1. Introduction
Onion (Allium cepa L.) is one of the most widely cultivated and consumed vegetables worldwide, recognized for its characteristic flavor and various health benefits (Stoica et al., 2023). In 2022, global onion production reached approximately 111 million tonnes, with India (28.6%), China (22.2%), Egypt (3.3%), and the United States (2.64%) being the top producers (FAO, 2023; Safari et al., 2024). Onions are mainly cultivated in temperate and subtropical regions (Park et al., 2024; Yang et al., 2020). In Korea, mid- to late-maturing cultivars are predominantly grown in Jeollanam-do and Gyeongsangnam-do (Park et al., 2024; Yang et al., 2020). Onions are rich in flavonoids, organosulfur compounds, and fructooligosaccharides, which are known to exhibit antioxidant, anti-inflammatory, and cardioprotective effects (Ren and Zhou, 2021; Sagar et al., 2022; Zhao et al., 2021). In addition, due to their distinctive flavor and nutritional value, onions are extensively used in culinary applications, including soups, stews, stir-fries, and sauces, as well as in the development of functional food products such as powders and beverages (Griffiths et al., 2002; Stoica et al., 2023). Due to these beneficial properties and their extensive use, maintaining onion quality during storage is crucial for maximizing both economic returns and ensuring consumer satisfaction.
In response to market demands, a wide range of cultivars has been developed globally with a focus on climate adaptability and postharvest storability (Havey, 2018). In East Asia, breeding efforts have targeted day-neutral and intermediate-day cultivars to improve local adaptability, yield, and storage quality (Kim et al., 2009). Despite these efforts, onions remain vulnerable to physiological deterioration during storage, including sprouting, browning, microbial spoilage, weight loss, and softening, which significantly reduce marketability (Petropoulos et al., 2017). These storage-related changes are closely linked to metabolic activities and the associated shifts in metabolite profiles (Chávez-Mendoza et al., 2016). Recent studies have applied metabolomics using analytical platforms such as nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (GC-MS), and liquid chromatography-mass spectrometry (LC-MS) has been employed to investigate metabolite profiles in onions across cultivars (Böttcher et al., 2017), red onion genotypes (Metrani et al., 2020), and during storage (Saviano et al., 2019), as well as in onion by-products (Elattar et al., 2024). However, despite the known effects of cultivar, cultivation region, and environmental conditions on quality and storability (Petropoulos et al., 2017; Vintila et al., 2014), metabolomics-based research integrating these variables remains limited. In particular, integrative methodologies assessing cultivar, cultivation region, and storage period in onion metabolomics are rarely studied.
Therefore, this study aimed to compare the quality changes during storage among onion cultivars grown in different regions and to elucidate the physiological and metabolic responses associated with cultivar- and region-specific variation through metabolite profiling at both early and late storage stages. The findings are expected to provide insights into the mechanisms underlying storage-related quality deterioration and inform strategies for improved postharvest management of onions. These findings may address current deficiencies and offer a novel structure for enhancing postharvest practices among producers and storage administrators.
2. Materials and methods
The onions used in this study included the cultivars Katamaru (K1 and K2) and Newmars (N1) cultivated in Mungyeong, Gyeongsangbuk-do, and the cultivars Newmars (N2) and Terius (T) cultivated in Hamyang, Gyeongsangnam-do, respectively. After harvest, the onions were cured under forced-air (ambient temperature for 2 weeks) and stored in cold storage maintained at 0-1°C and 80-90% relative humidity, and quality assessments were conducted every two months during storage. Quality assessments were performed at two-month intervals during the storage period.
During storage, the respiration rate of onions was measured by placing a single bulb in a sealed container (1,380 mL) inside the cold storage room and allowing it to respire for 1 h. A 1 mL sample of the accumulated headspace gas was collected using a gas-tight syringe and analyzed using gas chromatography (GC, HP7890B, Agilent Technologies, Santa Clara, CA, USA) equipped with an HP-5 column (30 m× 0.32 mm, Agilent Technologies) and a thermal conductivity detector (TCD). The GC oven temperature and carrier gas flow rate were set to 80°C and 5 mL/min, respectively. The respiration rate was expressed as mg CO2/kg · h (Yang et al., 2023).
The weight loss of onions was calculated by measuring the weight of ten bulbs per cultivar at two-month intervals and expressing the reduction as a percentage of the initial weight.
The firmness of onion flesh was measured at the equatorial zone using a texture analyzer (TA 1, LLOYD Instruments, Ametek Inc., Fareham, UK) equipped with a 5 mm-diameter cylindrical probe. Measurements were performed at a penetration speed of 2 mm/s with a strain of 10 mm. Ten samples were analyzed per treatment.
Soluble solids content (SSC) was measured by juicing the onion flesh and analyzing the extract using a digital refractometer (PAL-1, Atago Co., Ltd., Tokyo, Japan).
Moisture content was calculated as a percentage by measuring the weight loss of 5 g of onion flesh after drying at 105°C for 24 h.
The color of onion flesh was measured at the equatorial zone using a color meter (CR-300, Minolta Co., Tokyo, Japan) to obtain L* (lightness), a* (redness), and b* (yellowness) values. The whiteness index (WI) and browning index (BI) of the scales were calculated using the following equations (Kim et al., 2022):
Metabolites in onion flesh were extracted by adding 0.8 mL of 80% methanol containing an internal standard (zidovudine, Sigma-Aldrich) to 0.04 g of freeze-dried onion powder. The mixture was vortexed and homogenized using bullet blender. After centrifugation (14,000 ×g for 10 min), clear supernatant was injected to a UPLC-Q-TOF MS system (Waters Corp., Milford, MA, USA). Metabolites were separated on an Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm; Waters) with a mobile phase consisting of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The flow rate was 0.35 mL/min, and the column temperature was maintained at 40°C. Eluted metabolites were detected using a Q-TOF MS operated in negative electrospray ionization (ESI) mode. MS data were acquired under the following conditions: scan range of m/z 100-1,500, scan time of 0.2 sec, capillary voltage of 3 kV, sampling cone voltage of 30 V, desolvation gas flow of 800 L/h at 350°C, and source temperature of 120°C. Leucine-enkephalin ([M-H]− = 554.2615) was used as the lock mass at a flow rate of 20 μL/min and injected every 10 sec to ensure mass accuracy. To monitor instrument stability, a pooled quality control (QC) sample was injected after every 10 samples. MS/MS spectra were acquired with a collision energy ramp from 10 to 30 eV and a scan range of m/z 50-1,500 (Lee et al., 2023).
LC-MS data collection, normalization, and alignment were performed using MarkerLynx software (Waters). Peak detection parameters included a peak-to-peak baseline noise of 1, noise elimination level of 6, peak width of 1 sec at 5% height, and intensity threshold of 10,000. Peaks were aligned using a mass window of 0.05 Da and a retention time window of 0.2 min. All data were normalized using the internal standard. Metabolite identification was performed using the authentic standards, UNIFI platform (version 1.8.2.169, Waters) linked to the ChemSpider databases, and METLIN database (metlin.scripps.edu). Identification levels were assigned according to the Metabolomics Standards Initiative (MSI), with most compounds corresponding to Level 2 (Fiehn et al., 2007). No authentic standards were used unless otherwise stated.
SIMCA-P+ version 21.0.1 (Umetrics, Umeå, Sweden) was used to statistically analyze the dataset obtained from UPLC-Q-TOF MS. In particular, partial least squares discriminant analysis (PLS-DA) was performed to visualize classification patterns, and the model was evaluated using R2X, R2Y, Q2, and permutation tests. In addition, one-way analysis of variance (ANOVA) with Duncan’s multiple range test was conducted using SPSS version 28.0 (SPSS Inc., Chicago, IL, USA) to evaluate statistical significance at a level of p<0.05. Pearson correlation coefficients between metabolites and quality were calculated using GraphPad Prism 11.0 (GraphPad, San Diego, CA, USA).
3. Results and discussion
Changes in respiration rate during storage are presented in Fig. 1A. At the beginning of storage, the N1 onions exhibited the highest respiration rate (11.2±1.8 mg CO2/kg · h), which gradually declined to the lowest level among all samples by 8 months. In contrast, statistically significant differences were noted for K2 at 2 months and K1 at 4 months (p<0.05); however, the overall respiration rate variation among cultivars was minimal, between 5.1 and 5.7 μmol CO2/kg · h. The initially high respiration rate in N1 onions may have resulted from postharvest conditions such as temperature and humidity, harvest timing, or differences in curing duration (Benkeblia et al., 2000; Sharma et al., 2025). In general, onions with higher respiration rates tend to exhibit greater weight loss during storage and increased hormonal activity, which may induce physiological disorders such as sprouting and rooting (Sharma et al., 2025). Previous studies have also reported that even within the same cultivar, differences in climate at harvest and postharvest handling practices can significantly influence metabolic activity and respiration during storage (Benkeblia et al., 2000; Rahmah et al., 2023).
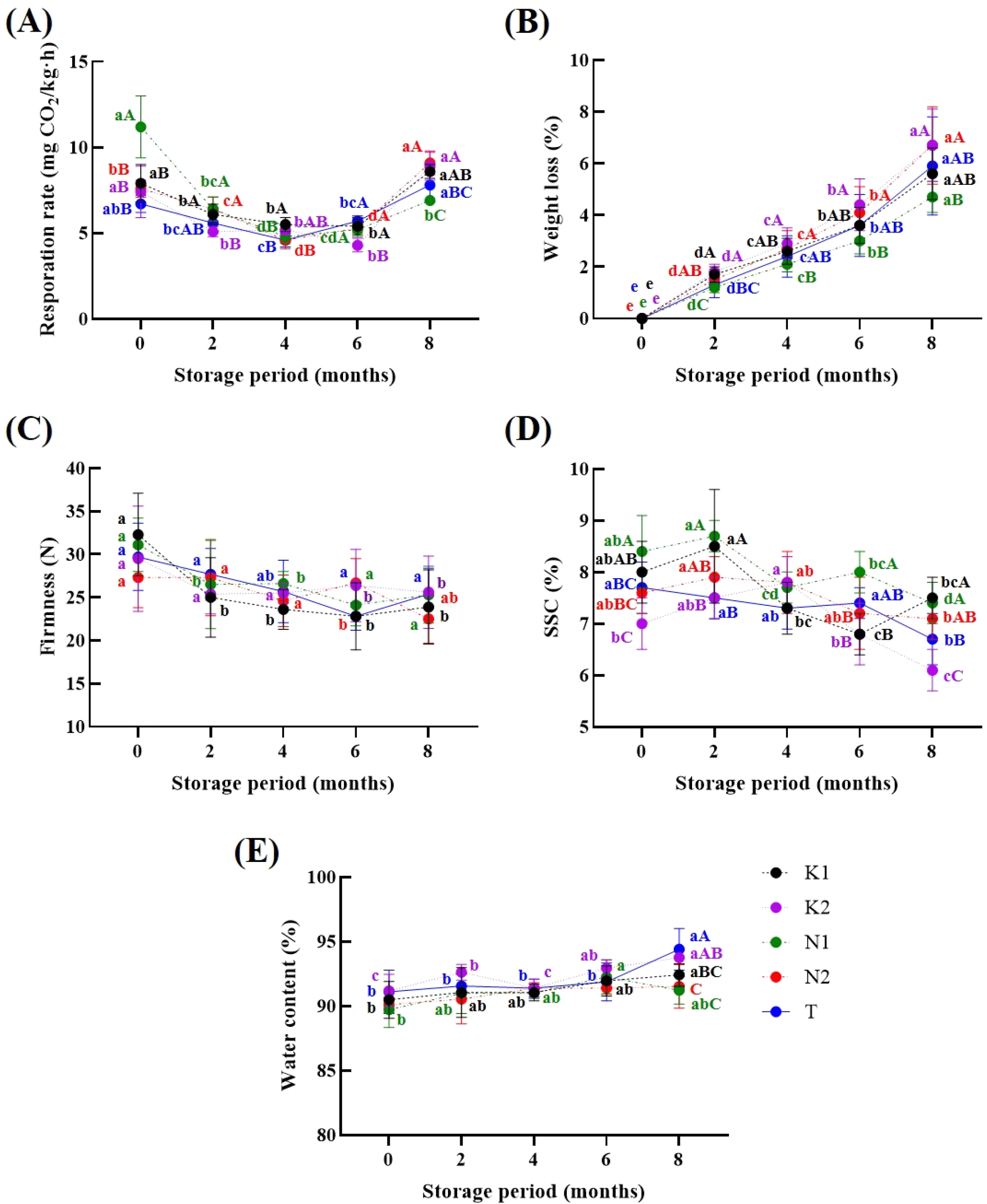
Under the storage conditions used in this study, decay rate remained below 3% in all samples throughout the 8 months of storage period (data not shown). In contrast, weight loss showed marked variation across cultivars and cultivation regions, particularly after 6 mon of storage. At 6 and 8 mon, cultivar T exhibited the lowest weight loss (2.9% and 4.7%, respectively), while K2 and N2 showed the highest losses (Fig. 1B). Weight loss during storage typically results from water loss due to transpiration (Sekara et al., 2017), and respiration is closely associated with this process (Suravi et al., 2024). The variations in weight loss among cultivars under uniform storage conditions may indicate inherent physiological and preharvest disparities among cultivars (Rahaman et al., 2021). Notably, the low respiration rate and weight loss in N1 onions at 8 mon imply that reduced respiration may have mitigated moisture loss during later storage phases.
Regarding firmness, no statistically significant differences were observed among cultivars or cultivation regions throughout the storage period, although all samples showed a general decrease in firmness (Fig. 1C). This reduction is primarily attributed to tissue softening caused by enzymatic degradation of structural polysaccharides by softening enzymes such as pectin methylesterase (PME), polygalacturonase (PG), and β-galactosidase (Coolong et al., 2008; Kang, 2024). Although enzymatic activity was not evaluated in this study, previous studies have shown that the activity of these enzymes can be affected by cultivar and cultivation conditions (Liu et al., 2019; Hou et al., 2022), and more prominently by postharvest treatments and storage temperature (Bu et al., 2013; Chope et al., 2012; Kang et al., 2024).
SSC increased slightly in K2 onion up to 4 months, followed by a decrease. In other cultivars, SSC generally decreased after 2 or 4 months of storage (Fig. 1D). At 8 months, K2 exhibited the lowest SSC value (6.1±0.4%). Such reductions in SSC during storage are typically attributed to the metabolic consumption of soluble carbohydrates as carbon sources and energy donors for the synthesis of amino acids, organic acids, and other intermediates (Romo-Pérez et al., 2020).
Moisture content ranged from 89.7% to 94.4% across all cultivars during storage (Fig. 1E). Although statistically significant differences were observed, these were attributed to biological variation among individual bulbs. Overall, moisture levels were consistent with previously reported ranges for stored onions (Jolayemi et al., 2018; Park et al., 2024).
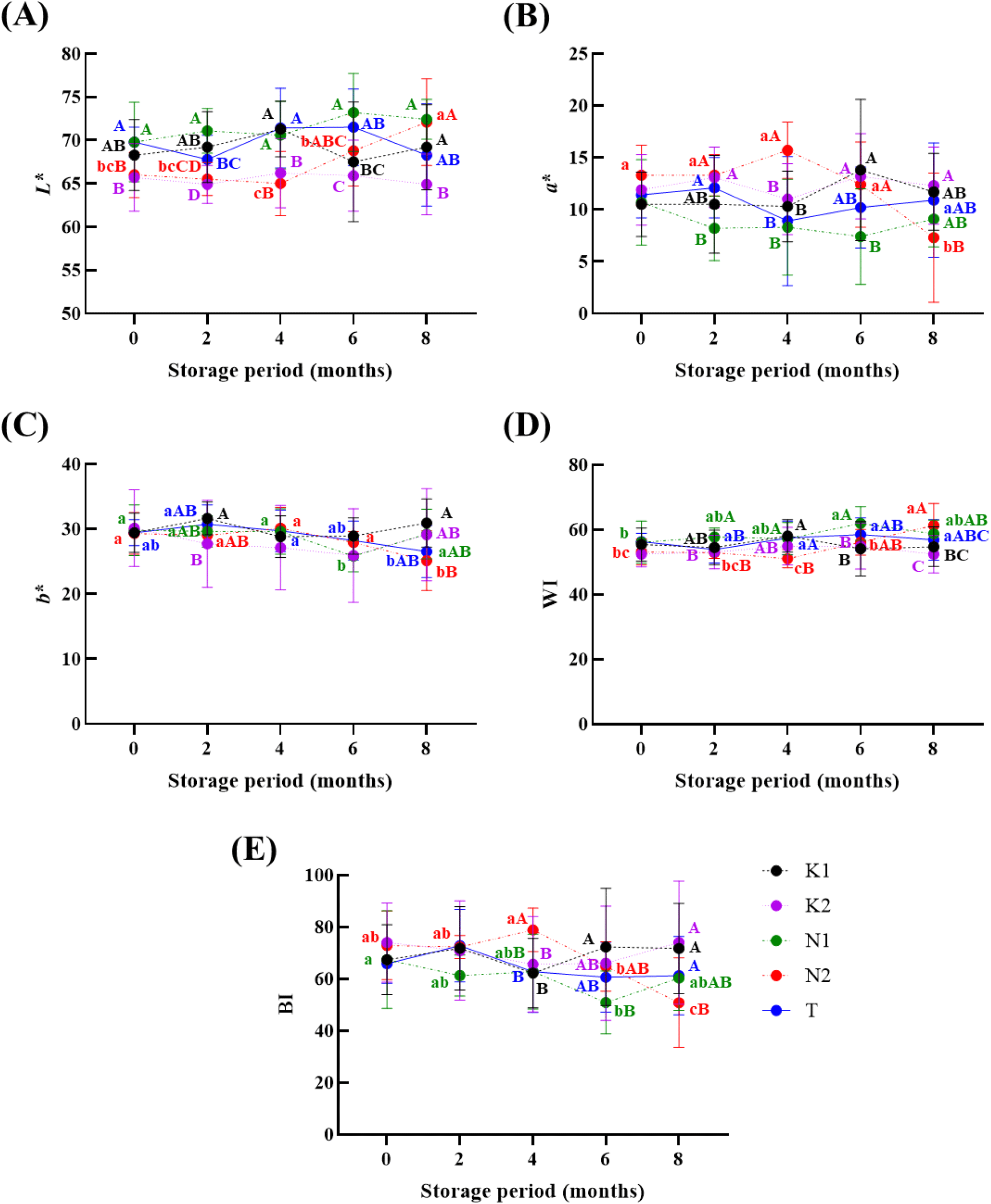
Color changes in onion during storage are shown in Fig. 2. L* values varied among onion cultivars during storage (p<0.05), with stability over time, except for N2 onions, which exhibited a significant increase after 4 months (p<0.05). Similarly, a* value decreased only in N2 onions after 6 months. The BI value of N2 onion also decrease after 4 months (p<0.05). Among the cultivars, K2 onion consistently exhibited lower L* values during storage, whereas N1 onion had relatively higher L* and lower a* values. While the complex mechanisms underlying these color changes were not specifically investigated, such variations may be linked to cultivar-specific physiological responses to extended storage, including changes in tissue hydration, cellular structure, or membrane integrity (Petropoulos et al., 2017; Szymańska et al., 2012).
Metabolite profiles in onion flesh were analyzed using UPLC-Q-TOF MS to compare metabolomic differences between the early and final stages of storage across cultivars and cultivation regions. Multivariate statistical analysis was used to visualize metabolomic differences between the early and late storage stages across different cultivars and regions (Fig. 3). Quality parameters of the applied PLS-DA models indicated that R2X and R2Y ranged from 0.420 to 0.734 and 0.191 to 0.984, respectively. The Q2 values ranged from 0.124 to 0.990, reflecting variation in model predictability across cultivars and storage stages. In particular, some groups (e.g., Fig. 3D) exhibited low Q2 and less typical permutation test distributions, suggesting that the statistical robustness of the separation may vary across models (Ström and Wheelock, 2024).
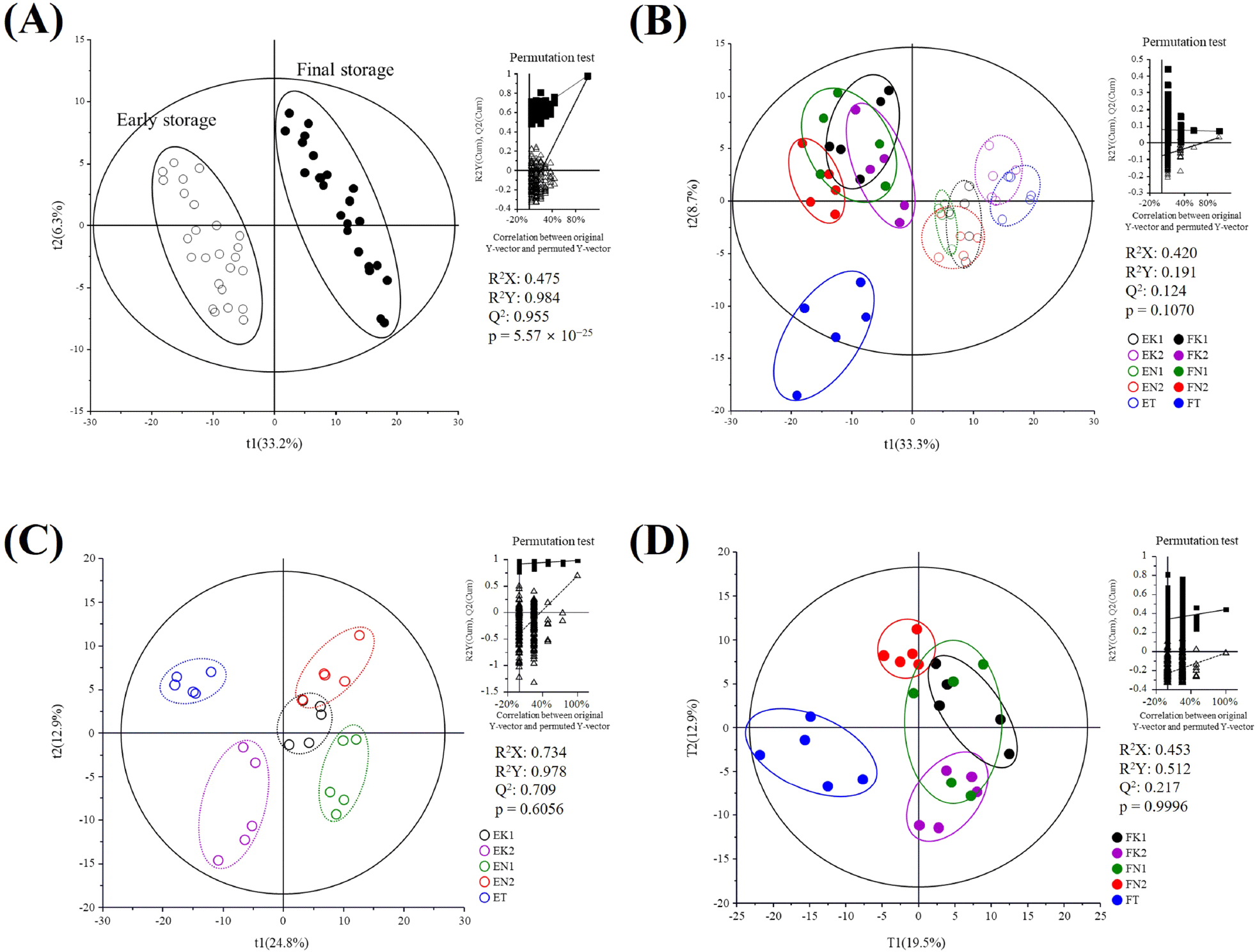
Despite these limitations, PLS-DA score plots showed discernible clustering trends in some cultivar and cultivation region. Based on variable importance in the projection (VIP) values (>0.7) and p-values (<0.05), a total of 28 metabolites, including sugars, amino acids, peptides, organic acids, phenolic compounds, and saponins, were identified as key contributors to these differences (Table 1). Moreover, nine metabolites were also identified that did not meet the VIP and p-value but were either abundant in onion samples or showed substantial variation during storage in specific groups. Similar metabolite profiles have been reported in previous studies on onion metabolomics and UPLC-Q-TOF MS analyses (Böttcher et al., 2017; Matsuse et al., 2022; Zhou et al., 2020).
Metabolomic pathways associated with changes during onion storage were proposed based on the identified metabolites using the KEGG database (https://www.kegg.jp/), and relative abundances were compared (Table 1 and Fig. 4). Among primary metabolites related to glycolysis and the TCA cycle, sucrose increased 3.09-fold in T onion at the final storage stage. oligosaccharides such as cellotriose, galactosyl pinitol, and maltopentose increased over 1.77-fold in all onions, with more than 4.65-fold increases in K2 and T onions. These sugar accumulations are likely linked to structural polysaccharide degradation (e.g., pectin), associated with firmness reduction. In this study, the structural polysaccharide degradation products di-galactosyl pinitol, cellotriose, and maltopentose showed a weak negative correlation with firmness (r=−0.328 to −0.220). Nonetheless, previous studies have demonstrated that oligosaccharides are produced as a result of enzymatic degradation, such as β- galactosidase, PME, and PG of structural polysaccharides, and these mechanisms have been associated with a reduction in firmness during the storage of onions (Bu et al., 2013; Chope et al., 2012; Coolong et al., 2008; Kang, 2024).
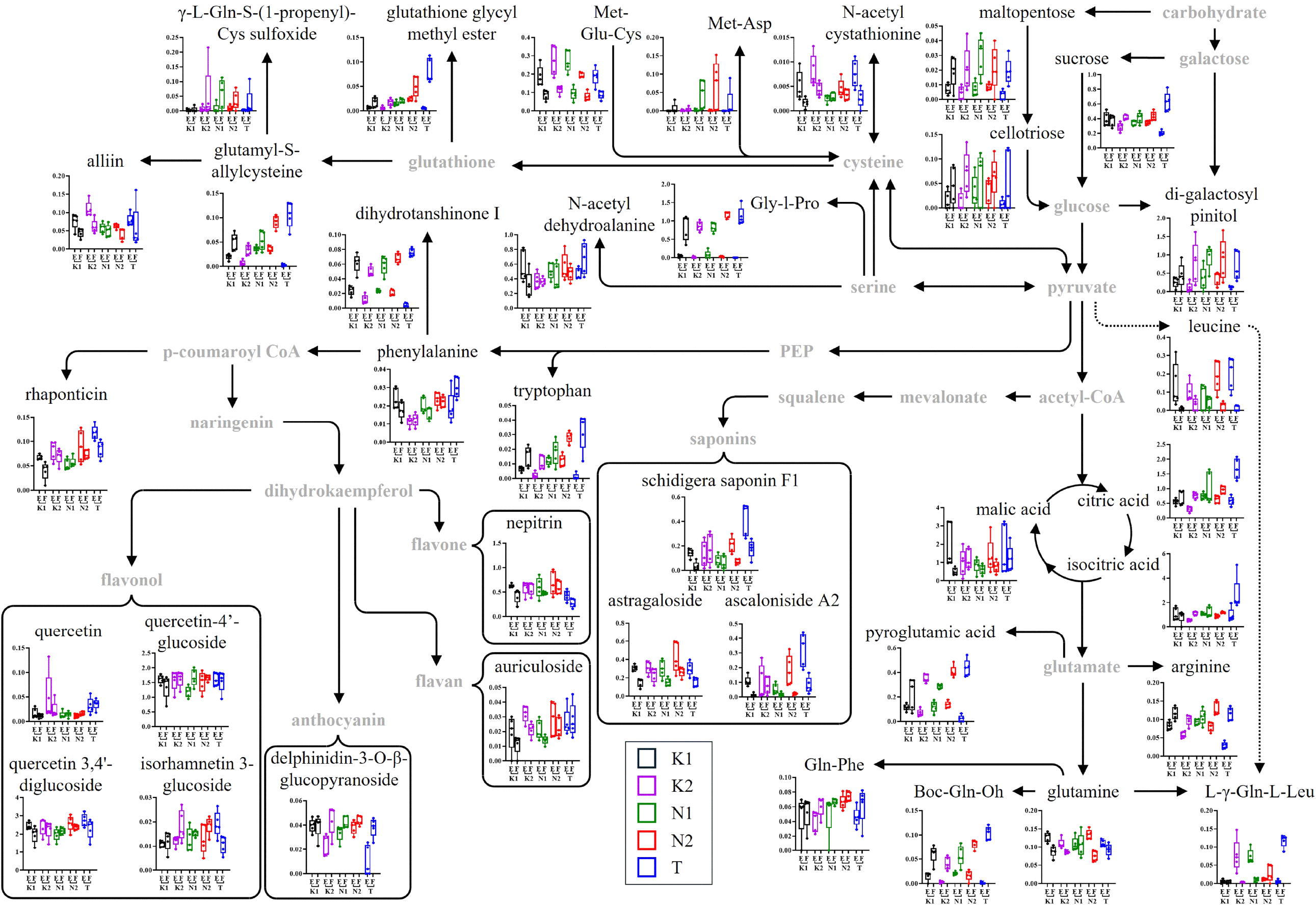
Regarding organic acids, malic acid decreased during storage in all onions except N1, whereas citric acid increased in all, with a sharp 2.87-fold rise in T onion. Isocitric acid also increased most in T onion. These trends reflect sustained TCA cycle activity and ongoing energy metabolism during storage (Akram, 2014; Shivakumar, 2014). Differential accumulation of intermediates like citric and isocitric acids may influence biosynthetic pathways and overall metabolic activity across cultivars and regions (Kumari, 2023).
Amino acids and peptides associated with glycolysis and the TCA cycle, including arginine, pyroglutamic acid, Boc-Gln-OH, and Gln-Phe, increased in all onions. In T onion, arginine, pyroglutamic acid, Boc-Gln-OH, and L-γ-Gln-L-Leu increased sharply (3.86 to 93.74-fold), while glutamine decreased in K1, K2, N2, and T onions. L-γ-Gln-L-Leu notably decreased in K2 (-19.39-fold). These shifts are linked to activation of stress-responsive metabolomic pathways (Chilukuri et al., 2018) and may be affected by initial cultivar and region-specific qualities (Böttcher et al., 2018; Hansen et al., 2001; Jo et al., 2016). Arginine and pyroglutamic acid contribute to osmotic and antioxidant responses, while changes in amino acids and peptides may influence onion flavor (Taglienti et al., 2020).
The phosphoenolpyruvate (PEP) pathway, associated with phenylpropanoid and flavonoid biosynthesis, plays roles in plant antioxidant defense and stress response (Kumar et al., 2023; Sharma et al., 2019). In this study, phenylalanine increased only in T onion, while tryptophan increased 4.97- and 26.11-fold in K2 and T onions. Dihydrotanshinone I increased over 1.5-fold in all samples, peaking in T onion (17.53-fold). Delphinidin-3-O-β-glucopyranoside, a minor anthocyanin in yellow onions, rose 3.74-fold in T onion. These changes suggest selective activation of defense pathways based on initial physiological traits. Meanwhile, auriculoside and quercetin decreased in K1 and K2 onions (1.71 and 2.02-fold), likely due to antioxidant utilization or conversion (Lachman et al., 2003; Sharma et al., 2015).
Saponin compounds such as astragaloside, ascaloniside A2, and schidigera saponin F1 generally showed a decreasing trend during storage across most onion samples. In particular, ascaloniside A2 markedly decreased in K1, N1, N2, and T onions by −9.49, −3.06, −8.30, and −4.12-fold, respectively, while schidigera saponin F1 decreased by −4.21, −2.90, and −2.39-fold in K1, N2, and T onions, respectively. Research on saponin accumulation and changes in onions is limited compared to other Allium species. However, saponin biosynthesis in plants is known to be strongly influenced by various environmental factors, including genotype, microclimate, soil fertility, and cultivation practices (Ariyanti and Latifa, 2021; Szakiel et al., 2011). Furthermore, due to their complex molecular structures, saponins can degrade over time if optimal storage conditions are not maintained (Kim et al., 2008).
Organosulfur compounds and some amino acids and in the cysteine-methionine pathway play roles in stress defense and flavor (Künstler et al., 2020). Alliin, N-acetylcystathionine, N-acetyldehydroalanine, and Met-Glu-Cys decreased during storage, with N-acetylcystathionine showing notable reductions in K1 and T onions (−3.05 and −2.94-fold). Met-Glu-Cys decreased more than −2.17-fold in all onions. In contrast, Gly-l-Pro, L-Gln-S-(1-propenyl)-Cys sulfoxide, Met-Asp, glutathione glycylmethyl ester, and glutamyl-S-allylcysteine increased significantly, especially in T onion. As these metabolites were not confirmed with authentic standards, future studies should validate their identities to strengthen confidence in their functional interpretation. Nevertheless, these increases reflect enhanced antioxidant defenses and physiological regulation under oxidative stress (Künstler et al., 2020). Decreased compounds may have been used as precursors or intermediates in thiosulfinate biosynthesis, linked to pungency development (Kamata et al., 2016). Pungency was not directly assessed in this study; however, previous studies have reported that sulfur-containing compounds, such as alliin, are positively associated with pungency in Allium species due to their involvement in the thiosulfinate biosynthetic pathway (Ammarellou, 2024; Benkeblia and Lanzotti, 2007). Additionally, such divergent changes across cultivars suggest directed regulation of sulfur metabolism, reflecting cultivar-specific physiological responses (Kamata et al., 2016).
Overall, metabolic changes during storage were primarily observed in glycolysis, the TCA cycle, phenylpropanoid, and cysteine-methionine pathways. The absence of Level 1 confirmation for key metabolites is a limitation that future studies should address. Despite this, the shifts in sugars, organic acids, and amino acids point to active energy metabolism and stress responses, especially in T onion. Selective accumulation of functional metabolites suggests differential regulation of antioxidant and defense-related pathways across cultivars and regions (Böttcher et al., 2017; Saviano et al., 2019).
4. Conclusions
This study investigated quality and metabolite changes in onion bulbs during storage by analyzing identified metabolites across different cultivars and regions. During storage, physiological indicators such as respiration rate, weight loss, and firmness showed a general decline, with slight but statistically significant differences depending on cultivar and cultivation region. Metabolomic analysis revealed that sugars, organic acids, amino acids, and organosulfur compounds were the major metabolite classes affected by storage, with particularly notable changes observed in the T onion. Among these, oligosaccharide accumulation coincided with firmness reduction, and significant changes were observed in organosulfur compounds involved in the biosynthetic pathway of pungency. These results revealed cultivar- and region-dependent alterations in primary and secondary metabolic pathways. Further studies are needed to elucidate how key metabolites contribute to texture and flavor regulation and how they respond to environmental and preharvest influences during storage. Nevertheless, the findings are expected to enhance understanding of storage-related quality deterioration and contribute to developing improved postharvest management strategies for onions.
