1. Introduction
The milkfish (Chanos chanos) is extensively cultivated in Indonesia and holds significant economic importance (Aris et al., 2021). South Sulawesi recorded the highest milkfish production in Indonesia, reaching 169,688 tons in 2017 and increasing to 183,356 tons in 2019. Compared to common cultured freshwater species such as goldfish, snakehead (cork fish), and catfish, milkfish belongs to a group of fish characterized by their high protein content (Aristiani et al., 2022). A 100 g serving of milkfish meat contains 129 kcal of energy, 20 g of protein, 20 mg of calcium, 4.8 g of fat, 0.1 g of iron, 150 mg of phosphorus, 0.05mg of vitamin B1, and 150 IU of vitamin A (Malle et al., 2019). Given its high protein content and substantial production in South Sulawesi, milkfish has strong potential for producing fish protein hydrolysate (FPH), offering an opportunity to enhance its economic value (Dinakarkumar et al., 2022).
Fish protein hydrolysate is the result of breaking down proteins into peptides and free amino acids through enzymatic hydrolysis, acids hydrolysis, or base hydrolysis (Fitriyani et al., 2021). FPH contains bioactive compounds with potential applications in the food and pharmaceutical industries (Slizyte et al., 2016). In addition, FPH can be used as a food supplement or consumed directly to increase protein intake (Utomo et al., 2014). FPH exhibits functional properties that display bioactive characteristics, including antioxidant activity (Slizyte et al., 2016). Antioxidants have a function to counteract free radicals and inhibit them through a hydrogen atom donor mechanism (Boligon, 2014).
Currently, various methods are available for producing FPH; however, enzymatic hydrolysis is considered superior, as it preserves amino acid content and is relatively fast (Utomo et al., 2014). Several studies have emphasized the important role of enzymes in the hydrolysis process of fish protein hydrolysates. Wijayanti et al. (2016) suggested that the concentration of bromelain enzyme has a significant effect on milkfish FPH, with the best results achieved at an enzyme concentration of 6% and a temperature of 55°C for 6 h. Similarly, Wijayanti et al. (2015) found that the optimal conditions for papain were 5% enzyme concentration at 55°C for 6 h. Previous research on milkfish protein hydrolysate using bromelain has primarily focused on liquid hydrolysates. Therefore, this study was conducted to develop a dried form of milkfish FPH by optimizing hydrolysis time and enzyme concentration. The best treatment was determined based on proximate composition, yield, soluble protein content, molecular weight distribution, amino acid profile, and antioxidant activity, with the goal of enabling its application in food products or dietary supplements.
2. Materials and methods
Samples of C. chanos (1.0-2.0 kg/fish) were obtained from aquaculture ponds in the Pattene area, Maros, South Sulawesi, Indonesia. Analytical-grade chemicals and solvents were used in this study, including glacial acetic acid (CH-COOH) and sodium hydroxide (NaOH), which were purchased from Merck (Merck, Darmstadt, Hessen, Germany) and Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). The bromelain enzyme (50 U/g) was obtained from Nanning Pangbo Bioengineering Co., Ltd. (Nanning, Guangxi, China).
Minced milkfish meat was mixed with distilled water (Bratachem, Jakarta, Indonesia) in a ratio of 1:2 (w/v). Bromelain enzyme was added to the mixture at concentrations of 5, 6, and 7% (w/w). The enzymatic hydrolysis was conducted at 55°C for 5, 6, and 7 h using a water bath (WB-11, Memmert, Schwabach, Bavaria, Germany). The pH of the reaction mixture was maintained at 7.0 by adjusting with CH3COOH and NaOH during the process.
After hydrolysis, the mixture was heated at 90°C for 20 min to inactivate the enzyme. The hydrolysate was filtered using Whatman filter paper No. 1 (Whatman, Maidstone, Kent, UK) and centrifuged at 4°C for 15 min at 10,000 rpm using a centrifuge (Model 5810R, Eppendorf, Hamburg, Germany) to obtain the fish protein hydrolysate filtrate. The resulting filtrate was freeze-dried using a freeze dryer (Alpha 1-2 LDplus, Martin Christ, Osterode am Harz, Lower Saxony, Germany) at −50°C for 49 h. The obtained hydrolysate powder was stored in a sealed container.
The proximate analysis of milkfish protein hydrolysate included the determination of moisture, ash, and fat content using standard methods of the Association of Official Analytical Chemists (AOAC, Washington, DC, USA). Moisture content was determined using AOAC method 14.004 (1984), ash content using AOAC method 14.009 (1984), and fat content using AOAC method 14.006 (1984). The analysis was performed using an oven (UN110, Memmert, Schwabach, Bavaria, Germany) for drying, a muffle furnace (Furnace 62700, Barnstead Thermolyne, Dubuque, IA, USA) for ash determination, and a Soxhlet apparatus (Soxtec 8000, FOSS, Hillerød, Denmark) for fat extraction. The yield of the hydrolysate was calculated based on the weight of the final product relative to the initial amount of fish meat substrate used.
Soluble protein content was determined using the Lowry method, adapted for fish protein hydrolysate (FPH) samples (Witono et al., 2020). A 0.1 g sample of FPH was dissolved in 100 mL of distilled water (Bratachem, Jakarta, Indonesia). From this solution, 1 mL was pipetted and diluted to 2 mL with distilled water. Then, 0.5 mL of the diluted solution was pipetted, mixed with 1.5 mL of distilled water, and subsequently mixed with 2.75 mL of Lowry reagent (Sigma-Aldrich, St. Louis, MO, USA). The mixture was then incubated for 15 min at room temperature. After incubation, 0.25 mL of Folin-Ciocalteu phenol reagent (Merck) was added, and the mixture was vortexed using a vortex mixer (VM-300, Gemmy Industrial Corp., Taipei, Taiwan). The mixture was allowed to stand for 30 min, and absorbance was then measured at 650 nm using a spectrophotometer (UV-1800, Shimadzu Corp., Kyoto, Japan).
A total of 20 mg of fish protein hydrolysate was added to 20 mL of 10% (b/v) trichloroacetic acid (TCA) solution (Merck). The mixture was allowed to stand for 30 min at room temperature and then centrifuged at 7,800 relative centrifugal force (RCF) or 15 min using a centrifuge (Model 5810R, Eppendorf, Hamburg, Germany). The resulting supernatant was analyzed for nitrogen content using the Kjeldahl method with a digestion unit (Kjeltec 8200, FOSS, Hillerød, Denmark) and a distillation unit (Kjeltec 8400, FOSS, Hillerød, Denmark).
Molecular weight determination was conducted using the SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) method (Witono et al., 2020). Two types of gels were prepared: a 12.5% separating gel and a 4% stacking gel. Electrophoresis was carried out using a vertical gel electrophoresis unit (Mini-PROTEAN Tetra System, Bio-Rad Laboratories, Hercules, CA, USA). Protein bands were visualized using silver staining. The gel was soaked in a fixation solution consisting of 25% methanol (Merck) and 12% acetic acid (Merck) for 1 h. It was then soaked in 50% ethanol (Merck) for 20 min, followed by soaking in 30% ethanol for 20 min, repeated twice. The gel was then treated with an enhancer solution containing sodium thiosulfate pentahydrate (Na2S2O3 ․ 5H2O) (Sigma-Aldrich, St. Louis, MO, USA) for 1 min and rinsed with distilled water (Bratachem, Jakarta, Indonesia). Afterward, the gel was immersed in silver nitrate (Sigma-Aldrich, St. Louis, MO, USA) and left for 30 min. Following a 40-sec rinse with distilled water, the developer solution containing sodium carbonate (Na2CO3) (Merck) and formaldehyde (Merck) was added. Finally, the gel was soaked again in the fixation solution to complete the staining process. Protein molecular weights were estimated by comparing the bands to a standard protein ladder.
The amino acid content of fish protein hydrolysate (FPH) was analyzed using an ultra-performance liquid chromatography system (UPLC) (Acquity UPLC H-Class, Waters Corp., Milford, MA, USA) and Bio H-Class amino acid analysis system following the method described by Nollet (2004). Briefly, 0.1 g of the sample was mixed with 5 mL of 6 N hydrochloric acid (HCl) (Merck) and stirred. The mixture was hydrolyzed at 110°C for 22 h, cooled to room temperature, transferred into a 50 mL volumetric flask, and diluted to volume with distilled water (Bratachem, Jakarta, Indonesia). The hydrolysate was filtered through a 0.45 μm membrane filter (Millex-HV, Millipore, Burlington, MA, USA). An aliquot of 500 μL of the filtrate was mixed with 40 μL of internal standard α-aminobutyric acid (AABA) (Sigma-Aldrich, St. Louis, MO, USA) and 460 μL of distilled water. From this mixture, 10 μL was reacted with 70 μL of AccQ-Fluo borate buffer (Waters Corp., Milford, MA, USA) and vortexed. Then, 20 μL of AccQ-Fluor reagent A (Waters Corp., Milford, MA, USA) was added. The solution was incubated at 55°C for 10 min before being injected into the UPLC system. Amino acid peaks were detected and quantified based on the height of each peak on the chromatogram.
The antioxidant activity of protein hydrolysates in inhibiting DPPH radicals was expressed as percent inhibition. The DPPH solution was prepared by dissolving 4 mg of DPPH crystals (Sigma-Aldrich, St. Louis, MO, USA) in 100 mL of methanol (Merck). Samples were prepared at various concentrations (500, 600, 700, 800, 900, and 1,000 ppm) were prepared using methanol as the solvent. For each concentration, 2 mL of the sample was mixed with 2 mL of DPPH solution, vortexed using a vortex mixer (VM-300, Gemmy Industrial Corp., Taipei, Taiwan), and incubated at 37°C for 30 min in an incubator (BEI-250, Yamato Scientific Co., Ltd., Tokyo, Japan). The absorbance was measured at a wavelength of 517 nm using a UV-VIS spectrophotometer (UV-1800, Shimadzu Corp., Kyoto, Japan). The antioxidant activity was calculated based on the percentage of DPPH radical inhibition (Taniyo et al., 2021).
3. Results and discussion
The chemical composition of protein hydrolysate from milkfish (C. chanos) is analyzed based on its proximate composition, including moisture, fat, and ash content. Additionally, the protein content is determined separately as soluble protein content. The proximate composition and resulting yield are detailed in Table 1.
The moisture content of milkfish protein hydrolysate obtained in the study ranged from 12.24% to 15.31%. The highest moisture content was observed in the hydrolysis treatment for 5 h with a 5% enzyme addition. Interestingly, hydrolysis time, enzyme concentration, and their interaction did not significantly affect (p>0.05) the moisture content, likely due to the consistent freeze-drying conditions (time and temperature) applied uniformly across all treatments. This is consistent with Kurniawati et al. (2019), which indicates that the moisture content of FPH is strongly influenced by the drying method. The moisture content observed in this study was higher than that reported by Wijayanti et al. (2015), who used papain enzyme for milkfish hydrolysis and obtained values ranging from 6.47% to 6.75%. The differences can be attributed to variations in the duration of the drying process, despite using the same method. This is further supported by the results of a study by Kurniawati et al. (2019), which obtained a catfish FPH moisture content of 3.77% with a drying treatment of −40°C for 96 h.
The moisture content of a material depends on both the drying conditions and the hydrolysis process, which can alter its water-binding capacity. According to Alahmad et al. (2022), hydrolysis can increase a substance’s ability to absorb water. This is because the hydrolysis process adds COOH and NH2 groups to the substance, which makes it able to absorb more water (Kristinsson and Rasco, 2000). The degree of hydrolysis test showed that after 5 h of hydrolysis, the substance could absorb more water compared to 6 and 7 h of hydrolysis.
The ash content obtained during this study ranged from 5.26% to 5.56%, with the highest ash content of 5.56% observed in the 7-h hydrolysis treatment with a 5% enzyme addition. Hydrolysis time, enzyme addition, and their interaction did not affect the ash content (p>0.05). This is attributed to the addition of NaOH during the hydrolysis process, which maintains stable pH conditions, and results in the formation of Na salts. This aligns with the assertion by Martínez et al. (2021) that the high ash content in FPH is due to the addition of NaOH, which leads to the formation of NaCl salt compounds through neutralization of the reaction of H+ with OH−.
The ash content observed in this study was lower than that reported in previous research by Wijayanti et al. (2016) on milkfish FPH using papain and bromelain enzymes, with ash content ranging from 10.53% to 10.67% and 10.61% to 12.91%. The variation in ash content is attributed to differences in the fish source, including habitat, age, and environmental conditions, which affect the mineral composition of milkfish meat, as well as differences in the amount of NaOH used to maintain pH during hydrolysis.
The fat content of milkfish FPH ranged from 0.18% to 0.23%, with the highest fat content of 0.23% observed in the 5-h hydrolysis treatment with 6% enzyme addition. The fat content was not significantly (p>0.05) influenced by hydrolysis time, enzyme addition, or their interaction. This lack of influence can be attributed to the hydrolysis process and treatments such as centrifugation, which effectively reduce fat content. According to Shahidi and Hossain (2022), enzymatic hydrolysis can rapidly modify the structure of fish tissue, resulting in a reduced fat content in FPH. Furthermore, the fat content in FPH is also affacted by the centrifugation and filtration processes carried out after hydrolysis. Martínez et al. (2021) noted that the centrifugation process, performed once the desired degree of hydrolysis is achieved, affects the fat content of FPH. During centrifugation, the aqueous phase, containing soluble protein, and the insoluble precipitate, containing protein and fat, are separated (de Amorim et al., 2016). Additionally, as noted by Alahmad et al. (2022), the fat content in the hydrolysate can be attributed to lipid particles present in the supernatant after centrifugation.
The fat content of the milkfish FPH obtained in this study is comparatively lower than that reported in previous research by Wijayanti et al. (2016), which showed fat contents of 2.34% using papain enzyme and 0.94% using bromelain enzyme. In contrast, the fat content obtained is higher than that of FPH bibisan at 0.03%, as reported by Witono et al. (2014), who utilized a mixture of papain and biduri enzymes. The content of low-fat in FPH can enhance the quality of the hydrolysate, leading to improved stability and better organoleptic properties, as noted by Nikoo et al. (2023). The discrepancies in fat content may be attributed to variations in substrate and enzyme types utilized, underscoring the importance of selecting the appropriate enzyme and substrate combination to produce economically valuable FPH with desirable biological and functional properties.
The yield obtained in this study ranged from 14.95% to 16.84%, with the highest yield value achieved in the 5-h treatment with 5% enzyme addition. Statistical analysis revealed that neither hydrolysis time, enzyme concentration, nor their interaction had significant influence on yield (p>0.05). This is closely related to the yield of soluble protein and the degree of hydrolysis achieved.
The results of this study indicate that the optimal conditions for hydrolyzing milkfish FPH are achieved after 5 h of hydrolysis with the addition of 5% enzyme. Further increasing enzyme concentration or hydrolysis time does not significantly increase yield, likely due to substrate depletion in the enzyme mixture. This aligns with the statement made by De Amorim et al. (2016) that various factors, such as the type of raw material, optimal enzyme conditions, and degree of hydrolysis, can influence FPH yield. A higher degree of hydrolysis leads to a higher yield. The yield obtained in this study exceeds that of previous research on FPH from milkfish using papain and bromelain enzymes by Wijayanti et al. (2015, 2016), who reported yields of 10.64% and 11.41%, respectively. This difference is attributed to variations in raw material composition, optimal enzyme duration, and degree of hydrolysis. Therefore, determining the optimal enzyme-to-substrate ratio and hydrolysis duration is crucial for achieving a high yield.
Proteins in fish protein hydrolysates can be expressed as soluble proteins due to the hydrolysis process that breaks down intact proteins into smaller peptides that are easily soluble in water (De Amorim et al., 2016). The soluble protein obtained in this study is presented in Table 2.
The study produced soluble protein content ranging from 37.27% to 44.73%. The ANOVA (p>0.05) results indicated that the hydrolysis time significantly influenced the soluble protein content of the FPH, while the addition of enzymes did not have a significant effect. The highest soluble protein content, 44.73%, was achieved in samples hydrolyzed for 5 h with the addition of 6% enzyme. However, the addition of 6% enzyme did not significantly impact the soluble protein content of milkfish FPH, indicating that 5% concentration is the optimal enzyme concentration.
Prolonged hydrolysis (>5 h) slightly decreased soluble protein content, in contrast to De Amorim et al. (2016), who reported an increase in soluble protein with time. Their study suggested that increasing the hydrolysis time would elevate the fraction of soluble protein in the solution, while decreasing the bound fat molecules.
The decline in soluble protein levels observed in this study is attributed to the optimal hydrolysis conditions achieved during the 5-h treatment, leading to the formation of non-polar amino acids and a subsequent decrease in soluble protein levels with prolonged hydrolysis. According to Witono et al. (2014), the reduction in soluble protein content can be attributed to the formation of non-polar amino acids, such as glycine, alanine, valine, leucine, isoleucine, and proline, from soluble peptides during the hydrolysis process.
The soluble protein content obtained was not significantly affected by the enzymes. This is probably due to the reduction in the hydrolysis rate caused by the lower substrate concentration and the interaction between the final product and the enzyme, which hinders the enzyme’s ability to obtain soluble protein. According to Martínez-Montaño et al. (2021), the decrease in hydrolysis rate is a result of substrate depletion and the negative impact of products that disrupt the enzyme’s catalytic activity. In this study, the soluble protein content obtained was lower than the soluble protein content of white mouth croaker processing waste FPH, which was obtained from offal and muscle, at 75.7% and 83.9%, respectively. Nevertheless, the results obtained are higher than the soluble protein content of raw fish, which is 3.509% (Witono et al., 2014).
The liquid part of enzyme-created protein breakdown is a great way to obtain lysine, arginine, glycine, alanine, and proline. It can be used to incorporate amino acids with useful functional characteristics into food items, thereby enhancing their quality by modifying them (de Amorim et al., 2016).
Based on the results of the analysis of variance (ANOVA), neither hydrolysis time nor enzyme concentration had a statistically significant effect on the degree of hydrolysis (p>0.05, Table 3). However, a trend was observed in which an increase in hydrolysis time was associated with a higher degree of hydrolysis. The highest degree of hydrolysis (32.71%) was obtained after 5 h of hydrolysis with the addition of 6% enzyme. However, since the increase from 5% to 6% enzyme concentration was not statistically significant, the optimal enzyme concentration was determined to be 5%. Further increases in hydrolysis time or enzyme concentration beyond optimal conditions did not significantly improve the degree of hydrolysis. This contradicts the statement by Alahmad et al. (2022), suggesting that increasing hydrolysis time and adding enzymes could enhance the degree of hydrolysis.
The rate of hydrolysis decreases over time due to substrate depletion and the interference of reaction products with the enzyme’s active site, which hinders its catalytic mechanism (Martínez-Montaño et al., 2021). Noman et al. (2018) found that the hydrolysis degree ranged from 14.93% to 24.89% between 0.25 to 6 h when producing protein hydrolysate from tuna. However, prolonging the hydrolysis time to 8 h did not produce a significant effect. Likewise, Alahmad et al. (2022) discovered that adding 4% and 5% enzymes did not significantly affect the degree of hydrolysis during carp hydrolysate preparation. This was due to enzyme aggregation, which impeded the catalytic active sites in the substrate during the hydrolysis process.
The polyacrylamide gel electrophoresis with sodium dodecyl sulfate (SDS-PAGE) method was used to determine the molecular weight distribution in milkfish FPH. Fig. 1 shows the molecular weight distribution of milkfish protein hydrolysate (FPH) based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The results indicate the presence of peptide fractions with molecular weights ranging from 10.03 to 98.80 kDa.
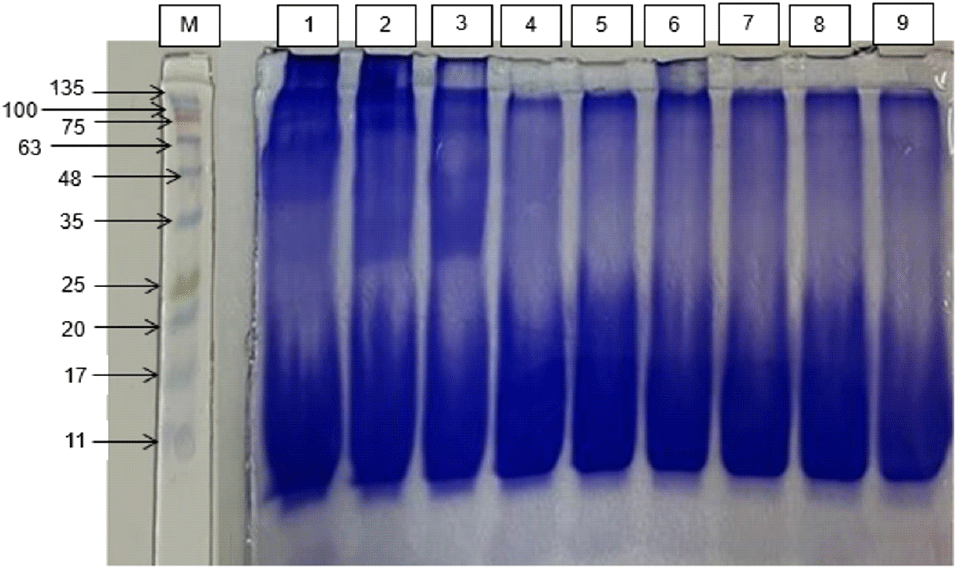
In the treatment lasting 5 h with the addition of enzymes (5%, 6%, and 7%), a total of 8 peptide bands were successfully identified, with molecular weights of 98.80 kDa, 92.88 kDa, 82.07 kDa, 58.42 kDa, 39.08 kDa, 12.45 kDa, 11.35 kDa, and 10.03 kDa. In the hydrolysis treatment lasting 6 and 7 h with the addition of enzymes (5%, 6%, and 7%), 4 peptides were identified with molecular weights of 82.07 kDa, 12.45 kDa, 11.35 kDa, and 10.03 kDa.
The absence of several peptides with larger molecular weights in the 6- and 7-h hydrolysis treatments with the addition of enzymes (5%, 6%, and 7%) indicates a further hydrolysis process occurring after the optimum time, resulting in the production of smaller peptides and free amino acids. This led to the reduction of peptides with larger molecular weights. These findings align with the research by (Mohanty et al., 2021) which observed a decline in antioxidant activity following a reduction of peptides with specific molecular weights to free amino acids after 90 min of hydrolysis.
The FPH derived from milkfish in this research has a molecular weight comparable to the results of Dinakarkumar et al. (2022), who acquired FPH with a molecular weight of 15-75 kDa from by-product fish caught with trawls using papain enzymes and proteinase k. This FPH exhibited favorable functional characteristics, including emulsifying, water-binding, and oil-binding properties. Similarly, Mohanty et al. (2021) documented the isolation of peptides with molecular weights ranging from <10-25 kDa in FPH from Labeo cohita fish waste using alkalase enzymes, which demonstrated antioxidant and metal chelating characteristics. According to Narikimelli et al. (2019), FPH with high nutritional value is recognized to be rich in small molecular weight peptides, offering beneficial functional characteristics and bioactivity. The results of our study indicate that the use of bromelain enzyme is an efficient approach to cleaving protein peptide bonds into smaller molecular weight peptides and amino acids, which possess advantageous functional characteristics and bioactivity.
The protein hydrolysate is a complicated blend of short-chain peptides and free amino acids. The interactions between the amino acids and peptides in protein hydrolysates have varied effects on their biological activity and functional properties (Fadimu et al., 2022). The amino acid composition of the milkfish FPH is detailed in Table 4.
The amino acid analysis of milkfish FPH showed that the total essential amino acid (EAA) content was 24.16 g/100 g, slightly exceeding the non-essential amino acid (NAA) content of 23.08 g/100 g, indicating a well-balanced and EAA-rich protein profile. Analysis of the data also revealed that the essential amino acid with the highest concentration is arginine (4.80 g/100 g), followed by leucine (4.14 g/100 g) and lysine (3.73 g/100 g). On the other hand, the non-essential amino acid with the highest concentration was found to be glutamic acid (6.93 g/100 g), followed by glycine (4.12 g/100 g), and alanine (3.36 g/100 g).
Milkfish FPH is rich in essential amino acids, making it a great source of high-quality protein. Amino acids such as arginine, leucine, and lysine are essential for human body growth, tissue repair, and metabolic functions A (Malle et al., 2019). Milkfish FPH also contains substantial amounts of non-essential amino acids, including glutamic acid and glycine. Glutamic acid contributes to energy metabolism and brain function, while glycine supports collagen formation and nerve function (Hertz and Zielke, 2004). Additionally, the presence of glutamic acid and aspartic acid in this FPH product gives it an umami taste. As noted by Ryu et al. (2021), these components are responsible for the savory and sour tastes in protein hydrolysates. Therefore, milkfish FPH is a valuable protein source that fulfills the body’s requirements for essential and non-essential amino acids. This quality makes milkfish FPH a promising raw material for use in the food industry, nutraceuticals, and other biomedical applications.
The composition of hydrophobic amino acids in milkfish FPH, such as valine, methionine, phenylalanine, isoleucine, leucine, and tryptophan, suggests that milkfish FPH possesses favorable antioxidant properties. This aligns with the assertion by (Samaranayaka and Li-Chan, 2011) that hydrophobic amino acids like histidine, tryptophan, phenylalanine, proline, glycine, lysine, isoleucine, and valine exhibit strong capabilities in neutralizing radicals during oxidative reactions. This behavior is especially noticeable in enzyme-catalyzed reactions, owing to the presence of the imidazole ring, which functions as a significant proton donor.
Hydrolysates have demonstrated antioxidant properties in various oxidative environments (Halim et al., 2016). The assessment of antioxidant activity was carried out using the 1,1-diphenyl-2-picryl hydrazyl (DPPH) free radical scavenging method. The antioxidant activity of milkfish FPH is detailed in Table 5.
The antioxidant activity, as measured by IC50, in our study ranged from 1.43-2.04 mg/mL. Our analysis of variance ANOVA (p>0.05) revealed that the hydrolysis time had a significant impact on the antioxidant activity of milkfish FPH. However, the addition of the enzyme and the interaction between the hydrolysis time and the enzyme did not yield a significant effect on the antioxidant activity of the milkfish FPH we obtained. The highest antioxidant activity recorded in our study was 1.43 mg/mL after 5 h of hydrolysis with a 5% enzyme addition, while the lowest activity was 2.04 mg/mL after 7 h of hydrolysis with a 7% enzyme addition. The decrease in the antioxidant activity of milkfish FPH as the hydrolysis time and enzyme addition increased can be attributed to the conversion of short-chain peptides to free amino acids due to prolonged hydrolysis past the optimal time. This finding aligns with the results of Mohanty et al. (2021), who observed a decline in the antioxidant activity of Labeo rohita visceral waste FPH after 90 min of hydrolysis, resulting in a predominance of free amino acids over short-chain peptides.
Following 6 and 7 h of hydrolysis, Fig. 1 shows that free amino acids began to appear. At the same time, larger peptide fragments started to disappear, indicating their conversion into smaller peptide fragments and free amino acids. According to You et al. (2009), an abundance of free amino acids might reduce antioxidant activity, as they have a limited ability to donate protons to free radicals. Additionally, the lack of influence of enzyme addition on antioxidant activity could be attributed to the enzyme becoming saturated with available substrates, as well as the potential negative effects of reaction products on the enzyme’s active site (Martínez-Montaño et al., 2021).
The potency of an antioxidant is categorized as very strong if its IC50 value is <0.05 mg/mL, strong if the IC50 value falls within 0.05-0.10 mg/mL, moderate if the IC50 value is in the range of 0.1-0.25 mg/mL, weak if the IC50 value is between 0.25-0.5 mg/mL, and very weak if it exceeds 0.5 mg/mL (Hermaya et al., 2021). The study’s findings indicate that milkfish FPH possesses an antioxidant capacity falling within the very weak category, as the highest IC50 value documented in the study was 1.58 mg/mL. The relatively low antioxidant activity observed is ascribed to the molecular weight of proteins and the amino acid composition present in milkfish FPH. According to Lassoued et al. (2015), the antioxidant capability is significantly influenced by the constituent amino acids, their sequence, and their hydrophobicity.
The research conducted by Saidi et al. (2014) on tuna fish by-product hydrolysates demonstrated significant superoxide radical activity and high reducing power. This was attributed to the presence of specific amino acids such as tyrosine, phenylalanine, proline, histidine, and leucine, which collectively accounted for 30.3% of the total constituent amino acids. Additionally, Cai et al. (2019) found that the amino acid sequence proline-tyrosine-serine-phenylalanine-lysine exhibited higher antioxidant activity compared to the sequence glycine-phenylalanine-glycine-proline-glutamate-and leucine. Furthermore, hydrophobic amino acids such as histidine, tryptophan, glycine, lysine, and valine were noted to contribute to strong radical scavenging activity due to the presence of an imidazole ring, which serves as a good proton donor (Samaranayaka and Li-Chan, 2011). This suggests that the composition, sequence, and hydrophobicity of amino acids have a significant impact on their antioxidant activity. On the other hand, milkfish FPH, as tested for amino acid content, lacks tyrosine and therefore have a limited impact on antioxidant activity in counteracting free radicals. This aligns with the statement by Halim et al. (2016), who emphasize the considerable contribution of tyrosine in counteracting free radicals, attributed to its phenolic chain acting as a strong electron donor that halts radical reactions.
The molecular weight of milkfish FPH peptides plays a crucial role in determining their antioxidant activity. The peptides found in milkfish FPH have molecular weights ranging from 10.03 to 98.80 kDa, resulting in a relatively low ability to neutralize free radicals. According to a study by Zou et al. (2016), peptides with a molecular weight of 1 kDa possess the ability to release hydrogen, effectively transforming free radicals into more stable products. Furthermore, Chiodza and Goosen (2023) suggested that fish protein hydrolysates with a molecular weight of less than 10 kDa exhibit high antioxidant properties, while those with a molecular weight of 10-30 kDa are more likely to possess good antihypertensive properties. These findings provide valuable insights into the use of milkfish protein hydrolysate in the development of food products with beneficial antihypertensive properties.
4. Conclusions
The findings revealed that variations in enzyme concentration did not significantly impact the moisture content (12.24%-15.31%), ash content (5.26%-5.56%), fat content (0.18%-0.23%), or yield (14.95%-16.84%) of milkfish protein hydrolysate (FPH), indicating a consistent basic compositional stability across various treatments. In contrast, the duration of hydrolysis significantly influenced key functional characteristics, with a hydrolysis period of 5 h being identified as optimal. This specific treatment yielded a soluble protein content of 44.73%, a degree of hydrolysis of 32.71%, and an antioxidant activity of 1.43 mg/mL. The peptides generated exhibited a molecular weight range between 10.03 and 98.80 kDa, reflecting a diverse distribution of molecular sizes. This diversity suggests a blend of both small and moderately sized peptides, which can enhance bioavailability, digestibility, and potential functional applications, including nutraceuticals and dietary supplements. Peptides with lower molecular weights (below 10-15 kDa) are particularly associated with antioxidant, antihypertensive, and other bioactive effects. Their smaller size also contributes to greater stability, as they are less prone to aggregation or precipitation over time, suggesting that FPH could be effectively integrated into functional foods or health-related products. The presence of both essential (His, Thr, Val, Met, Phe, Ile, Leu, Arg, Trp, Lys) and non-essential amino acids (Cys, Glu, Asp, Ser, Gly, Pro) enhances the nutritional quality and potential of FPH as a premium protein source. The absence of any significant interaction between enzyme concentration and hydrolysis duration suggests that optimal hydrolysis conditions can be standardized, which supports the prospects of scalability for industrial applications and consistent product quality.
