dioxin (TCDD) or B[a]P, to cytosolic aryl hydrocarbon receptor (AhR) causes dissociation of AhR from the multiprotein complex and its translocation into the nucleus where it dimerizes with AhR nuclear translocator (ARNT) (Reyes et al., 1992; Rowlands and Gustafsson, 1997). The AhR/ARNT heterodimer induces gene expression by binding to specific xenobiotic response element (XRE, 5’-GCGTG-3’) in promoter regions of a variety of target genes, such as the cytochrome P450 (CYP) enzymes CYP1A1 and CYP1B1, and increases their expression through transcriptional activation (Backlund and Ingelman-Sundberg, 2005; Nebert and Dalton, 2006; Petrulis and Perdew, 2002). These target genes encode proteins involved in growth, differentiation, and inflammation (Sutter et al., 1991; Yin et al., 1994). Beside the transcriptional induction of CYP1A1, AhR activation leads to a c-Src-dependent stimulation of epidermal growth factor receptor (EGFR) and its downstream targets MAPK signaling pathways (Kwon et al., 2003; Randi et al., 2008).
Ligand binding of the cytosolic AhR, leads to the dissociation of the multiprotein complex and the subsequent release of c-Src in the cytoplasm (Enan and Matsumura, 1996; Kohle et al., 1999). c-Src functions as a co-transducer of transmembrane signals emanating from a variety of polypeptide growth factor receptors, including EGFR (Luttrell et al., 1988). The c-Src kinase translocates to the cell membrane where it can interact with the EGFR in a bidirectional fashion; c-Src can bind and phosphorylate of EGFR, and vice versa (Belsches et al., 1997). The binding of downstream signaling proteins to phosphorylated EGFR tyrosines initiates several signaling cascades involved in cell proliferation, migration, differentiation, and tumor formation (Carpenter, 1999).
Matrix metalloproteinases (MMPs), as a hallmark in aging, are a family of over 20 different proteolytic enzymes that degrade the protein components of the extracellular matrix (ECM) (Mueller, 1996). MMP expression is essential for the skin remodeling and inappropriate expression and activity of MMPs is associated with various pathologies such as rheumatoid arthritis and tumor metastasis (Luca et al., 1997; Wandel et al., 2000). The expression of MMP-1 (collagenase), MMP-3 (stromelysin) and MMP-9 (gelatinase) is increased by various stimuli such as UV irradiation and inflammatory cytokines (Dasu et al., 2003; Fisher et al., 1996). Especially, in skin fibroblasts, active MMP-1 induces collagen fragmentation and functional changes similar to aged human skin (Xia et al., 2013). Tobacco smoke extracts impaired collagen biosynthesis significantly in cultured skin fibroblasts (Yin et al., 2000). In addition, B[a]P-induced AhR pathway activation increases the expression of MMP-1 mRNA and MMP-1 protein and promotes ECM degradation in HaCaT keratinocytes and human periodontal ligament cells (HPDLCs), vascular smooth muscle cells (VSMCs) (Meng et al., 2009; Tomokiyo et al., 2012). However, in human skin, the molecular mechanism of B[a]PDE-induced MMP-1 expression via AhR translocation has not been fully understood.
In this study, we investigated the molecular mechanisms of how AhR and c-Src are involved in MMP-1 expression by inducing B[a]PDE directly in HaCaT cells.
Materials and methods
B[a]PDE, benzo[a]pyrene-7,8-diol-9,10-epoxide was purchased from MRIGlobal Chemical Carcinogen Repository (Kansas City, MO, USA). The AhR antagonist α-naphthoflavone and mouse monoclonal anti-β-actin were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Anti-human MMP-1 antibody was purchased from Neomarkers (Fremont, CA, USA). Primary antibodies recognizing phosphorylated c-Raf (Ser338), phosphorylated MEK1/2 (Ser217/221), phosphorylated JNK (Thr183/Tyr185), phosphorylated MKK4 (Ser257/Thr261), phosphorylated MKK3/6 (Ser189/207), phosphorylated EGFR (Tyr845), phosphorylated Src Family (Tyr 416), total c-Raf, total MEK1/2, total MKK4, total MKK3/6 and total EGFR were purchased from Cell Signaling Biotechnology (Danvers, MA, USA). The antibody against phosphorylated p38 (Thr180/Tyr182) was purchased from BD bioscience (San Jose, CA, USA). Primary antibodies recognizing phosphorylated ERK1/2 (Tyr 204), total ERK1/2, total p38, total c-Src and gefitinib (EGFR inhibitor) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Specific inhibitor of MEK (U0126), JNK (SP600125) and Src Family (PP2) were purchased from Tocris Bioscience (Bristol, UK). SB203580, a p38 inhibitor was obtained from Merck Millipore’s Calbiochem® (Billerica, MA, USA).
The human immortalized keratinocyte cell line HaCaT was purchased from the American Type Culture Collection (Manassas, VA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin-streptomycin and 0.25% trypsin-EDTA were purchased from GIBCO® (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Sigma-Aldrich Co.
The HaCaT human keratinocytes were cultured in DMEM supplemented with 10% FBS and 2 TM penicillin/streptomycin at 37℃ in humidified atmosphere with 5% CO2.
Cells (1×105) were cultured in a 60 mm in diameter dish for 24 h, then, they were starved in serum-free medium for a further 24 h to eliminate the influence of FBS on the kinase activation. Cells were treated with various concentrations of inhibitors for 1 h, and then treated with 0.5 μM B[a]PDE. The harvested cells were disrupted and the supernatant fractions were boiled for 5 min. The protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) as described in the manufacturer’s manual. Total cell lysates were separated by 8-10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to a polyvinylidene fluoride (PVDF) microporous membrane (EMD Millipore Co., Billerica, MA, USA). After blotting, the membrane was incubated with the specific primary antibodies at 4℃ overnight. After hybridization with secondary antibodies, the protein bands were visualized using an Amersham ECL prime western blotting detection system (GE Healthcare, Buckinghamshire, UK).
Results and discussion
Previous studies have shown that tobacco smoke extract accelerates skin aging through abnormal induction of MMPs. In the present study, we first investigated B[a]PDE-induced MMP-1 expression in HaCaT cells. The treatment with B[a]PDE at 1 μM for 1-6 h significantly induced MMP-1 expression in a time-dependent manner (Fig. 1A and 1B). Next, treatment with B[a]PDE at 0.1-1 μM for 6 h significantly induced MMP-1 expression at 0.5 μM (Fig. 1C). Taken together, these data clearly show that MMP-1 expression was increased by B[a]PDE at 0.5 μM for 6 h.
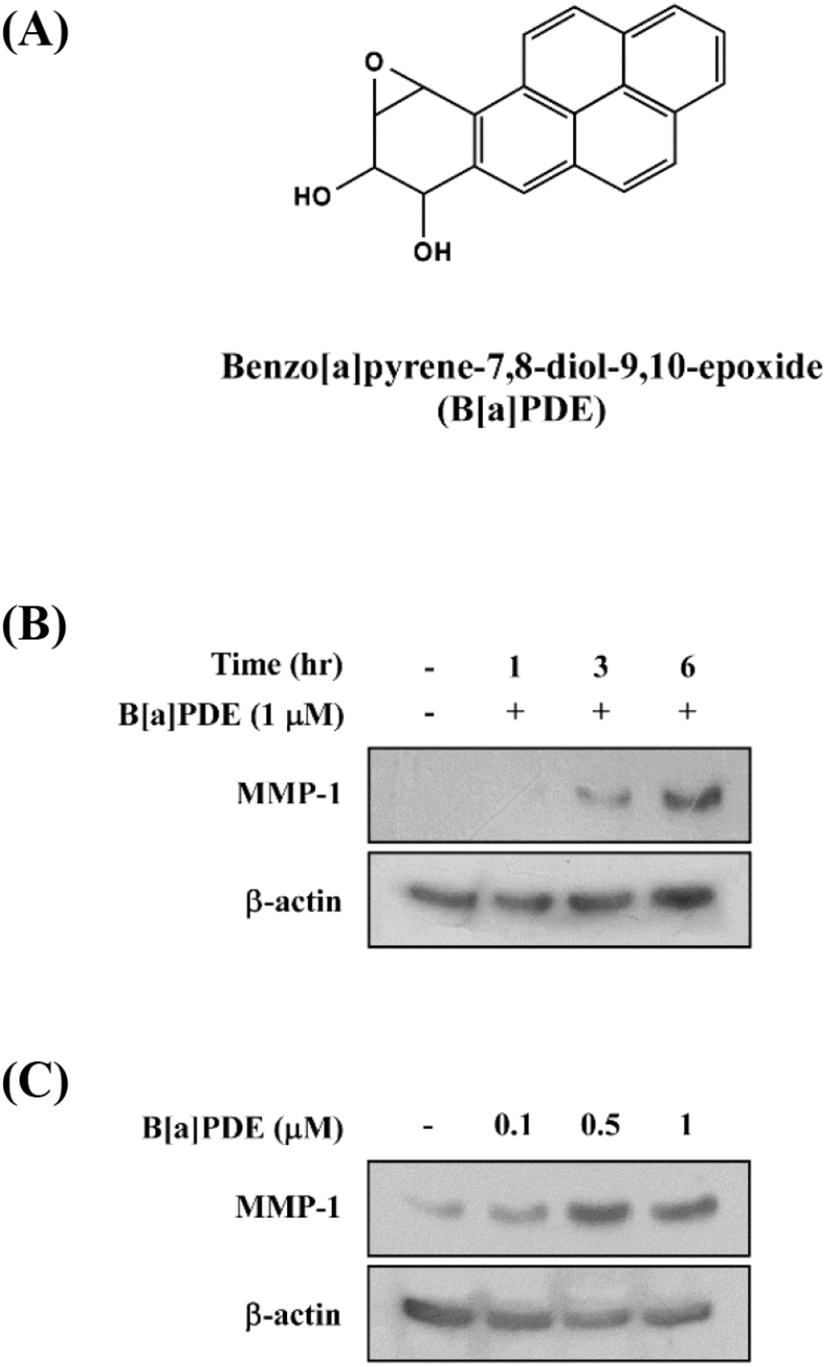
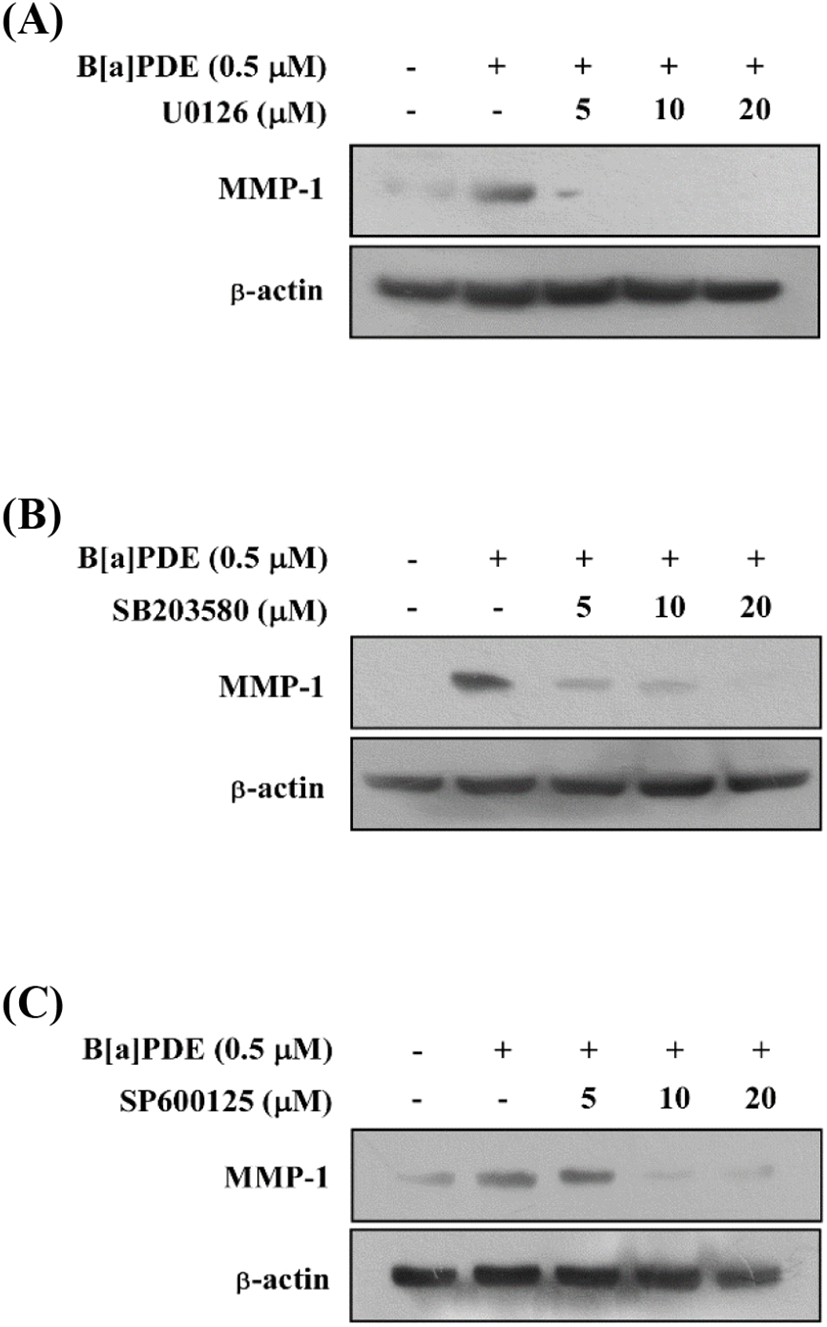
The MAPK signaling pathway is necessary for the regulation of MMP expression. Additionally, the previous study demonstrated a novel signaling pathway by which AhR agonists elevated intracellular calcium levels leading to increased MMP-1 expression through MAPK pathways in bronchial epithelial cell lines (Lee et al., 2015). To determine whether the activation of MAPK pathways increases MMP-1 expression in HaCaT cells exposed to B[a]PDE, the cells were pretreated with MAPK inhibitors at 5-20 μM for 1 h before being exposed to B[a]PDE at 0.5 μM. U0126 (MEK inhibitor) completely blocked B[a]PDE-induced MMP-1 expression (Fig. 2A). In addition, SB203580 (p38 inhibitor) and SP600125 (JNK inhibitor) significantly suppressed B[a]PDE-induced MMP-1 expression in a dose-dependent manner (Fig. 2B and C). These results indicated that up-regulation of B[a]PDE-induced MMP-1 occurs through the MAPK pathways in HaCaT cells.
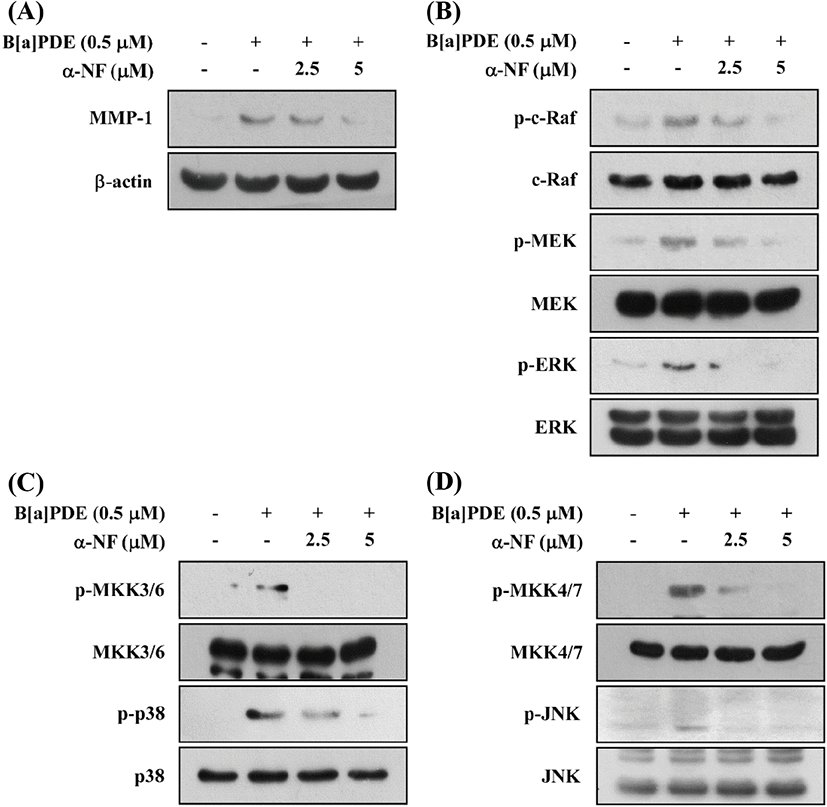
Previous reports demonstrated the stimulation of MMP-1 expression through the activation of the AhR pathway in melanoma cells, vascular smooth muscle cells, and urothelial carcinoma cells (Ishida et al., 2010; Meng et al., 2009; Vogeley et al., 2019). To find out whether B[a]PDE is dependent on AhR to activate the MAPK pathways, we determined MMP-1 expression in HaCaT cells treated with B[a]PDE in the presence of α-Naphthoflavone (α-NF), a classical AhR antagonist. These results suggested that α-NF inhibits the B[a]PDE-induced MMP-1 expression (Fig. 3A) and phosphorylation of c-Raf/MEK/ERK, MKK3/6/p38, and MKK4/7/JNK signaling pathways in a dose-dependent manner (Fig. 3B, C and D). Consequently, AhR has been shown to play a major role in B[a]PDE-induced MMP-1 expression and c-Raf/MEK/ERK, MKK3/6/p38, and MKK4/ 7/JNK phosphorylation.
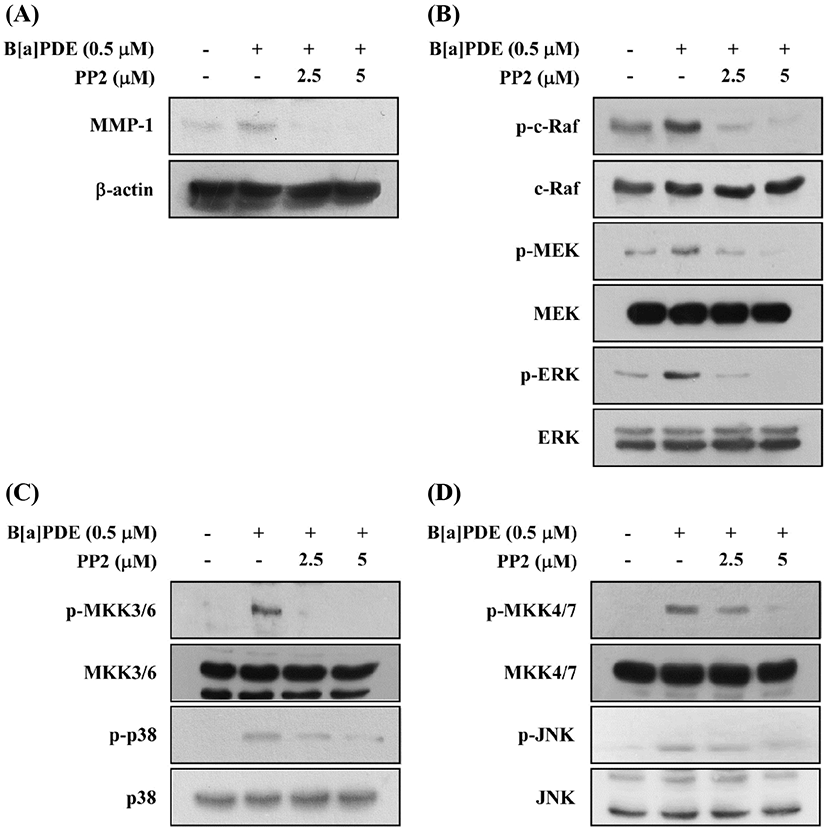
c-Src kinase is at least one of the earliest and the most upstream components of toxic signaling of the AhR activated by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the post-transcriptional process. TCDD were mediated through a c-Src-dependent activation of the extracellular signal-regulated kinases 1/2 (ERK1/2) in MCF10A cells (Mazina et al., 2004). To confirm whether c-Src involved in B[a]PDE-induced MMP-1 expression and phosphorylation of MAPK, HaCaT cells were cultured in the presence of the PP2 (c-Src inhibitor) at 2.5 and 5 μM. Pretreatment of the cells with PP2 reduced B[a]PDE-induced MMP-1 expression (Fig. 4A). Furthermore, PP2 significantly reduced B[a]PDE-induced phosphorylation of c-Raf, MEK and ERK (Fig. 4B), MKK3/6/p38 and MKK4/7/JNK (Fig. 4C and D).
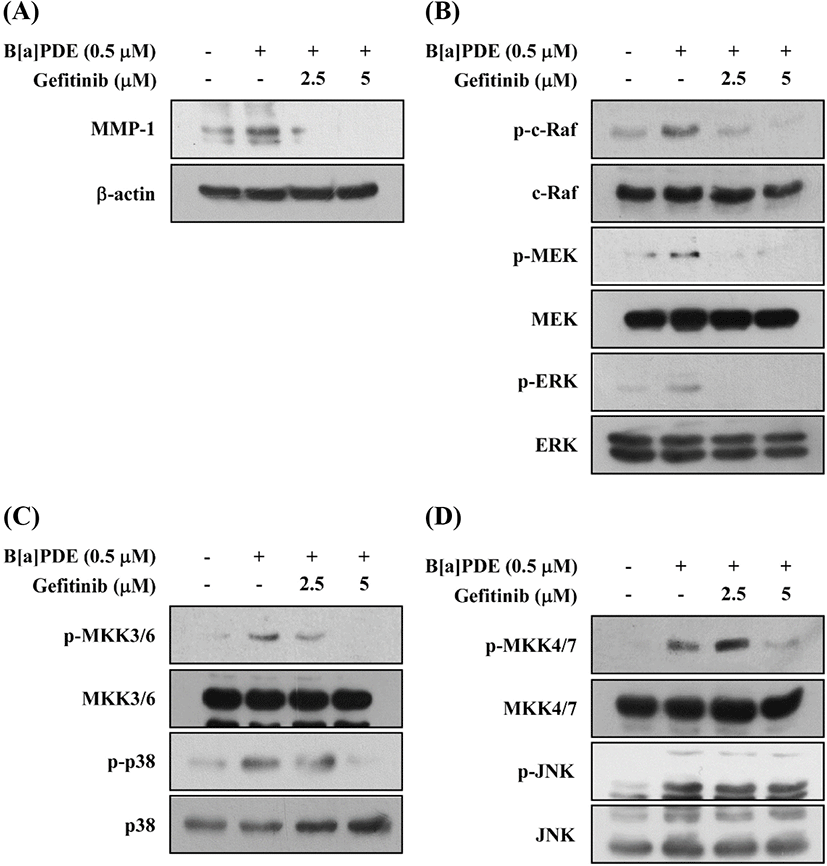
Activation of the AhR leads to rapid activation of soluble tyrosine kinase c-Src. Activated c-Src is capable of phosphorylating cell-surface receptors such as EGFR, which induce subsequent stimulation of downstream MAPK signaling to regulate gene transcription (Xie et al., 2012). Several other studies have reported that exposure to TCDD and related xenobiotics like B[a]P or hexachlorobenzene results in activation of the mitogen-activated signaling pathways, which is mostly a downstream targets of EGFR (Kwon et al., 2003; Randi et al., 2008). In this study, we examined the effect of gefitinib (EGFR inhibitor) on B[a]PDE-induced MMP-1 expression and phosphorylation of MAPKs. Pretreatment with Gefitinib inhibits the B[a]PDE-induced MMP-1 expression (Fig. 5A) and phosphorylation of c-Raf/MEK/ERK, MKK3/6/p38, and MKK4/7/JNK and their upstream kinases (Fig. 5B, C and D). These results suggest that c-Src and EGFR are required for the B[a]PDE-induced MMP-1 up-regulation and activation of MAPK pathways in HaCaT cells.
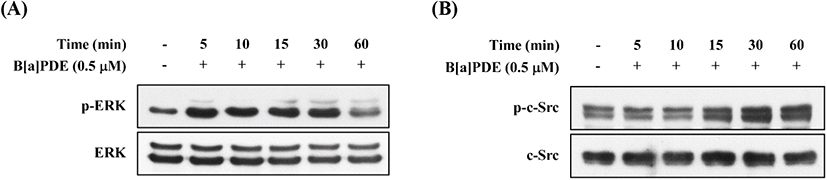
EGFR can be transactivated by other agents, such as activation of Src family, and is known as mediators of c-Src-dependent ERK activation (Zhang et al., 2007). To identify c-Src association to the EGFR in HaCaT cells, we investigated the action of B[a]PDE on the phosphorylation status of ERK and c-Src. As shown in Fig. 6A, B[a]PDE-induced ERK phosphorylation was detected at 5-30 min and returned to close baseline until 60 min. In addition, B[a]PDE-induced c-Src phosphorylation was detected at 15 min and persisted for ≥60 min (Fig. 6B). As anticipated, these results confirmed that B[a]PDE stimulates phosphorylation of ERK via c-Src-dependent EGFR transactivation.
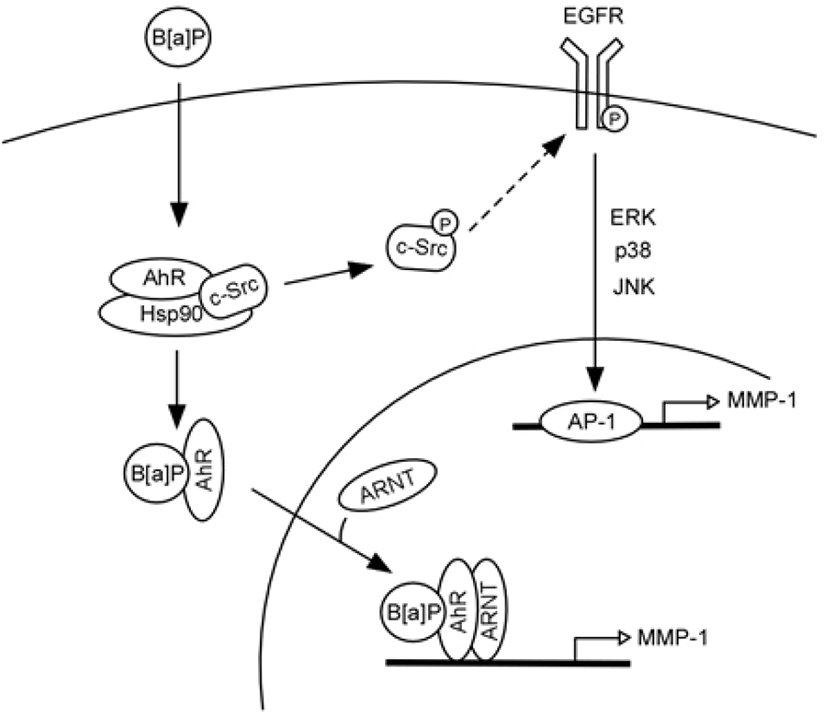
Based on the current findings, in Fig. 7, we can propose a model that represents signaling pathways for B[a] PDE-induced MMP-1 expression in human keratinocytes. In unstimulated cells, c-Src and AhR coexist in a protein complex that also likely contains heat-shock protein 90 (HSP90) (Bock and Kohle, 2006). Binding of B[a]PDE to AhR activates c-Src (Tyr416 phosphorylation), which in turn activates EGFR (Tyr845 phosphorylation). Activated EGFR promotes MAPK signaling and MMP-1 transcription and consequently stimulates ECM degradation. Activated AhR can also be translocated to the nucleus and acts as a transcription factor, directly regulating MMP-1 gene expression.
In conclusion, the results of this study show a novel mechanism by which B[a]PDE induces MMP-1 expression through activation of AhR, c-Src and EGFR in non-genetic pathways. Taken together, it can be suggested that the mechanism of B[a]PDE-induced MMP-1 expression via multiple signaling plays an important role in promoting cellular processes such as skin aging.
