1. Introduction
The economic living standards of modern society have increased compared to the past, along with the social status and activity of women, which led to increased interest for better quality of life. As a result, more interest in physiological and mental health, as well as maintaining healthy skin in all generations and genders (Jin et al., 2013). In our current society, skin disorders such as inflammation, melasma, freckles, wrinkles, and acne have been reported to often affect social relationships and activities (Yuan et al., 2015). Thus, interest in preventive and therapeutic methods, such as UV protection, dietary management, stress reduction, and cosmetic products to improve skin health has significantly grown (Yuan et al., 2015). Although the skin surface has an acid barrier to protect the skin, exposure to ultraviolet ray (UV) can promote excessive production of reactive oxygen species (ROS) (Kim and Choe, 2015). These ROS are known to induce lipid peroxidation, protein oxidation, DNA oxidation, disrupt the collagen-hyaluronic acid structure to form wrinkles, and melanogenesis, which leads to skin aging (Kim and Choe, 2015). As interests in skin have grown, extensive efforts have been made to develop cosmetic products that contains bioactive compounds derived from food or natural sources. In particular, various natural compounds that has been used in traditional herbal medicine or therapy have been extensively studied and reported their antimicrobial, antioxidation, whitening, moisturization, and skin anti-aging effect (Son et al., 2024). Natural sources, such as plants, contain various bioactive compounds that can potentially regulate its physiological functions to maintain homeostasis upon disruption, including deficiency or excessive secretion of biomolecules (Oh and Mo, 2011). Many of these bioactive compounds have been known to show antioxidation, anti-viral, and anti-inflammation effect (Oh and Mo, 2011). Recently, many researchers have studied various phytochemicals, bioactive compounds derived from plants, has significantly expanded, which led to the development of numerous pharmacologically active materials (Jo and Cho, 2012; Joo, 2013; Kwon et al., 2016).
Lonicera insularis Nakai (L. insularis) is known to be a deciduous shrub species, which is native to Ulleung-do and Dok-do islands in South Korea (Lee et al., 2024). Previous studies on L. insularis have reported anti-obesity effect, immunostimulatory activity, physiological properties of seed germination, biosynthetic pathway of argininosecologanin, which is an alkaloid compound derived from roots of L. insularis, DNA based molecular biology, and analyzed the genetic variation and phylogenetic correlation (Jeong, 2014; Kang et al., 2018; Kim, 2010; Lee et al., 2024; Yu et al., 2022). Although Lee et al. (2020) have reported the potential health beneficial effects of L. insularis extract, studies on its functionality and efficacy as a bioactive compound for functional cosmetics or beauty food applications remain unclear.
Therefore, this study aimed to determine the potential of L. insularis leaves as a source of beauty food and cosmetic product material by measuring the antioxidation, whitening, skin pore-tightening, anti-wrinkle, and anti-inflammation effect. Furthermore, the results are expected to serve as a foundation for future cellular-biology studies on anti-inflammation, melanogenesis, and collagen homeostasis using various in vitro models of macrophage cell, melanoma cell, and fibroblast cell.
2. Material and methods
The leaves of L. insularis from Ulleung-do were collected and taxonomically identified based on previous reports (Lee, 1979; Nakai, 1938). After removing foreign materials, only leaves were collected and dried at 45°C in a drying oven (FO600M, Jeiotech, Daejeon, Korea). Once the leaves were dried, leaves were ground and powdered to 40 mesh powder.
For extraction, a hot-water extract (LWE) was obtained by adding 1 g of L. insularis powder into 200 mL of distilled water (DW) and heated until the volume was reduced to 100 mL and extracted for 24 h in a shaking incubator at room temperature. The ethanol extract (LEE) was prepared by adding 1 g of L. insularis powder into 100 mL of 40% ethanol (EtOH) and extracted for 24 h in a shaking incubator at room temperature (Lee et al., 2020). The extracts were filtered through Whatman No. 1 filter paper (Whatman Inc., Maidstone, UK) to remove residues and then concentrated using a rotary evaporator (R200, Büchi, Flawil, Switzerland). The concentration of each extracts were adjusted into adequate concentration for bioactivity assay based on total phenolic content.
2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity was measured according to Cano et al. (2023). A mixture of 50 mL of 7 mM ABTS and 0.88 mL of 140 mM K2S2O8 was vortexed and left in the dark for 15 h to form radicals. The solution was then mixed with 100% EtOH at a ratio of 1:88 and adjusted to an absorbance of 0.7±0.02 at 734 nm to be used as ABTS solution. For measurement, 0.2 mL of sample solution and 4 mL of ABTS solution were vortexed. For blank, 0.2 mL of distilled water and 4 mL of ABTS solution were mixed and vortexed. The mixtures were reacted for 2 min at room temperature, and absorbance was measured at 734 nm. Butylated hydroxytoluene (BHT) was used as a positive control. The ABTS radical scavenging activity (%) was calculated as:
Thiobarbituric acid reactive substances (TBARS) inhibition activity was measured according to Christodoulou et al. (2022). First, an emulsion solution was freshly prepared by mixing 5% linoleic acid and 1% Tween 40 (100 mL each) using a hand mixer. For preparation of the stock solution, 15% trichloroacetic acid, 0.375% thiobarbituric acid (TBA), and 4 mL of 0.25 M hydrochloric acid (HCl) were mixed. The TBA reagent was prepared by adding 2% BHT to the stock solution. For the blank, 0.2 mL of distilled water and for the sample group, 0.2 mL of sample solution were each mixed with 0.8 mL of emulsion and vortexed, followed by incubation at 50°C for 17 h, after which 4 mL of TBA reagent was added. The mixtures were reacted in boiling water for 15 min, cooled in an ice chamber for 10 min, centrifuged (FLETA 40, Hanil Science Inc., Gimpo, Korea) at 2,000 rpm for 20 min, and left standing for 10 min. The absorbance was then measured at 532 nm. BHT was used as a positive control, and TBARS inhibition activity (%) was calculated as:
Elastase inhibition activity was measured according to AlShaikh-Mubarak et al. (2023). The reaction mixture containing 0.1 mL of sample solution, 0.1 mL of 0.8 mM N-succinyl-(Ala)3-p-nitroanilide in 0.2 M Tris-HCl buffer (pH 8.0), and 1 mL of 0.2 M Tris-HCl buffer (pH 8.0) was prepared. To this mixture, 0.1 mL of 0.3125 Unit/mL porcine pancreatic elastase in 0.2 M Tris-HCl buffer (pH 8.0) was added, vortexed, and reacted at 37°C for 20 min. The mixture was then kept in ice water for 5 min, and measured the absorbance at 410 nm. For the blank, 0.1 mL of distilled water was used and ascorbic acid was used as a positive control. The elastase inhibition effect (%) was calculated as:
Tyrosinase inhibition activity was measured according to Kumar et al. (2011). The substrate solution was prepared with 1.5 mM L-tyrosine in 0.1 M sodium phosphate buffer (pH 6.8). To 0.4 mL of the substrate solution, 0.2 mL of sample extract and 2.3 mL of the same buffer (pH 6.8) were added and mixed, followed by the addition of 0.1 mL of 250 Kunit/mL tyrosinase from mushroom (Sigma-Aldrich Co., St. Louis, MO, USA). The mixture was incubated at 37°C for 20 min and cooled in ice water for 5 min. The absorbance was measured at 475 nm. For the blank, 0.2 mL of distilled water was used and kojic acid was used as a positive control. The tyrosinase inhibition effect (%) was calculated as:
The astringent activity was measured to determine skin pore-tightening effect according to Kim et al. (2024). Same amount of sample and hemoglobin solution (Sigma-Aldrich Co., St. Louis, MO, USA) were added to a centrifuge tube and mixed. The mixture was then centrifuged at 3,500 ×g for 3 min using a centrifuge (FLETA 40, Hanil Science Inc., Gimpo, Korea) to precipitate the blood proteins. The absorbance was measured at 576 nm and the skin pore-tightening effect (%) was calculated as:
The hyaluronidase inhibition activity was measured to determine anti-inflammatory effect according to Jung (2020). 0.25 mL of sample solution and 0.25 mL of hyaluronidase (1,000 Unit/mL) in 20 mM sodium phosphate buffer (pH 6.9) was mixed and incubated at 38°C for 5 min. Then, 0.25 mL of hyaluronic acid (4 mg/mL) in 0.3 M phosphate buffer (pH 5.3) was added and reacted at 38°C for 45 min. After incubation was complete, 2.5 mL of albumin solution in 0.04 M acetate buffer (pH 3.7) was added and the mixture was left standing for 5 min. The transmittance was measured at 600 nm. For the blank, 0.25 mL of distilled water was used and ascorbic acid was used as a positive control. The hyaluronidase inhibition effect (%) was calculated as:
The results were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test with GraphPad Prism ver. 10.3.1 (GraphPad Software, Inc., San Diego, CA, USA). Data were presented as mean±standard deviation and statistical significance was determined at a 95% confidence level.
3. Result and discussion
Phenolic compounds in plants are known to be a factor to provide unique color and distinctive taste of plants or food. It has been reported that the physicochemical properties and the amount of phenolics extracted from plants can vary depending on the type of extraction solvent (Cho et al., 2012).
L. insularis leaves were extracted using water and 40% EtOH as solvents based on the previous study by Lee et al. (2020). As shown in Table 1, the hot-water extract showed a phenolic content of 3.53±0.73 mg/g with a yield of 0.35 %, while the ethanol extract showed phenolic content of 2.82±0.19 mg/g with a yield of 0.28%. While the lyophilized hot-water extract exhibited a brown color, EtOH extract showed light green color.
| Total phenolic contents (mg/g of dried Lonicera insularis leaf powder) | |
|---|---|
| Water extract | Ethanol extract |
| 3.53±0.731)b2) | 2.82±0.19a |
Phenolics are secondary metabolites with various physiological properties, which are known to be directly associated with biological functions (Jin et al., 2016). Based on these results, further investigations on measuring antioxidative effect and skin beauty associated bioactivity of phenolic compound in L. insularis were performed.
ABTS changes its blue-green color and fades when reacted with antioxidant compounds. The degree of decolorization is used to calculate antioxidative effect (Cano et al., 2023). The ABTS radical scavenging activity of L. insularis leaf extracts is shown in Fig. 1A. At 50 μg/mL of phenolics, LWE and LEE each showed high scavenging activity of 98.7% and 90.47% in a concentration-dependent manner. Compared to 44.35% of positive control BHT at the same concentration, the L. insularis extracts showed higher ABTS radical scavenging activity even at low concentrations. According to Jeong et al. (2009), hot-water extracts of various teas, including green tea, Pu-erh tea, oolong tea, and black tea, showed ABTS radical scavenging activities of 92.09%, 80.29%, 82.67%, and 48.07% respectively. Also, Kim (2016) reported that EtOH extract of yacon leaves showed 24.10% ABTS radical scavenging activity at 50 μg/mL. Thus, the L. insularis extracts showed significantly higher ABTS radical scavenging activity (p<0.05) from the lower concentrations, which suggests that the antioxidative effect may depend more on hydrophilic compounds.
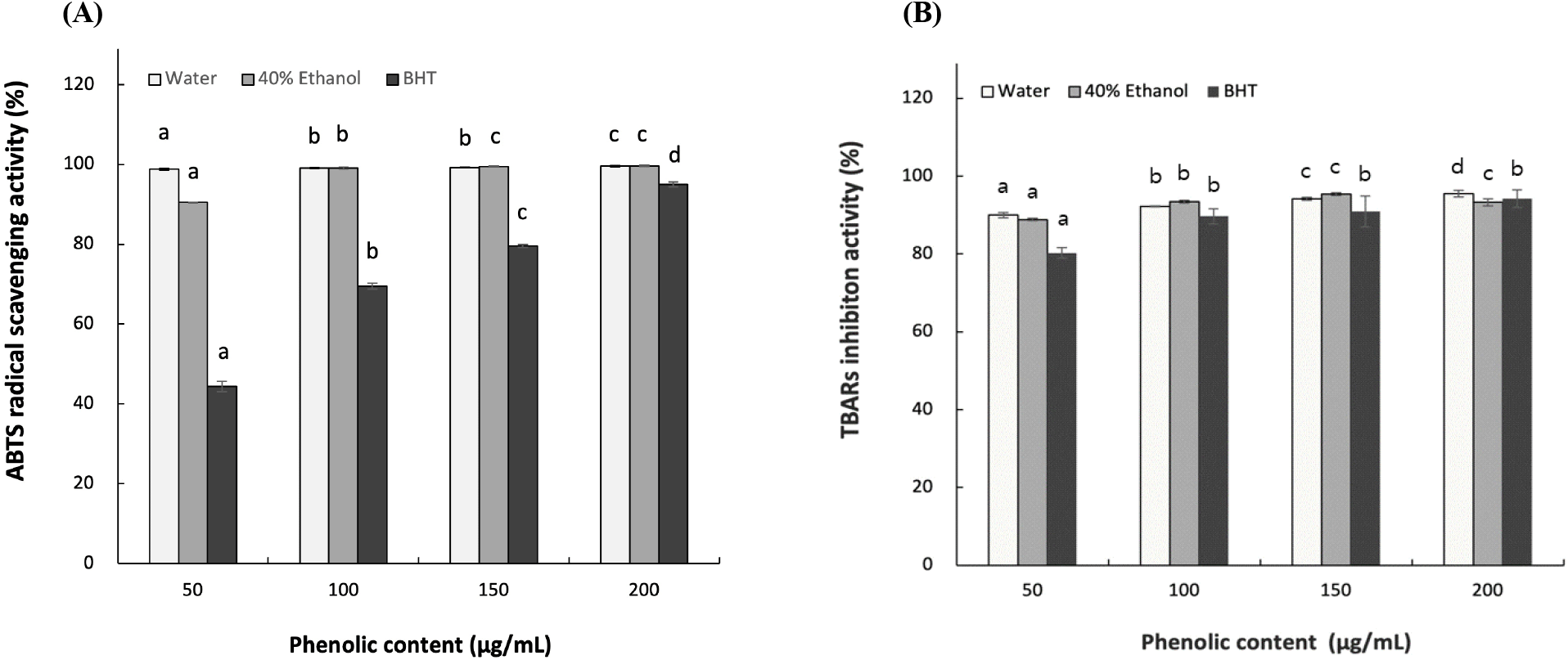
The TBARS assay measures the extent of lipid oxidation by quantifying the red color formed from the reaction between malondialdehyde and TBA (Christodoulou et al., 2022). To further determine the antioxidative effect of L. insularis, TBARS assay was used to measure antioxidative effect. As shown in Fig. 1B, each LWE and LEE showed 90.0-95.52% and 88.85-93.26% inhibition activity at 50-200 μg/mL phenolics. Both extracts showed significant antioxidative effect in lipid soluble system in a concentration-dependent manner. When compared to the positive control BHT, the L. insularis extracts showed significantly higher antioxidative effect, which suggest that L. insularis has a strong inhibition effect on lipid peroxidation. It was previously reported by Kim et al. (2015) that hot-water and EtOH extract of Chionanthus retsus leaves showed approximately 85% and 95% of TBARS inhibition effect at 150 μg/mL of phenolics. However, L. insularis leaf extracts showed higher inhibition effect, which confirms the significant inhibition effect on lipid oxidation. Our results suggests that the antioxidative effect of L. insularis extracts increases in a concentration-dependent manner as the phenolic concentration increases (Jin et al., 2016). Thus, L. insularis leaf extracts show high antioxidative effect in both hydrophilic and hydrophobic conditions, which suggests a potential as a functional material.
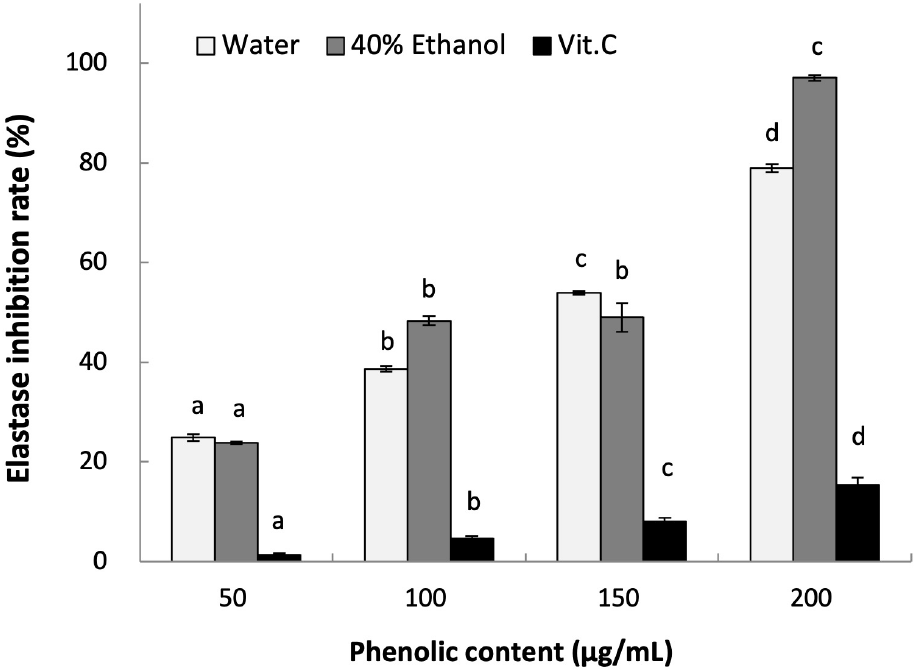
ROS generated by UV exposure can promote photoaging, which is often followed by inflammatory responses, melanin pigmentation, and wrinkle formation in the skin (Kim and Na, 2013). Elastase is an enzyme that degrades elastin, which is a structural protein in the dermal layer that is responsible for maintaining skin elasticity. As a result, the degradation of elastin can cause dermal tissue damage, loss of elasticity, and wrinkle formation (Cho et al., 2010).
As shown in Fig. 2, the elastase inhibition effects of L. insularis leaf extracts increased in a concentration-dependent manner. At 200 μg/mL of phenolics, LWE and LEE each showed inhibition effect of 78.96% and 97.01% (p<0.05). The positive control vitamin C showed 15.37% at 200 μg/mL, which was lower than L. insularis leaf extracts. Compared to other hot-water and ethanol extracts of Albizia julibrissin at the concentration range between 300-2000 μg/mL showing 4.94-23.46% and 6.67-21.67%, L. insularis leaf extracts showed significantly higher elastase inhibition effect even at lower concentrations. These results suggest that L. insularis have a high potential for improving skin wrinkles.
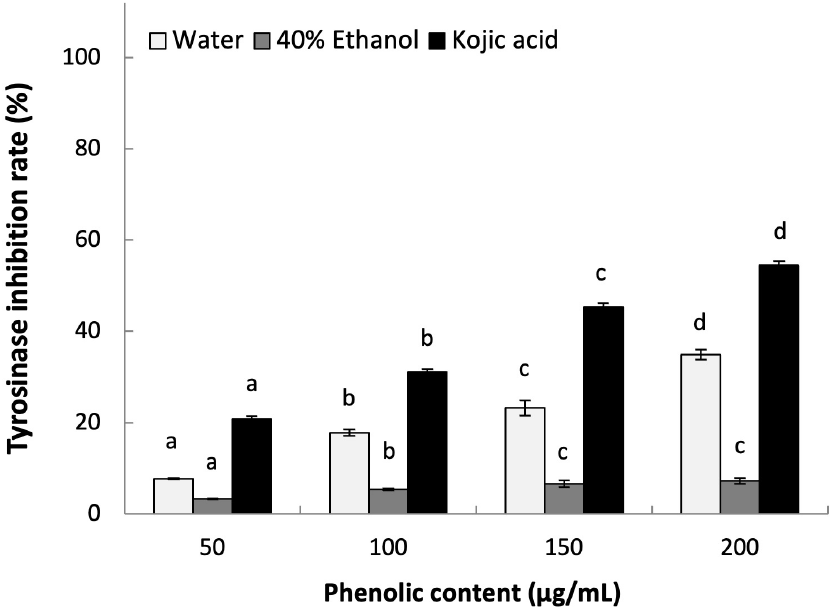
Tyrosinase is one of the key enzyme in the melanin synthesis, which can produce melanin pigments by inducing oxidation of tyrosine (Kumar et al., 2011). We determined the whitening effect of L. insularis leaf extracts by measuring the inhibition effect on tyrosinase activity. As shown in Fig. 3, LWE showed 34.94% of tyrosinase inhibition effect at 200 μg/mL of phenolics in a concentration-dependent manner (p< 0.05). However, LEE showed only 7.22% of tyrosinase inhibition effect at 200 μg/mL of phenolics, which was lower than LWE. Previously, it was reported that EtOH extract of Pyrrosia lingua, safflower seed (Carthamus tinctorius), nutgrass (Cyperus rotundus), and Schizonepeta tenuifolia each showed tyrosinase inhibition effect of 40.70%, 27%, 6%, and 23% at 200 μg/mL or higher concentration of 1,000 μg/mL. Based on these results, hot-water extract of L. insularis showed high tyrosinase inhibition effect in much lower concentration in a concentration-dependent manner compared to previously reported medicinal plants. Thus, L. insularis suggests a high potential functional ingredient for cosmetics for whitening purposes or beauty food applications.
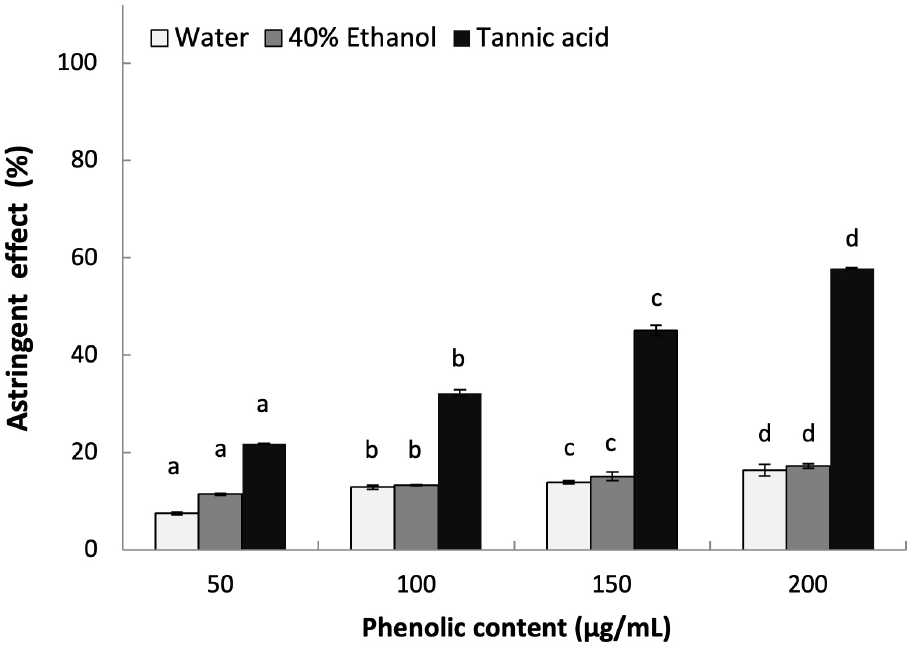
Astringency is known to form an insoluble layer on the skin surface, which can protects the skin by tightening the skin tissue to maintain moisture to the stratum corneum and improve the skin texture by tightening the skin pores (Kim et al., 2024). In addition, tightened skin pore can prevent bacterial invasion and protect the skin structure to inhibit wrinkle formation (Kim et al., 2024). The astringent effect of L. insularis leaf extracts were measured as shown in Fig. 4. Both extracts showed similar effect of 16.37% and 17.23% astringent effect at 200 mg/mL phenolics in a concentration-dependent manner (p<0.05). Compared to previous studies that reported the astringent effect of Rheum palmatum and Kalopanax septemlobus stem extracts showing low astringent effect, it is suggested that the phenolic compounds in L. insularis is responsible for the astringency by showing a similar trend (Kwan, 2011; Lee, 2011).
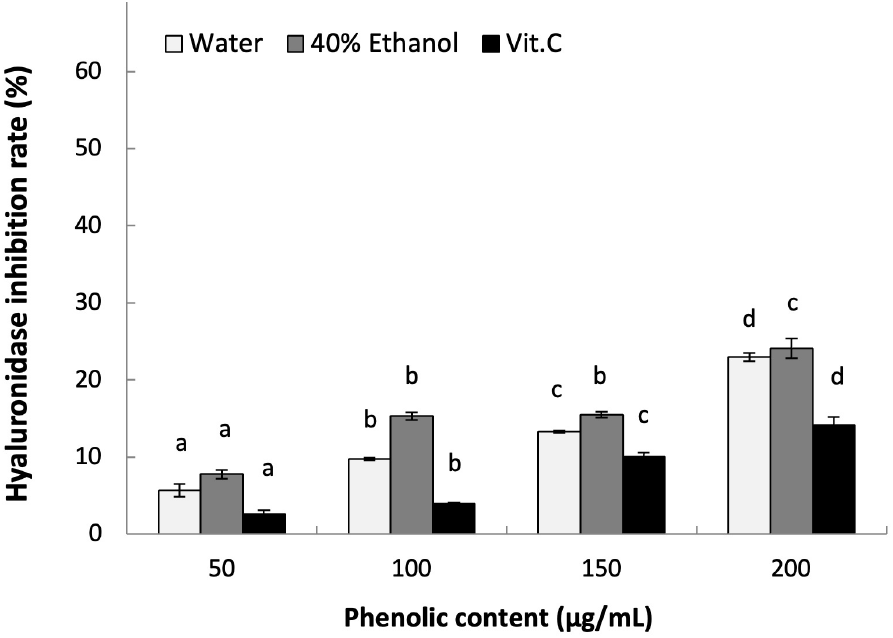
Hyaluronic acid is a polysaccharide polymer produced by fibroblasts, which can be degraded by hyaluronidase to induce inflammation. Thus, inhibition of hyaluronidase activity is expected to prevent inflammation by maintaining the structure of hyaluronic acid (Lee et al., 2015). As shown in Fig. 5, the hyaluronidase inhibition effect of LWE and LEE at 200 μg/mL phenolics were each 22.97% and 24.11% in a concentration-dependent manner (p<0.05). In addition, both L. insularis extracts showed higher hyaluronidase inhibition effect compared to the positive control ascorbic acid at all concentrations. Compared to a previous report on the hyaluronidase inhibition effect of Prunella vulgaris extracts showing 17.24% with hot-water extract and 25.35% with EtOH extract at 200 μg/mL phenolics, L. insularis showed a similar result (Kim et al., 2013). In another study on reporting the hyaluronidase inhibition effect of Curcuma longa extracts, both hot-water and EtOH extracts showed inhibition effect of 5-10% at 300 μg/mL, which suggests that L. insularis leaf extracts have significantly higher inhibition effect even at lower concentrations. Therefore, L. insularis extracts are suggested to show anti-inflammation effect by inhibiting the activity of hyaluronidase to prevent the degradation of hyaluronic acid and regulate inflammatory responses.
To this date, studies have been focused on seed germination physiology, immunostimulatory, anti-obesity effect, biosynthetic pathway of argininosecologanin, DNA-based molecular biology, genetic variation, phylogenetic correlation, and functional food properties (Jeong et al., 2014; Kang et al., 2018; Kim, 2010; Lee et al., 2020; Lee et al., 2024; Yu et al., 2022). Despite these efforts, no studies have been investigated on beauty-associated biological properties.
Based on our findings, additional studies on identifying the underlying mechanisms on anti-inflammation by measuring the expression of inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-α, interleukin-1β, interleukin-6, and monocyte chemoattractant protein-1 using macrophage cell (Lee et al., 2024); mechanisms on melanogenesis by measuring melanin contents, microphthalmia-associated transcription factor, tyrosinase-related protein 1, tyrosinase-related protein 2, cellular tyrosinase expression protein expressions, as well as gene expression of melanocortin 1 receptor, transforming growth factor beta (TGFß) 1, Ras-related protein Rab-27A, and myosin VA using melanoma cell (Cho et al., 2025, Park et al., 2018); mechanisms on collagen homeostasis by measuring protein expression of collagen type I alpha 2 (COL1A2), matrix metalloproteinase-1 (MMP-1), matrix metalloproteinase-9 (MMP-9), and tissue inhibitor of metalloproteinases (TIMP), as well as gene expression of COL1A2, MMP-1, MMP-9, TIMP1, hyaluronan synthase 2, and TGFß are in progress to provide further understanding on cellular-biology perspectives of L. insularis.
4. Conclusions
In conclusion, we have investigated the beauty-associated properties of L. insularis leaf extracts by measuring antioxidation, anti-wrinkle, whitening, anti-inflammation, and astringent effects with in vitro assays. Although the current research is limited to in vitro experiments, this research highlights the potential of L. insularis as a source for therapeutic and cosmetic products aimed at improving skin health and beauty.
