Introduction
Okra (Abelmoschus esculentus L.), originating in Africa, is widely cultivated in several Asian countries. However, this plant is only recently being cultivated in South Korea (Olawuyi et al., 2020). Fruits (which contain seeds), stems, leaves, and flowers of okra can be consumed either raw or cooked. The green leaves of okra are spirally arranged, long-petioled (up to 50 cm) with soft hair on the upper side, transversally elliptical to orbicular in outline (up to 20 cm long and broad), and have 5-7 palmate lobes (Fig. 1). They are considered as vegetables and can be consumed like spinach or as a salad ingredient. They can also be boiled to make tea, used as a seasoning in dried powder form, or added to soup as a thickener (Bawa and Badrie, 2016; Lamont, 1999; Roy et al., 2014). Similar to the fruits, the okra leaves (OKL) also contain a thick mucilaginous liquid due to the presence of polysaccharides and are suitable for medicinal and industrial applications (Ghori et al., 2017). The okra fruit contains polyphenolic compounds such as p-coumaric acid, ferulic acid, sinapic acid, catechin, and quercetin and its derivatives (Arapitsas, 2008; Huang et al., 2007; Xia et al., 2015). OKL are rich in fiber, vitamins A and C, calcium, potassium, iron, and β-carotene (Gemede et al., 2015).
Besides, OKL contain polyphenolic compounds and antioxidants (Geng et al., 2015; Liao et al., 2012), although these compounds in the OKL have not yet been identified.
Polyphenols are active components predominant in fruits and vegetables. They are natural antioxidants and have remarkable health benefits (Altemimi et al., 2016; Ojulari et al., 2019). Since polyphenols are bioactive components, generous consumption of vegetables rich in polyphenols can neutralize the effect of free radicals and lower the risk of chronic diseases such as obesity, cancer, and cardiovascular diseases (Ojulari et al., 2020; Olawuyi and Lee, 2019). Polyphenolic compounds are accumulated in various parts of the plant and are often isolated for their biological activity. The extraction efficiency, yield of polyphenols, and their antioxidant potencies and stabilities are typically influenced by the extraction technique and extraction conditions (Chandini et al., 2011; Vuong et al., 2013). Owing to the drawbacks of the conventional extraction methods such as high energy requirement, time and poor efficiency, several novel techniques, including supercritical fluid extraction, pressurized fluid extraction, and microwave- and ultrasound-assisted extraction (UAE) have been employed for the extraction of polyphenols. In particular, UAE has been widely used to extract polyphenolic compounds from plant sources (Albu et al., 2004; Ali et al., 2018; Herrera and De Castro, 2005; Hromadkova et al., 2008) due to the relatively low cost of equipment and the highly efficient and easy mode of operation. UAE uses acoustic cavitation to disrupt plant cell walls, which facilitates the penetration of solvent and the release of compounds into the extraction solution (Altemimi et al., 2016; Vinatoru, 2001). The conditions may vary according to the extraction process (material and technique), and their influence on the extraction of polyphenolics have been extensively reported (Ali et al., 2018; Alu datt et al., 2011; Chandini et al., 2011; Pinelo et al., 2005; Vuong et al., 2013).
Despite such extensive studies, there are no reports on the effect of extraction conditions on the yield of polyphenols from OKL. This study represents the first attempt to investigate the influence of some important parameters such as solvent concentration, solid-to-solvent ratio, temperature, and time on the efficiency of UAE of OKL polyphenolics. The yield of the polyphenolics and the antioxidant activity of the OKL extracts obtained under the optimal ultrasonic extraction conditions were determined in this study. Furthermore, the polyphenolic compounds in the OKL extract were also identified and quantified.
Materials and methods
Fresh okra leaves were collected from a local farm (Danggin, Korea), cleaned by washing in water, then dried at 40°C for 24 h. Dried leaves were pulverized (RT-04, Mill Powder Tech., Taiwan), sieved (425 μm aperture), and stored in an air-tight bag at −20°C until use. Extraction solvents were purchased from Duksan Chemicals (Ansan, Korea), while other experimental reagents and standards were purchased from Sigma Aldrich Co. (St. Louis, USA).
UAE was carried out on an ultrasound bath (JAC-3010, KODO, Hwaseong, Korea) at a frequency of 45 kHz and power of 490 W. Ethanol was selected as the extraction solvent, and absolute ethanol (99%) was referred to as 100% ethanol in this study. A single-factor design consisting of varying levels of ethanol concentration, solid-to-solvent ratio, UAE temperature and time was employed. To evaluate the effect of solvent concentration on the polyphenolic yields and antioxidant activities, ethanol was diluted with distilled water to prepare 20, 40, 60, 80, and 100% ethanol. The extraction yields, total polyphenolic contents (total phenolics and flavonoids), and antioxidant activity for the extraction using various ethanol concentrations were determined at a fixed solid-to-solvent ratio (1:20 g/mL), temperature (30°C), and time (30 min). The optimal solvent concentration was further used to determine the optimal solid-to-solvent ratio for the extraction of OKL polyphenolics. OKL powder (1 g) was extracted with 10, 25, 20, 30, and 40 mL, by fixing the temperature and time as before. The optimal solvent concentration and solid-to-solvent ratio were further used to determine the influence of temperature (10, 25, 40, 55, and 70°C) on the UAE of OKL polyphenolics. Finally, the optimal solvent concentration, solid-to-solvent ratio, and temperature were used to determine the effect of UAE time on the yields, total polyphenolic contents (total phenolics and flavonoids), and antioxidant activities of OKL extracts. Extracts were concentrated by rotary evaporator and freeze-dried to obtain the extract powder. The extraction yield was calculated on a dry basis as the percentage ratio of dried weight of extract to the weight of OKL powder.
The total phenolic content (TPC) of the OKL extracts was determined using Folin-Ciocalteu’s reagent, as described by Olawuyi et al. (2019). One hundred microliters of the diluted extract was reacted with 50 μL Folin-Ciocalteu’s reagent and 300 μL 2% Na2CO3. The mixture was allowed to stand for 15 min in the dark. Following this, 1 mL of distilled water was added, and the absorbance at 725 nm was recorded using a UV-spectrophotometer (UV-2550, Shimadzu Co., Tokyo, Japan). The results were expressed as mg gallic acid eq. per g (mg GAE/g).
The total flavonoid content (TFC) was estimated using the aluminum-colorimetric assay (Teng et al., 2009). Seventy microliters of the extract was mixed with 430 μL of distilled water, 50 μL of 5% NaNO2, and 50 μL of 10% Al(NO3)3·9H2O. The solution was allowed to stand for 6 min in the dark. Following this, 500 μL of 1 N NaOH was added, and the absorbance at 510 nm was measured. The TFC of the OKL extract was expressed as mg rutin eq. per g (mg RE/g).
TThe 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2’- azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay were used to estimate the antioxidant activities of the OKL extracts. DPPH (Blois, 1958) and ABTS (Olawuyi et al., 2020) reagents were prepared as described in these studies. For DPPH, 100 μL of diluted OKL extract was mixed with 900 μL of DPPH reagent and kept in the dark for 30 min. The absorbance at 517 nm was measured, and the DPPH radical scavenging capacity (RSC) was expressed as mg ascorbic acid eq. per g (mg AAE/g) (Blois, 1958). For the ABTS assay, 50 μL of diluted OKL extract was mixed with 950 μL of ABTS reagent and allowed to react in the dark for 30 min. The absorbance at 734 nm was measured, and the ABTS radical scavenging activity was expressed as mg ascorbic acid eq. per g (mg AAE/g) (Arnao et al., 2001).
The phenolic acids and flavonoids in OKL extract were identified and quantified using high-performance liquid chromatography (HPLC) previously described (Seal, 2016). Phenolic acids and flavonoids were identified using an HPLC system detector (Jasco International Co., Ltd, Tokyo, Japan) equipped with an Athena C18 reversed-phase column (250 mm×4.6 mm, 5 μm), a UV/VIS detector (UV-2075 plus, Jasco International Co., Ltd, Tokyo, Japan), and a quaternary gradient pump (PU-2089 Plus, Jasco International Co., Ltd., Tokyo, Japan). The mobile phase contains 1% acetic acid aqueous solution (A) and acetonitrile (B), a flow rate of 0.7 mL/min, column temperature was set at 25°C and the injection volume of 20 μL extract. Gradient elution was carried out by varying the proportion of mobile phases B and A in the eluent. The gradient elution was changed from 10 to 40% B in a linear fashion for the duration of 28 min, from 40 to 60% B in 39 min, from 60 to 90% B in 50 min. The UV detector was set at 272 nm and each compound was identified by comparing the retention time and UV/VIS spectra with those of standards. Compounds were quantified from the calibration curve of the corresponding standards.
Experiments were conducted in triplicate, and the results were expressed as mean±SD. Significant difference (p<0.05) in mean were separated by analysis of variance (ANOVA) and Duncan’s multiple range tests using SPSS software (Version 20.0, SPSS Inc., Chicago, IL, USA). Principal component analysis (PCA) was carried out using STATISTICA 8.0 (StatSoft Inc., Tulsa, OK, USA) to further explain the variation in the data.
Results and discussion
The effect of ethanol concentration on the extraction of OKL polyphenols and their antioxidant activities is shown in Fig. 2. Significant differences (p<0.05) in the extraction yield (8.10-27.13%), TPC (4.33-7.51 mg GAE/g), TFC (8.36-28.27 mg RE/g), DPPH RSC (1.13-8.70 mg AAE/g) and ABTS activity (3.32-14.15 mg AAE/g) were observed for different ethanol concentrations. At absolute and lower ethanol concentrations, the extraction yield, polyphenolic contents (TPC and TFC), and antioxidant activities (DPPH RSC, ABTS) were relatively lower, compared to 40% and 60% ethanol concentrations. This is consistent with previous studies, wherein the aqueous organic solvents were found to exhibit higher extraction efficacy compared to low-concentration and absolute organic solvents (Fu et al., 2016; Metrouh-Amir et al., 2015; Wijekoon et al., 2011). Water and ethanol have specific roles in the extraction of phenolic compounds. The presence of ethanol in the extraction solvent creates a concentration gradient depending on the amount of ethanol, and this aids the diffusion of the solvent into the solute, subsequently improving the mass transfer (Ali et al., 2018). Besides, water assists the swelling of solute, while ethanol disrupts the polyphenolic bonds in the solute (Cujic et al., 2016; Yang et al., 2017). Extraction yield of phenolic compounds. A combination of these two phenomena is probably responsible for the higher polyphenolic yield, as observed in this study. Thus, 60% ethanol was the optimal solvent for the extraction of OKL polyphenolic compounds.
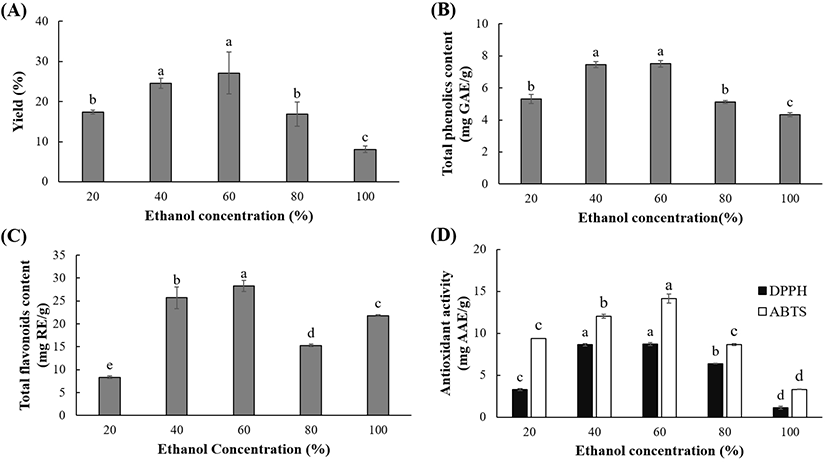
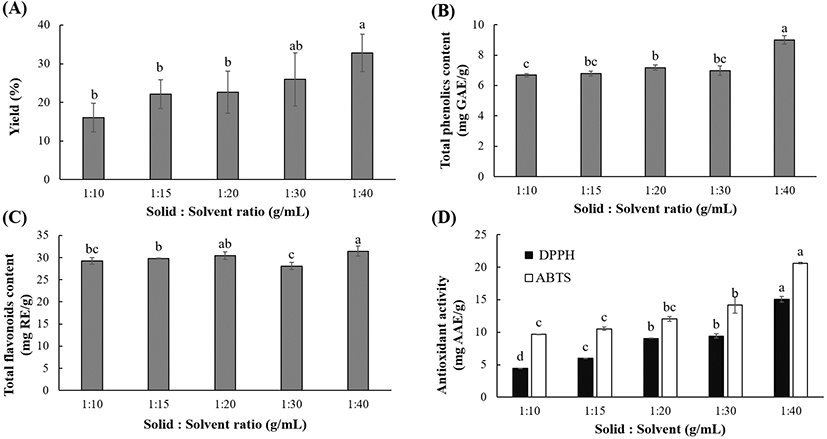
Fig. 3 shows that the solid-to-solvent ratio significantly (p<0.05) affects the extraction yield, polyphenolic content, and antioxidant activity. The yield (16.02-32.80%), TPC (6.68-9.00 mg GAE/g), and TFC (28.07-31.44 mg RE/g) were higher when the solvent ratio was increased from 10 to 40 g/mL. Likewise, the DPPH RSC (4.42-15.05 mg AAE/g) and ABTS activity (9.66-20.63 mg AAE/g) progressively increased with increasing solvent ratio. This could be attributed to the increased contact area, which improved the solubility and further increased the dissolution of polyphenolics into the solvent during the UAE. Similar findings have been reported for polyphenolics extracted from other natural materials (Vuong et al., 2011; Vuong et al., 2013; Xu et al., 2017). Furthermore, the mass transfer is considered dependent on the gradient between the solid and solvent (Belwal et al., 2016). At higher solvent-to-solid ratios, the concentration gradient between the polyphenols trapped in the particles and surface of the leaf powder is larger, which favors the mass transfer and accelerates the extraction kinetics (Vuong et al., 2013). Moreover, since UAE uses acoustic cavitation to generate and disrupt bubbles for facilitating the mass transfer, it has been hypothesized that solvent ratio could increase the the ultrasonic extraction efficiency (Moorthy et al., 2017).
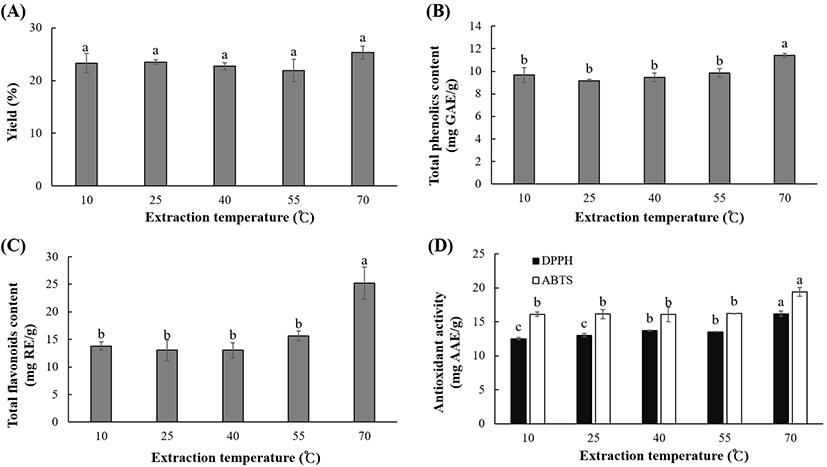
The effect of temperature (10-70°C) on the extraction yield, polyphenolic content, and antioxidant activity of OKL extracts is shown in Fig. 4. The extraction yield was slightly higher at 70°C; however, there were no significant differences (p<0.05) in the yield at different temperatures. The extraction temperature significantly (p<0.05) influenced the polyphenolic content, with TPC and TFC ranging between 9.16 and 11.42 mg GAE/g and 13.04 and 25.24 mg RE/g, respectively. At temperatures between 10 and 55°C, the DPPH RSC (12.52-13.76 mg AAE/g) and ABTS activity (-16 mg AAE/g) activity were similar. However, the antioxidant activities of OKL extract were significantly higher at a higher temperature of 70°C (16.22 mg AAE/g and 19.41 mg AAE/g for DPPH RSC and ABTS activities, respectively). This suggests that high temperatures favor the UAE of OKL polyphenolics. Optimal extraction of polyphenolics from various materials such as pawpaw leaves (Vuong et al., 2013), green tea (Vuong et al., 2011), piper betle (Ali et al., 2018), and grape pomace (Pinelo et al., 2005) has been reported to be temperature-dependent in the same temperature range observed in this study. Increase in the extracted polyphenolic content, as observed in this study, may be due to the enhanced solubility in the solvent and the decreased intermolecular interactions owing to the elevated temperature (Jianming et al., 2013). Furthermore, a high temperature promotes the formation of ultrasonic cavitation and mass transfer (Entezari and Kruus, 1996), thereby improving the extraction efficiency and enhancing the solvent penetration into the cell components of OKL (Filgueiras et al., 2000).
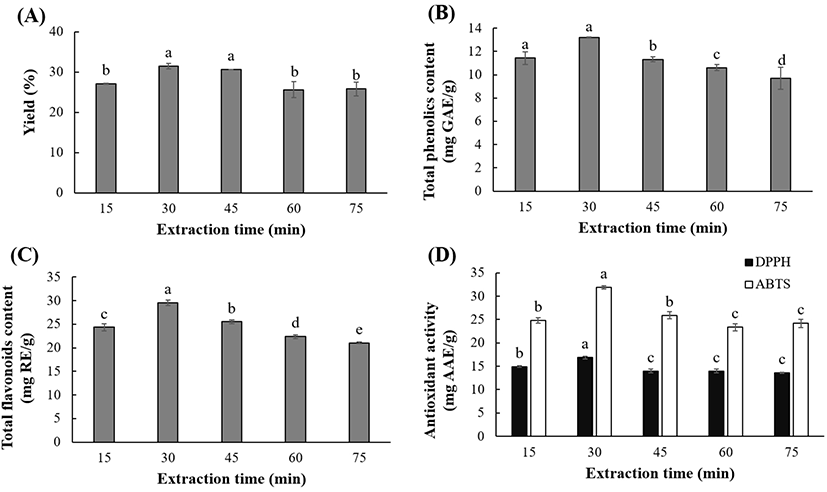
The extraction time is an important factor as it relates to process efficiency rating and energy-saving. UAE times of 15 to 75 min were considered in this study. The extraction yield (25.60-31.50%), TPC (9.70-13.21 mg GAE/g), TFC (21.05-29.57 mg RE/g), DPPH RSC (13.58-16.86 mg AAE/g), and ABTS activity (23.37-31.90 mg AAE/g) were significantly (p<0.05) affected by the extraction time (Fig. 5). The TPC, TFC, and antioxidant activities increased significantly (p<0.05) upon increasing the extraction time from 15 to 30 min. Beyond 30 min, the yield of polyphenolics and the antioxidant activities of the OKL extracts decreased. The decreased polyphenolic contents can perhaps be attributed to factors such as chemical decomposition, loss of mass transfer, solvent vaporization, and reduced solvent permeability, which are associated with prolonged ultrasonic extraction (Ali et al., 2018; Tan et al., 2013).
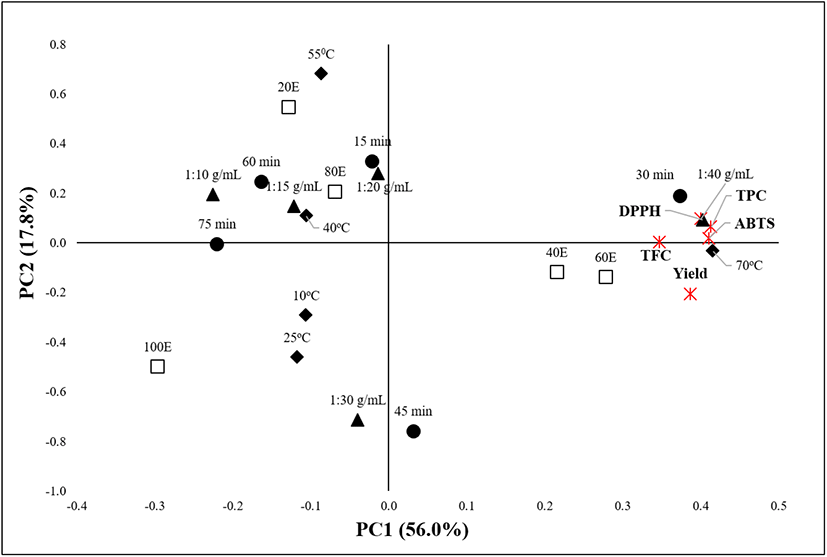
The principal component analysis (PCA) is a useful tool applicable to determine correlative relationships between variables and optimal parameters for the extraction process (Tarko et al., 2017). It incorporates highly related variables into a principal component (PC) that accounts for the maximum variance in the observations (Ahmadian-Kouchaksaraie et al., 2016). The data of this study were subjected to PCA. The PCA described 73.8% of the data, with PC1 and PC2 accounting for 56.0% and 17.8% of the total variations, respectively (Fig. 6). PC1 positively correlated with the yield, polyphenolic contents (TPC and TFC), and antioxidant activities (DPPH RSC and ABTS), with the following extraction variables occupying the same axis: 60% ethanol, 1:40 g/mL solid-to-solvent ratio, 70°C, and 30-45 min. PC2 positively correlated with the TPC, TFC, DPPH RSC, and ABTS activity but negatively correlated with the yield, implying a distinct relationship between the polyphenolic compounds and their antioxidant activities. Thus, it can be concluded that OKL polyphenolics actively contribute to the antioxidant activity of the extract. Overall, the PCA confirmed that the experimentally determined optimal extraction conditions (60% ethanol, 1:40 g/mL, 70°C, and 30 min) indeed favor the UAE of OKL polyphenolics.
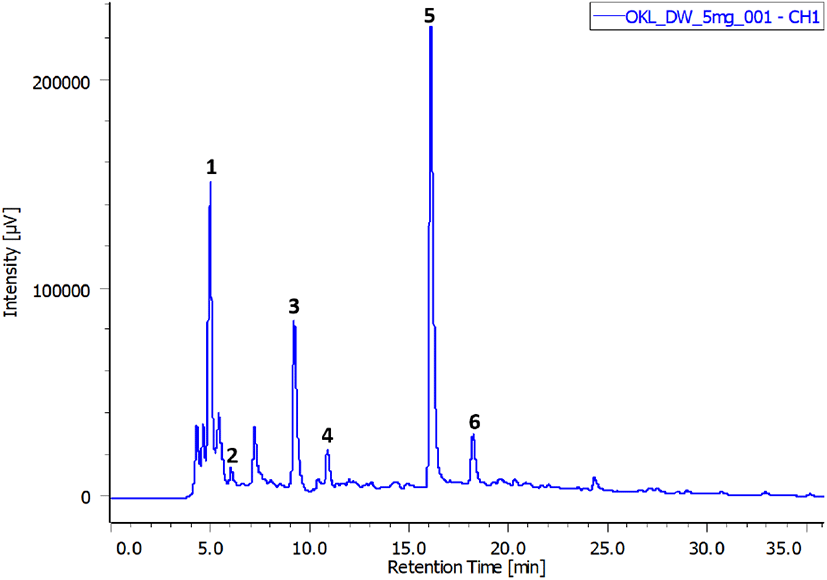
Freeze-dried extract obtained under the optimal extraction conditions (60% ethanol, 1:40 g/mL, 70°C, and 30 min) was used for this assay. Fig. 7 demonstrates the polyphenolic profile of OKL, and Table 1 lists the concentrations of the identified compounds in the extract. Six compounds were identified, namely, chlorogenic acid, gallic acid, catechin, caffeic acid, p-coumaric acid, and ferulic acid, and their contents were 25.91, 3.94, 52.83, 1.04, 16.29, and 6.12 mg/g, respectively. Chlorogenic acid, p-coumaric acid (phenolic acid), and catechin (flavonoid) were the predominant polyphenolic compounds in OKL. Catechin, which is the most abundant compound in OKL, is a natural antioxidant present in many plants such as green tea (Vuong et al., 2011). Similarly, p-coumaric acid and catechin have also been identified in substantial amounts in okra fruits (Arapitsas, 2008).
In conclusion, polyphenolic compounds were successfully extracted from OKL by UAE under different extraction conditions. This is the first study revealing the effects of solvent concentration, solid-to-solvent ratio, extraction temperature, and extraction time on the OKL polyphenolic recovery and antioxidant activities to obtain the optimal extraction conditions. Optimal conditions for OKL extraction were 60% ethanol, solid-to-solvent ratio of 1:40 g/mL, temperature of 70°C, and extraction time of 30 min. High polyphenolic contents (TPC and TFC) and antioxidant activities (DPPH and ABTS) were obtained under the optimal conditions. Furthermore, the PCA established a positive correlation between the polyphenolic compounds in OKL and the antioxidant activity. Finally, phenolic acids including chlorogenic acid, gallic acid, caffeic acid, p-coumaric acid, and ferulic acid and flavonoids such as catechin were identified as the major polyphenolic compounds in the OKL extract. This study could lay the foundation for utilizing OKL as a functional material.