1. Introduction
Fermentation is a natural process that has been used for thousands of years to preserve food and enhance its taste, texture, and nutritional value (Sharma et al., 2020). Fermented foods have also been associated with reduced risk of chronic diseases such as obesity, diabetes, and heart disease (Chan et al., 2019; Gille et al., 2018). They are a significant part of many traditional diets worldwide, from kimchi in Korea to sauerkraut in Germany and kefir in the Middle East (Dimidi et al., 2019). In recent years, there has been growing interest in fermented foods, both for their unique flavors and their potential health benefits (El Sheikha and Hu, 2020; Ilango and Antony, 2021).
Cabbage is a low-calorie, mineral-rich cruciferous vegetable and an excellent source of vitamins C and K, as well as dietary fiber (Park et al., 2014). In addition to its nutritional content, cabbage provides various health benefits, including improved digestion, antioxidant effects, and immune function enhancement (Draghici et al., 2013; Nilnakara et al., 2009; Zou et al., 2021). Although cabbage can be consumed raw or as juice, fermentation can enhance its functional compounds, making it a more beneficial food (Patra et al., 2016; Siddeeg et al., 2022). Sulforaphane, a compound abundant in cruciferous vegetables including cabbage, is clinically recognized for its role in the prevention and treatment of chronic diseases and is stored in the form of glucoraphanin (Mazarakis et al., 2020). When the plant tissue is physically damaged, glucoraphanin is converted into sulforaphane by the enzyme myrosinase, which is also produced by probiotics and certain microorganisms (Luang In et al., 2018). Due to its structural instability, it is difficult to obtain sulforaphane directly from plant tissue (Sangkret et al., 2019), but its production can be increased through microbial fermentation (Cai et al., 2019).
Bacillus spp. are naturally abundant microorganisms that have been widely used in traditional fermented foods (Li et al., 2023). Various enzymes such as proteases produced by Bacillus spp. enhance the umami taste, release functional ingredients, and act as beneficial enzymes (Lee et al., 2023). Moreover, their wide growth temperature range enables the production of heat-stable enzymes (Berendsen et al., 2016), which can withstand food processing heat treatment (Seo et al., 2013). Additionally, the exopolysaccharides (EPS) produced by Bacillus are non-toxic biopolymer secreted into the environment, contributing to biofilm formation, cell growth, and desiccation protection (Diaz Cornejo et al., 2023; Nwodo et al., 2012). Due to these characteristics, Bacillus species hold great potential for applications not only in food but also in medicine, cosmetics, agriculture and environmental fields.
In this study, Bacillus spp. with excellent enzyme activity applicable to food production were identified, and a fermented cabbage powder with enhanced activities of multiple enzymes and improved functional properties was successfully developed.
2. Materials and methods
Keumjeong sanseongnuruk (Sanseongnuruk, Busan, Korea) and Soyulgok (Songhakgokja, Gwangju, Korea) were used as a source of nuruk for microorganism isolation. For comparison of enzyme activity, the type strain Bacillus subtilis ATCC6051 was used as the control. Microbial isolation was performed using Luria-Bertani (LB) agar plates containing 2% agar and enzyme activity substrates. The isolated strains were cultured in LB broth.
2.2. Isolation of Bacillus spp.
One gram of each nuruk sample was weighed and suspended in 10 mL of distilled water, followed by serial dilution to an appropriate concentration. After transferring 1 mL of the suspension to a 1.5 mL Eppendorf tube, the sample was heated at 100°C for 30 minutes using a heat block, and 100 μL of the heated suspension was spread onto an LB agar plate and incubated at 37°C. Single colonies were subsequently streaked onto fresh LB agar plates to obtain pure isolates, which were used in the following experiments.
Qualitative analysis of enzyme activity was assessed by observing clear zones on each agar plate. Protease activity was determined by inoculating a single colony onto an agar plate supplemented with 2% skim milk in LB broth and incubating at 37°C for 48 h, after which clear zones were observed (Abbasi Hosseini et al., 2011). To evaluate α-amylase activity, a single colony was cultured on LB agar supplemented with 1% soluble starch, incubated at 37°C for 48 h, and then stained with 1% Lugol’s iodine solution (0.5% I2 and 1% KI, w/v). Colonies that formed clear zone due to starch degradation were selected for further analysis (Ashwini et al., 2011).
For quantitative analysis of α-amylase activity, 1% (w/v) soluble starch was dissolved in 50 mM phosphate buffer (pH 6.0) and used as the substrate. A total of 200 μL, comprising equal volumes (100 μL each) of culture supernatant and 1% starch solution, was mixed and incubated at 30°C for 20 minutes. The amount of reducing sugar produced was quantified by measuring absorbance at 560 nm using the di-nitrosalicylic acid (DNS) method (Miller, 1959). One unit of amylase activity was defined as the amount of enzyme that produces 1 nmol of reducing sugar per minute.
Protease activity was measured by a modified azo-casein assay (Coêlho et al., 2016). Specifically, 100 μL of culture supernatant was added to 100 μL of 1% (w/v) azo-casein dissolved in Tris-HCl buffer (pH 8.0) and incubated at 40°C for 1 hour. The reaction was terminated by adding 200 μL of 20% (w/v) trichloroacetic acid, followed by chilling on ice for 5 minutes. After centrifugation at 13,000 rpm and 4°C, 160 μL of the supernatant was mixed with 40 μL of 1.8 M NaOH, and absorbance was measured at 420 nm using a spectrophotometer (SpectraMax iD3, Berthold Technologies, San Jose, CA, USA). One unit of protease activity was defined as the amount of enzyme that increases OD at 420 nm by 0.001 per minute at 40°C. For freeze-dried powder samples, enzyme activity was measured after rehydration to the original volume using distilled water.
Identification of the isolated strain was performed through phylogenetic analysis based on the nucleotide sequence of the 16S rRNA region. The sequences were obtained by genome amplification using the 27F and 1492R primers. Reference sequences for constructing the phylogenetic tree were retrieved from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/). The sequence alignment was conducted using the Clustal X program, followed by manual adjustments. A phylogenetic tree was constructed using the Kimura two-parameter model (Kimura, 1980) and neighbor-joining method (Saitou and Nei, 1987) implemented in MEGA 5 software. Bootstrap analysis with 1,000 replicates was performed to assess the confidence level at each node.
Commercial cabbage powder (Dusonaeyagcho, Yeongcheon, Korea) was purchased and used for the fermentation experiments. Distilled water was added to the cabbage powder to achieve a 10% (w/v) solid loading, and the mixture was sterilized by autoclave (SANYO, MLS-3781L, Osaka, Japan) at 121°C for 15 minutes. Bacillus strains pre-cultured in LB broth at 30°C and 250 rpm for 20 h were inoculated into the sterilized cabbage medium to an initial OD600 of 1.0, and fermentation was conducted at 30°C and 250 rpm. Fermentation was terminated when glucose and fructose were completely consumed, as determined by HPLC analysis.
The fermented cabbage was freeze-dried at −80°C for 96 h using a freeze dryer (FDS8518, ilShinBioBaseCo., Yangju, Korea). For the samples with added excipients, 5% (w/v) sucrose or mannitol was added prior to freeze-drying. After drying, the powder was weighed to determine the freeze-drying yield.
A total of 100 μg of each freeze-dried powder was placed in a 1.5 mL Eppendorf tube and heat-treated using a heat block at 60°C for 12 h or 90°C for 4 h. After heat treatment, the samples were rehydrated with distilled water, and protease activity was measured as described above. Enzyme stability (%) was calculated as the percentage of protease activity retained after heat treatment relative to the protease activity before heat treatment.
The entire freeze-dried product obtained from 50 mL of 10% fermented cabbage was used for analysis. Sulforaphane was extracted using a modified extraction method (Liang et al., 2006). Specifically, 50 mL of dichloromethane containing 2.5 g of anhydrous sodium sulfate was added to the freeze-dried powder, and ultrasonic extraction was performed at 30°C for 5 minutes. The supernatant was collected by centrifugation at 3,134 ×g for 5 minutes. The remaining pellet was re-extracted by adding 50 mL of dichloromethane, followed by the same ultrasonic extraction and centrifugation steps to obtain a second supernatant. The combined supernatants were vacuum-filtered (11 μm), and the solvent was evaporated at 30°C. The resulting residue was dissolved in 4 mL of acetonitrile and filtered through a 0.2 μm syringe filter for HPLC analysis.
HPLC analysis was performed to determine the fermentation profile and sulforaphane content. The fermented sample was diluted 10-fold, centrifuged at 15,928 ×g for 10 minutes, and filtered through a 0.2 μm syringe filter prior to analysis. HPLC was conducted using an Agilent 1260 system (Agilent Technologies, Santa Clara, CA, USA) equipped with a Rezex-ROA Organic Acid H+ (8%) column (150 mm×4.6 mm; Phenomenex Inc., Torrance, CA, USA). The mobile phase was 0.005 N H2SO4 at 50°C, with a flow rate 0.6 mL/min (Kim et al., 2012).
Sulforaphane was analyzed using HPLC (Alliance 2796 separation system, Waters, Milford, MA, USA) equipped with a 2996 photodiode array detector and a Kinetex C18 100Å column (5 μm, 150 mm×4.6 mm: Phenomenex Inc.). The filtration extract was analyzed using a water/acetonitrile (3:7, v/v) mobile phase at a flow rate of 0.6 mL/min. Sulforaphane was detected at 205 nm.
All data were analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test to determine significant differences among treatments. Statistical analysis was performed using SPSS Statistics 22 software (IBM Corp., Armonk, NY, USA), and significance was considered at p<0.05.
3. Results and discussion
A total of 24 Bacillus strains were isolated from two types of traditional nuruk collected from different production regions. Nuruk, a saccharification and fermentation starter produced by steaming starch-based materials such as wheat and rice, naturally harbors microorganisms and serves as a valuable source for screening strains with high enzymatic activity (Bal et al., 2015). Because non-spore forming strains are eliminated by heat treatment at 100°C, Bacillus spp., which form spores, could be selectively isolated (Lucking et al., 2013). Amylase activity was qualitatively confirmed in all strains by observing clear zones on LB agar plates supplemented with soluble starch, while protease activity was detected only in S1-S12 strains. This difference likely reflects variations in microbial communities present in nuruk, influenced by production method and regional characteristics (Park et al., 2018). Quantitative analysis of enzyme activities was performed on the strains that showed positive results in qualitative assay, and the results are presented in Table 1. The α-amylase activity ranged from 17.21 to 236.42 U/mL, showing substantial variation among strains; notably, eight strains exhibited higher α-amylase activity compared to the control strain, B. subtilis. Protease activity was observed in 12 strains, ranging from 26.42 to 118.50 U/mL, consistent with the clear zone results in the qualitative analysis.
Four Bacillus spp. strains exhibiting excellent α-amylase and protease were selected, and the results of their identification and phylogenetic analysis based on 16S rRNA sequencing are presented in Fig. 1. Two strains were identified as Bacillus amyloliquefaciens (S2, S11) and two strains of Bacillus subtilis (S4, S9), showing 99-100% sequence similarity to reference strains. Both species are generally recognized as safe (GRAS) organisms and are widely used in various fermented foods, including soybean paste and natto. Therefore, these four isolated strains are considered suitable as host strains for cabbage fermentation.
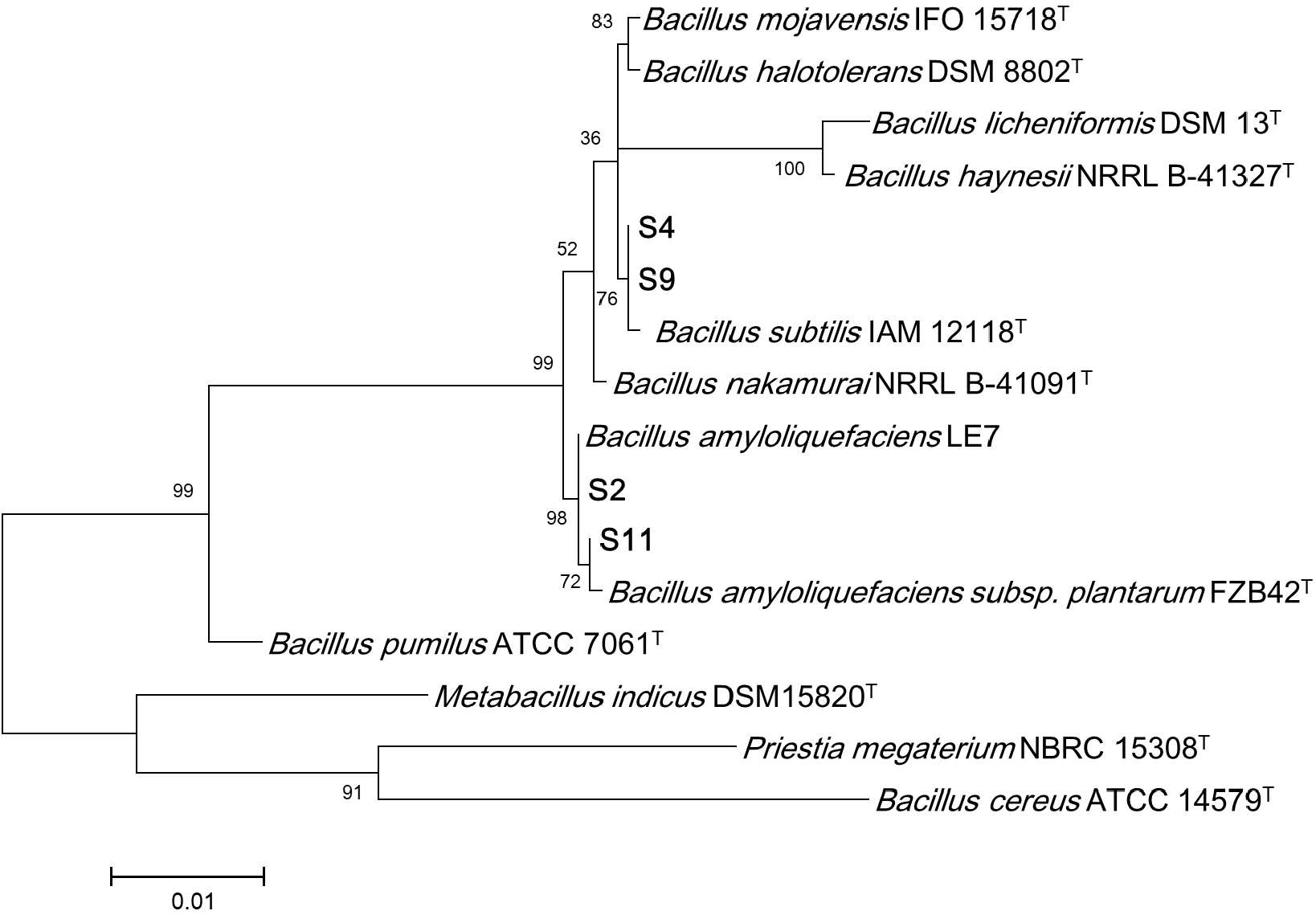
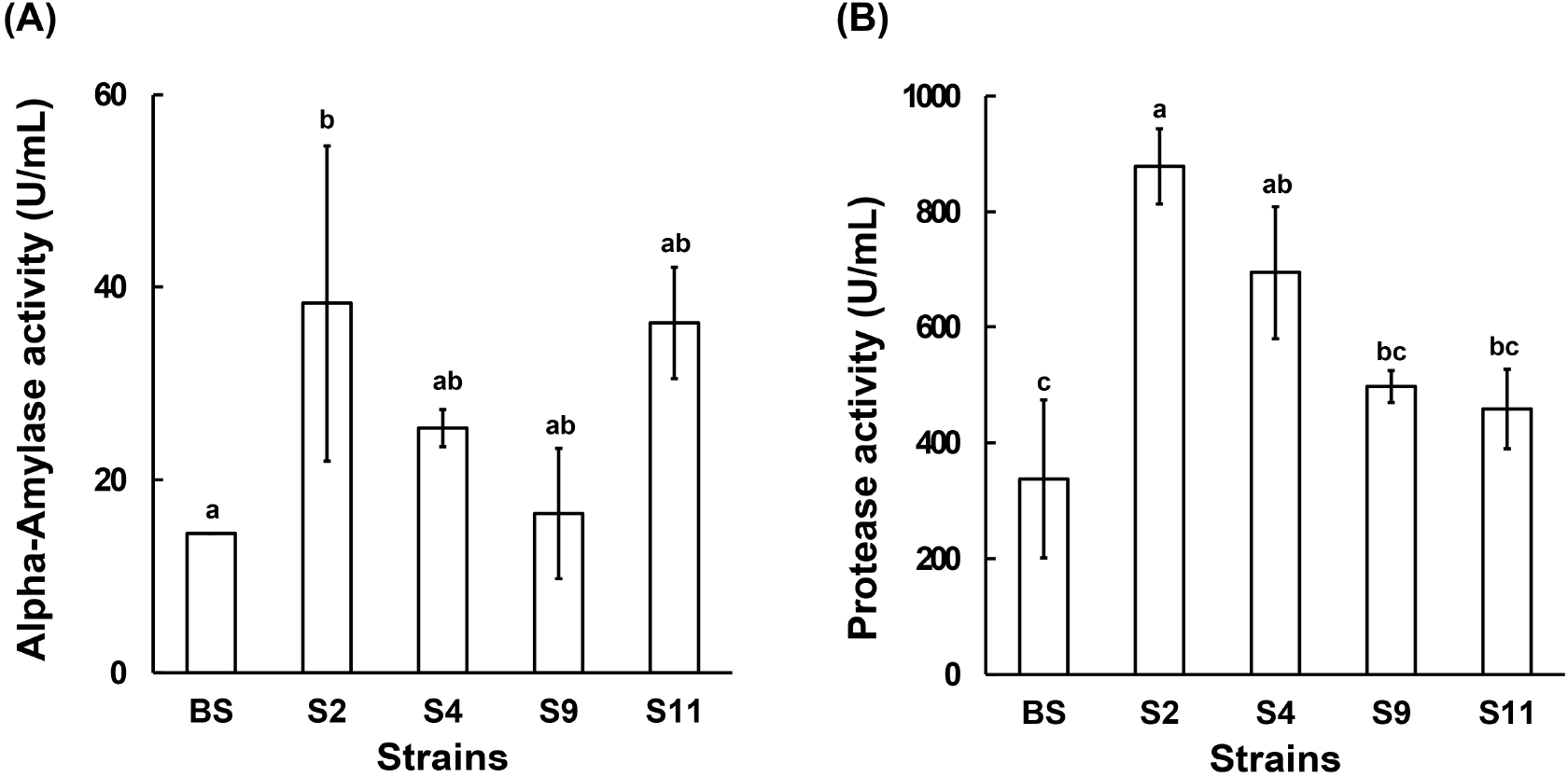
The enzymatic activities of the four selected Bacillus spp. strains in cabbage fermentation are shown in Fig. 2. Since residual reducing sugar could affect the α-amylase measurements, enzyme activity analysis was conducted after 36 h of fermentation, when all sugars were depleted (data not shown). Regarding α-amylase activity, B. amyloliquefaciens S2 and S11 exhibited activities of 38.30 and 36.26 U/mL, respectively. Notably, S2 showed the highest protease activity among the strains tested. Therefore, B. amyloliquefaciens S2 was selected as the most suitable strain for cabbage fermentation.
Bacillus amyloliquefaciens S2 was inoculated into 10% cabbage powder solution and aerobically fermented for 24 h. The metabolic profile before and after fermentation is shown in Table 2. According to previous studies, enzyme production, including amylase, is generally more efficient under aerobic conditions than anaerobic conditions, and fermentation time can be shortened due to rapid cell growth (Gangadharan et al., 2011; Milner et al., 1996). After 24 h of fermentation, S2 completely consumed the available glucose (decreased from 12.94±1.65 g/L to 0.14±0.01 g/L) and fructose (from 21.13±3.59 g/L to below the detection limit), while producing xylose (3.14±0.76 g/L), acetate (2.00±0.70 g/L), and ethanol (0.90±0.07 g/L). B. amyloliquefaciens possesses multiple enzymatic activities, including xylanase, β-glucosidase, and pectinase, in addition to amylase and protease, which can lead to the breakdown of cabbage cell wall components and release of sugars such as xylose (WoldemariamYohannes et al., 2020). Furthermore, organic acids such as acetate and minor amounts of ethanol can be produced through central carbon metabolism pathways (Yan et al., 2013).
| Units (g/L) | Before fermentation | Fermentation |
|---|---|---|
| Glucose | 12.94±1.651)a2) | 0.14±0.01b |
| Xylose | ND3) | 3.14±0.76 |
| Fructose | 21.13±3.59 | ND |
| Glycerol | 0.30±0.43 | ND |
| Acetate | 0.67±0.10 | 2.00±0.70 |
| Ethanol | ND | 0.90±0.07 |
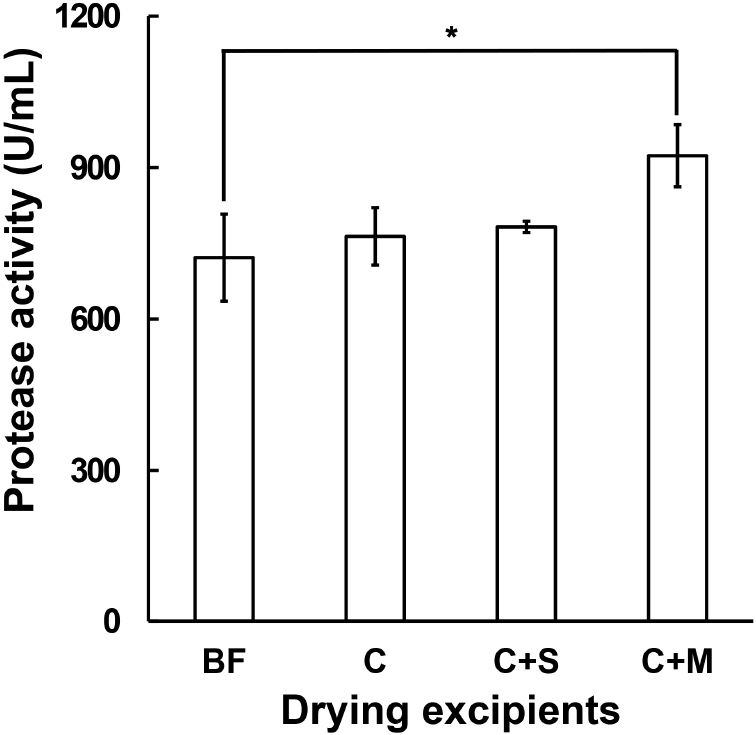
Fig. 3 shows the protease activity of samples before and after freeze-drying with or without the addition of excipients. The enzyme activities of the samples without excipient and with 5% sucrose addition were similar to the levels observed before freeze-drying. This indicates that freeze-drying is a highly suitable drying method for preserving enzyme activity and suggests that even without the addition of excipients, high preservation can be achieved, possibly due to the formation of natural polymers such as exopolysaccharides (EPS), which act as natural cryoprotectants (Kim and Yim, 2007). Notably, B. amyloliquefaciens is known for its high EPS production capacity (Deka et al., 2019), which may have contributed to the observed enzyme stability without excipient addition. Among the tested excipients, the addition of 5% mannitol resulted in the highest protease activity after freeze-drying. Mannitol is chemically inert and non-hygroscopic, making it effective at reducing freeze-drying time and cost, and it is widely used as an excipient in pharmaceutical formulations (Thakral et al., 2022). Additionally, as a sugar alcohol and alternative sweetener (Ghosh et al., 2011), mannitol serves as a highly suitable excipient for producing fermented cabbage freeze-dried products.
The thermal stability of protease activity in fermented cabbage powder is shown in Fig. 4. When treated at 60°C for 12 h, both the control sample (without excipient) and the sample with 5% mannitol showed enzyme stability close to 100%. This is consistent with previous reports that protease produced by B. amyloliquefaciens exhibits high activity and thermostability around 60°C (Hassan et al., 2013), providing an advantage over enzymes from other microorganisms. However, after heat treatment at 90°C for 4 h, enzyme stability decreased to approximately 52-57% in both samples. This reduction can be attributed to the known thermal sensitivity of Bacillus protease, which maintains stability up to around 60°C but shows significantly reduced activity above 80°C (Moradian et al., 2009). These results suggest that residual enzyme activity after fermentation was maintained not only due to the intrinsic thermostability of B. amyloliquefaciens protease but also due to the protective effect of extracellular polysaccharides (EPS) produced during fermentation, which may stabilize enzyme structures under heat stress (Deka et al., 2019; Kim and Yim, 2007).
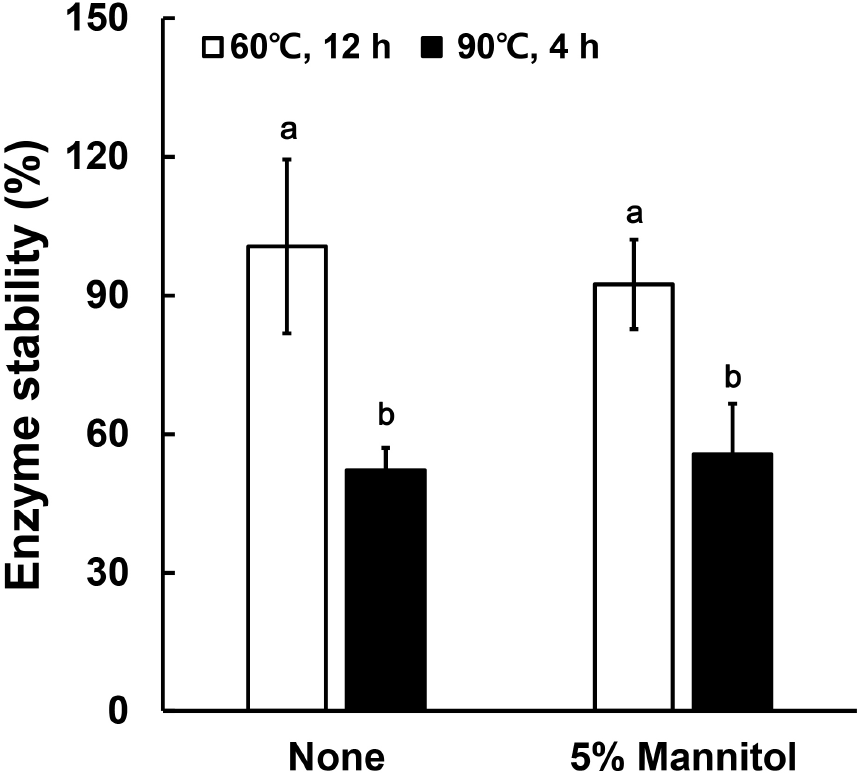
Table 3 shows the functional properties of cabbage powder before and after fermentation. Enzyme activity and sulforaphane contents were compared per gram of cabbage powder used for fermentation. The fermented freeze-dried cabbage powder showed α-amylase activity ranging from 1,948.9±544.1 U/g (without excipient) to 2,407.7±298.2 U/g (with 5% mannitol), with no statistically significant difference between the two treatments. However, the addition of 5% mannitol resulted in significantly higher protease activity (3,428.5±47.5 U/g) compared to the sample without excipient (2,902.0±108.8 U/g). Sulforaphane content increased markedly through fermentation, reaching over 100 μg/g, which corresponds to approximately a 150-fold increase compared to the unfermented powder (0.5±0.9 μg/g). This increase is attributed to the conversion of glucoraphanin to sulforaphane by the myrosinase activity of B. amyloliquefaciens S2 (Luang In et al., 2018; Youseif et al., 2022). The enhanced protease activity and sulforaphane content observed in the 5% mannitol-added group may be attributed to mannitol’s role as a compatible solute, which stabilizes protein conformation and protects microbial cells under fermentation and drying stress (Ghosh et al., 2011; Thakral et al., 2023). Additionally, mannitol may influence redox homeostasis and enzyme activity, thereby indirectly promoting the conversion of glucoraphanin to sulforaphane (Luang In et al., 2018).
4. Conclusions
Cabbage has long been consumed as a fermented food to enhance its flavor and functionality. Advances in microbial characterization and analytical techniques have facilitated the development of fermented products tailored to specific purposes. In this study, several Bacillus species with excellent enzymatic activity were isolated from Korean traditional nuruk, a source rich in diverse microbial communities, and the most suitable strain for cabbage fermentation was selected. The selected strain, B. amyloliquefaciens, is a food-grade microorganism widely used in various fermented foods due to its high protease activity. Freeze-drying was identified as an effective method for preserving enzyme stability. Moreover, mannitol contributed to improving the texture and sweetness of the final powder product. The fermentation process not only enhanced α-amylase and protease activities but also significantly increased the levels of functional compounds such as sulforaphane. Overall, the results of this study suggest that B. amyloliquefaciens is a promising host strain for the production of functional fermented cabbage powders.
