1. Introduction
Adequate human nutrition is dependent on leafy vegetables due to their numerous micronutrient and food security roles. Some of these leafy vegetables produce seeds, which can also be explored for other food uses. For example, the seed of amaranth has been explored for consumption as a snack and as a major source of gluten-free flour (Solarov et al., 2022). Celosia argentea plant is a relished source of leafy vegetables that is widely consumed for its nutritional benefits (Adegbenro et al., 2021). A part of the Celosia argentea plant is its seed, which also serves as a means of propagation (Olawuyi et al., 2024). The Celosia argentea seed (CAS) has been indicated as a pseudocereal due to its numerous components (Nagar et al., 2022).
Pseudocereals have been mostly explored as alternatives to cereals that belong to the grass family (Poaceae) due to their unique nutraceutical and nutritional properties (Nandan et al., 2024; Zhang et al., 2024). For example, amaranth, quinoa, and buckwheat seeds are well known to be nutrient-dense and have been found suitable for developing foods void of gluten such as soup, pastries, and noodles (Sofi et al., 2023). Celosia argentea seed, derived from a leafy vegetable commonly known as Lagos spinach or “soko yokoto” in Nigeria, is a small, dark seed traditionally underutilized despite its nutritional potential (Ayodele, 2021). So far, CAS use has been limited to folklore preparation of antidiabetic decoction (Duarte et al., 2023; Luo et al., 2024).
Celosia argentea seeds possess promising nutritional and functional properties that make them valuable for the food industry. The seeds are rich in proteins, essential amino acids, unsaturated fatty acids, and micronutrients, including iron and zinc (Ibrahim et al., 2024). Their antioxidant and anti-inflammatory phytochemicals also offer health-promoting benefits, supporting their incorporation into functional foods, flours, and nutraceuticals. Additionally, C. argentea seed oil has been explored as a potential edible oil source, while the flour can enhance the nutritional profile of baked and weaning foods (Azeez et al., 2024). These attributes underscore its potential use in tackling malnutrition and developing value-added products, especially in regions where the crop is underutilized.
Despite its potential, several barriers hinder the broader adoption of Celosia argentea seeds in mainstream food processing. These include limited agronomic data and standardization in cultivation, low public awareness, lack of commercial-scale processing technologies, and minimal research on its processing and regulatory approval. Furthermore, consumer acceptance is affected by unfamiliarity and cultural preferences. Addressing these challenges through targeted research, food safety assessments, and value chain development is necessary for scaling up the use of C. argentea seeds in the food industry.
Pseudocereals, like other cereals, undergo mild and severe processing operations, which depend on the desired product. Processing unit operations, depending on the level of severity, potentially influence the nutritional, antinutritional, and functionality of raw materials (Singh et al., 2023). Processing operations have been adopted to enhance or reduce the bioavailability of micro- and macro-nutrients. Utilisation and nutritional benefit of pseudocereals have been increased by adopting various food processing unit operations such as fermentation, germination, and roasting, among others. As such, CAS can attain its full utilisation domestically and commercially by evaluating the effect of pretreatment unit operations on the nutritional and bioactive properties of its flour. Hence, our study evaluated the influence of processing on the nutritional and bioactive properties of Celosia argentea seed flour.
2. Materials and methods
Celosia argentea seed was obtained from local vegetable farmers in Sango market, Saki West, Oyo state, Nigeria. All chemicals used were of analytical grade.
The matured CAS were separated from the immature seeds and other extraneous materials. The sorted CAS were washed with clean water and allowed to drain in a sieve, followed by being thinly spread on trays. The cleaned and drained CAS were thinly spread on trays and allowed to dry for 2 days at ambient temperature. The dried CAS were subdivided into 8 parts with each weighing 500 g. Each part was subjected individually to nixtamalization, germination, autoclaving, blanching, roasting, fermentation, and defatting, while the last 500 g was stored in the Ziploc bag kept inside a plastic container at ambient temperature.
Nixtamalization of CAS was done following the method of Ogunbusola et al. (2021) with slight modification. Analytical grade Calcium hydroxide (20 g) was dissolved in 2,000 mL of clean water, followed by the steeping of 500 g of CAS before being allowed to boil for 30 minutes. The cooked CAS was drained of water before drying at 50°C for 6 h. The dried CAS were milled into flour, cooled, and sieved with a mesh size of 80 μm. The nixtamalized celosia flour was packaged in a Ziploc bag before being placed in an airtight plastic container at ambient temperature until when needed for analysis.
Cleaned CAS was germinated using the method described by Chima and Fasuan (2021) with slight changes. The cleaned CAS was steeped in hot water for 4 h and immediately spread thinly on a previously cleaned and moist jute bag. The CAS thinly placed on the moist jute bag was well covered with another jute bag which was also kept moist. The jute bags with the CAS were moistened twice a day and allowed to germinate for 8 days at ambient temperature. The germinated CAS were dried at 50°C for 6 h. The radicules and plumules from the germinated CAS were removed by handpicking winnowing before milling into flour. The milled CAS flour was sieved using 80 μm mesh before being packaged in a Ziploc nylon and subsequently placed in an airtight plastic container at ambient temperature.
Autoclaving of CAS was done by soaking 500 g of previously cleaned CAS in water for 1 h, autoclaving at 121°C for 15 min, and cooling. The autoclaved CAS was dried at 50°C for 6 h, milled into flour, cooled, and sieved with 80 μm mesh before packaging into Ziploc nylon. The packaged autoclaved CAS flour was placed in an airtight plastic container until required for analysis.
The method of Mandliya et al. (2023) was used for the blanching of CAS with slight modifications. The cleaned CAS was dipped in a hot water bath operating at 80°C for 5 min. Before being ground into flour, the blanched CAS was drained, allowed to cool, and then put in a hot air oven set to 50°C for eight hours. After being sieved using an 80 μm mesh screen, the CAS flour was wrapped in a Ziploc nylon. The blanched CAS flour that was packaged was kept at room temperature in an airtight container.
The cleaned Celosia argentea seeds were spread uniformly in pre-heated trays and roasted at 160°C for 25 min. The Celosia argentea roasted seed was ground into flour. The flours were then passed through an 80 μm mesh screen, packaged in Ziploc nylon, and stored in an air-tight plastic container for further use.
The fermentation of CAS was done using the method of Olawoye and Gbadamosi (2020) with modifications. Briefly, the previously cleaned CAS was boiled for 3 h. The boiled CAS were then transferred into banana leaves wrapped in a sack and allowed to ferment for 72 h. Following fermentation, the seeds were dried for eight hours at 50°C in a hot-air oven. The dried CAS was ground into flour, allowed to cool, sieved through an 80 μm mesh screen, and then sealed in Ziploc nylon and stored in an airtight plastic container until used.
The cold extraction procedure highlighted by Manickam and Kuca (2024) was used to defat Celosia argentea flour. The defatted flour was packed in a Ziploc nylon bag and stored in an airtight plastic container on the laboratory shelf.
The proximate composition of Celosia argentea seed flours—including moisture content, crude protein (determined by the Kjeldahl method), crude fat (solvent extraction), crude fibre, and ash—was analysed following the standard methods of the Association of Official Analytical Chemists (AOAC, 2005). N-free extract = 100 − (% moisture + % crude protein + % crude fat + % crude ash + % crude fiber) was used to calculate the content of nitrogen (N)-free extract.
The sample ash was dissolved in 10 mL of 2 M HNO3, boiled for five minutes, and filtered into a volumetric flask. The filtrate was then diluted to 50 mL with distilled water. Magnesium (Mg), manganese (Mn), iron (Fe), and zinc (Zn) concentrations were determined using an atomic absorption spectrophotometer (Model 220GF, Buck Scientific Inc., Norwalk, CT, USA). Standard curves for each mineral were prepared using known concentrations (AOAC, 2005). A Jenway flame photometer was used to measure the sodium levels.
The procedure of Osunrinade et al. (2024) that utilises methanol to extract bioactive content was adopted for CAS flour samples. The CAS extracts were used to quantify total phenolic content (TPC), and total flavonoid content (TFC) of CAS as influenced by processing. The Folin-Ciocalteu reagent was used to quantify the TPC of the CAS extract with the use of a garlic acid-fitted standard curve (Osunrinade et al., 2022). Also, the Osunrinade (2021) method was used to evaluate the TFC of CAS extracts spectrophotometrically.
The antioxidant potential of CAS extracts was established by the spectrophotometric measurement of the 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and total antioxidant capacity (TAC) as outlined by Azeez et al. (2024).
Bioactive compounds present in the methanolic extract of processed CAS were profiled using the method described by Azeez et al. (2024). Briefly, 1 μL of methanolic extract of CAS samples was injected into the Pegasus 4D model of Shimadzu gas chromatography-mass spectrometer (GC-MS) instrument (LECO Corporation, based in St. Joseph, MI, USA) that used a fused silica column and helium carrier gas at a 1 mL/min flow rate. The temperature increase was set at 10°C each minute, and the injector temperature was set at 250°C. By comparing mass spectra with the spectra databases of the National Institute of Standards and Technology, substances were identified. Literature survey was used to establish the bioactivity of detected compounds.
Analysis of variance (ANOVA) was performed on the gathered data in triplicate using IBM-SPSS (version 21.0). The Duncan’s multiple range test was used to distinguish treatment means, and p<0.05 was used to establish significant differences. Software from SAS Institute Inc. (NC, USA) called JMP Pro 17 was used to analyze principal component analysis.
3. Results and discussion
Table 1 displays the outcome of how processing techniques affected the proximate composition of Celosia argentea seed flour. The moisture content of CAS samples ranged from 5 to 7% with a roasted sample having the lowest moisture content. When compared with the untreated CAS, all pretreatment reduced the moisture content of the CAS. This could be linked to the alteration in the cellular structure of the CAS that led to the ease of removal of moisture. This is like the findings of Olawoye & Gbadamosi (2020), who outlined a decrease in the moisture content of amaranth flour due to autoclaving, blanching, and defatting. More importantly, the moisture content of less than 10% reported in all samples is an indication of the storability of CAS irrespective of the pretreatment used.
IXT, nixtamalized Celosia argentea seed flour; INA, germinated Celosia argentea seed flour; TOL, autoclaved Celosia argentea seed flour; CHG, blanched Celosia argentea seed flour; OAS, roasted Celosia argentea seed flour; MER, fermented Celosia argentea seed flour; FTD, defatted Celosia argentea seed flour; RMC, untreated Celosia argentea seed flour.
The processed Celosia argentea seed sample had a protein content ranging from 13.18 to 17.18%; the fermented CAS had the highest protein content, while the untreated CAS had the lowest (13.18%). Generally, CAS samples had significantly higher protein content when compared to the untreated CAS. The high protein content in fermented CAS could be due to the activity of microorganisms that synthesise amino acids from carbohydrates. Hence, fermented CAS can be used to enrich cereal-based or plant-based foods, helping combat protein-energy malnutrition, especially in low-income populations. The protein content of CAS as influenced by pretreatment is in the range of protein content (8.98-18.01%) reported by Olawoye and Gbadamosi (2020) for pretreated amaranth seed.
The fat content of Celosia argentea ranged from 2.14-9.66%, where defatted CAS flour had the least fat, while roasted CAS had the highest fat content. Generally, processing increased the fat content of CAS except for the case of defatting. The significant increase in fat extracted from roasted CAS results from the thermal degradation of cellular membranes and structural components during roasting. This process releases bound lipids, enhancing their mobility and facilitating extraction from the flour. A similar result was reported by Olawoye & Gbadamosi (2020), who observed the lowest percentage of fat (1.95%) in defatted amaranth flour. Among the pretreatments, germination resulted in significantly lower fat content in CAS flour. The activity of lipolytic enzymes during germination, which resulted in the breakdown of fat into fatty acids and glycerol, may be the source of this decrease in fat content. Hence, germinated CAS flour could be a source of low-fat flours that are less prone to rancidity, thus enhancing the storage stability and shelf life of products made from the flour without the need for preservatives. The germinated CAS flour could be useful in low-fat diets for individuals with conditions, such as pancreatitis, gallbladder disease, or hyperlipidemia.
The crude fibre content of the processed CAS flour ranged from 1.26-2.13%, where fermented CAS flour had the highest fibre content, while roasted CAS flour had the lowest fibre content. Pretreatments, which included fermentation, germination, and blanching, increased the fibre content of CAS flour. As reported by Adebo et al. (2022), the increase in crude fibre due to fermentation could be due to the production of cellulases and hemicellulases by lactic acid bacteria and yeasts, which degrade grain cell walls, releasing insoluble fibres (cellulose, hemicellulose) that were previously bound to other compounds. Singh et al. (2015) also reported that fermentation converts insoluble fibres into soluble fibres (e.g., β-glucans, fructans), which are more bio-accessible and measurable as total fibre. During germination amylases and proteases break down starch and proteins, increasing the relative proportion of fibre, while brief heat treatment could soften cell walls, making insoluble fibres (e.g., cellulose) more detectable by breaking matrix bonds (Hübner and Arendt, 2013).
The ash content of nixtamalized, blanched, roasted, and fermented CAS flour was significantly higher than the untreated CAS flour. Blanched CAS flour had the highest ash content (15.22%) while defatted CAS flour had the lowest value (2.43%). The high ash in blanched CAS flour is an indication of post-blanching mineral retention, possibly due to reduced leaching of ash and increased leaching of non-mineral components. The removal of the oil-bound minerals (phospholipids) during defatting could have been responsible for the decrease in the ash content of defatted CAS flour (Grasso et al., 2019). The ash content results of CAS flour obtained in this study are higher than the ash content of whole-grain amaranth flour (2.28-2.53%) reported by De Bock et al. (2021). Higher ash content in CAS flour suggests that the seed is a good source of essential minerals such as calcium, iron, magnesium, and zinc, contributing to bone health, oxygen transport, and immune function when included in food products.
The N-free extract ranged from 51.93-72.91%, with fermentation having the greatest reducing effect on the CAS flour carbohydrate content, followed by blanching and nixtamalization. The significantly lower N-free extract content observed in fermented Celosia argentea seeds compared to raw or processed forms can be attributed to the biochemical and microbial mechanisms where lactic acid bacteria (LAB) metabolize available carbohydrates as energy sources, converting them into organic acids and simple sugars. A similar decrease in N-free extract due to fermentation was reported for amaranth seed (Olawoye and Gbadamosi, 2020). As expected, due to the removal of fat components, defatted CAS flour had the highest N-free extract content. The changes in proximate composition, such as increased protein and reduced moisture, are important for improving the storage stability and nutritional value of CAS flour. These improvements can enhance its use in the food and pharmaceutical industries.
Table 2 shows the findings of the mineral content analysis of the processed Celosia argentea seed flour. Calcium (0.26-0.92 mg/g), magnesium (0.32-0.41 mg/g), sodium (0.94-1.61 mg/g), iron (0.31-2.4 mg/g), and zinc (0.03-0.05 mg/g) were the minerals identified in the processed CAS flour. Nixtamalized CAS flour had the highest values of calcium, magnesium, and sodium, while the blanched sample had the highest iron content (4.14 mg/g). Nixtamalization enhances mineral content through alkaline infusion, structural modifications, and antinutrient reduction, while the higher iron content due to blanching could be associated with the fact that blanching increases measurable iron primarily by removing inhibitors and the disruption of the CAS cell walls, releasing bound iron from the aleurone layer and bran fractions. The result from these studies was lower when compared with the report of Olawoye and Gbadamosi (2020) who reported the magnesium, sodium, calcium, and iron of processed amaranthus flour to be 0.60-1.02 mg/g, 1.02-2.52 mg/g, 1.03-2.26 mg/g and 3.03-7.13 mg/g respectively. Minerals support the body’s anabolic and catabolic functions; thus, the minerals found in processed Celosia argentea are nutritionally significant. Additionally, sodium, iron, and calcium are crucial for preventing anaemia, preventing osteoporosis, and managing hypertension, respectively (Martiniakova et al., 2022; Yang et al., 2023), while zinc is essential for immune response, and it plays a key role in cell growth and repair.
IXT, nixtamalized Celosia argentea seed flour; INA, germinated Celosia argentea seed flour; TOL, autoclaved Celosia argentea seed flour; CHG, blanched Celosia argentea seed flour; OAS, roasted Celosia argentea seed flour; MER, fermented Celosia argentea seed flour; FTD, defatted Celosia argentea seed flour; RMC, untreated Celosia argentea seed flour.
To assess the antioxidant properties of Celosia argentea seed flour produced from different processing methods, various parameters, including total antioxidant capacity, total flavonoid content, total phenolic content, ferric reducing antioxidant power (FRAP), and DPPH, were evaluated, and the results are presented in Table 3. Pre-treatment of CAS significantly (p<0.05) influenced all the antioxidant properties studied. The total antioxidant capacity of Celosia argentea seed flour ranged from 0.93-6.49 mg GAE/g, where the highest TAC (6.49 mg GAE/g) was obtained from the nixtamalized sample, while the lowest value (0.93 mg GAE/g) was from the roasted sample. Nixtamalization could have increased the TAC by releasing bound antioxidants (phenolics, flavonoids), neutralizing anti-nutrients (tannins, phytates), and generating new antioxidants through Maillard reactions (Gaytán-Martínez et al., 2017). Whereas roasting degrades heat-sensitive phenolic acids (e.g., chlorogenic acid, ferulic acid) and flavonoids, which are primary contributors to antioxidant activity in grains (Mestanza et al., 2023).
IXT, nixtamalized Celosia argentea seed flour; INA, germinated Celosia argentea seed flour; TOL, autoclaved Celosia argentea seed flour; CHG, blanched Celosia argentea seed flour; OAS, roasted Celosia argentea seed flour; MER, fermented Celosia argentea seed flour; FTD, defatted Celosia argentea seed flour; RMC, untreated Celosia argentea seed flour.
Secondary plant compounds called flavonoids have a variety of pharmacological properties, including antiviral, anti-inflammatory, anticancer, and capillary fragility effects (Farhan et al., 2023). The total flavonoid content ranged from 1.35 to 3.34 mg/g, with a significant difference (p<0.05) between the unprocessed and processed CAS flour. Among the processed samples, germinated CAS flour had the highest TFC (3.24 mg/g) while roasted CAS flour had the lowest (1.34 mg/g) value of TFC. The high TFC could be because germination triggers the expression of enzymes like phenylalanine ammonia-lyase (PAL), chalcone isomerase (CHI), and flavonol synthase (FLS), which drive flavonoid synthesis through the phenylpropanoid pathway (Zhang et al., 2022). The result of TFC further explained the reducing effect of roasting, which could be primarily through thermal degradation of polyphenols and oxidative losses that might have occurred during roasting.
The main components of cereal grains’ antioxidant capacity are phenolic chemicals, which are also crucial for the prevention and management of degenerative disorders (Fărcaş et al., 2021). The maximum total phenolic compound (TPC) was found in fermented CAS flour (36.74 mgGAE/g), according to changes in TPC as influenced by various treatments. Blanching of CAS had a reducing effect on the TPC. The observed reduction in the total phenolic compound could be the result of some thermal processing-induced phenolic compound degradation. Olawoye and Gbadamosi (2020) also reported the total phenolic content of blanched amaranthus seed to be lower compared to other processing methods.
The FRAP assay quantifies the reducing potential of an antioxidant that produces a coloured ferrous tripyridyl triazine by reacting with a ferric tripyridyl triazine (Fe3+-TPTZ) complex. In general, the existence of chemicals that break the chain of free radicals by donating a hydrogen atom is linked to their reducing characteristics. At low pH, the Fe3+-TPTZ combination is reduced to a blue-coloured Fe2+-TPTZ (Medalcho et al., 2025). There was a significant difference (p<0.05) in the FRAP value of CAS flour samples based on the various pretreatments. In this study, only fermentation and germination caused a significant increase in the CAS flour ferric reducing antioxidant power, while other processes led to a significant reduction. Fermentation increases FRAP by releasing bound phenolics, while germination increases FRAP by activating enzymes that enhance antioxidant availability. Thermal degradation and leaching are major factors causing FRAP decline during processing (Collins et al., 2024).
A stable free radical molecule, DPPH, is frequently used to evaluate a variety of samples’ capacity to scavenge free radicals. It has an adsorption peak at 517 nm and is a stable organic free radical. When an electron or a free radical species is accepted, adsorption vanishes, causing a significant discolouration from purple to yellow (Girish et al., 2023). The scavenging activity of processed CAS flour samples was significantly different at p<0.05. The highest percentage inhibition of the DPPH radical determined for defatted, autoclaved, germinated, and fermented CAS flour was 50.28, 42.50, 40.89, and 32.85%, respectively. The extractability of bioactive antioxidants brought on by thermal processing may be the cause of autoclaving’s increased DPPH radical inhibition (Sharma et al., 2022).
Table 4 presents the bioactive compounds detected in CAS flour as influenced by processing. A total of 58 bioactive compounds with a library similarity above 90% were identified in CAS flour samples. Specifically, 15 compounds were detected in the unprocessed CAS flour, while the remaining 43 compounds were detected only after processing. Compounds unique to the untreated CAS flour (RMC) included cetene, 1-heptadecene, carbonic acid prop-1-en-2-yl tridecyl ester, bromoacetic acid pentadecyl ester, and linoleic acid ethyl ester. Most of the compounds detected in the CAS samples exhibited significant health benefits, including antioxidant, anti-inflammatory, antimicrobial, and antifungal properties.
IXT, nixtamalized Celosia argentea seed flour; INA, germinated Celosia argentea seed flour; TOL, autoclaved Celosia argentea seed flour; CHG, blanched Celosia argentea seed flour; OAS, roasted Celosia argentea seed flour; MER, fermented Celosia argentea seed flour; FTD, deffated Celosia argentea seed flour; RMC, untreated Celosia argentea seed flour.
n-Hexadecanoic acid was detected in all CAS samples except the fermented and germinated ones. This compound has been reported to possess various health benefits, including anti-inflammatory, antioxidant, antimicrobial, and anticancer properties (Aparna et al., 2012; Ganesan et al., 2024). The presence of 9,12-octadecadienoic acid methyl ester in defatted, nixtamalized, and blanched CAS samples suggests its potential health benefits, which include anti-inflammatory, antimicrobial, anticholesterolemic, and anticarcinogenic effects (Ayoola et al., 2020; Krishnaveni et al., 2014). Notably, this compound was identified in bread made from whole and refined wheat supplemented with Celosia argentea seed flour (Azeez et al., 2024).
Hexadecanoic acid methyl ester, detected in defatted, nixtamalized, blanched, and germinated CAS samples, exhibits therapeutic potential against oxidative stress-related diseases, fibroids, and possesses antioxidant, anti-inflammatory, and other health-beneficial properties (Abubacker and Deepalakshmi, 2013; Shaaban et al., 2021). Nixtamalization facilitated the presence of pentadecenoic acid, 14-methyl-, methyl ester in CAS samples. This compound has demonstrated potential antifungal and antimicrobial effects, along with antioxidant properties, and serves as a food flavour component (Godwin et al., 2015; Momodu et al., 2022). Additionally, pentadecanoic acid has been reported to exhibit anti-obesity effects (Robinson et al., 2024) and anti-inflammatory properties, which may improve insulin sensitivity and reduce the risk of type 2 diabetes (Robinson et al., 2024; Venn-Watson and Schork, 2023). Furthermore, Trieu et al. (2021) identified pentadecanoic acid as a reliable biomarker for dairy fat intake, with an inverse association with cardiometabolic disease risk.
Hexadecanoic acid butyl ester, which has been reported to exhibit antibacterial, anticancer, and anti-inflammatory properties, was detected in germinated, untreated, fermented, and blanched CAS samples. The hexadecane compound, present in nixtamalized, fermented, untreated, and blanched CAS samples, has demonstrated antibacterial and antioxidant activities (Shaaban et al., 2021). Additionally, 10-methylnonadecane, identified in untreated, nixtamalized, fermented, and blanched CAS samples, has been reported to possess antimicrobial and antioxidant properties. This compound has also been associated with other biological effects, including antiviral, anticancer, hypocholesterolemic, and neurostimulant activities (Kumosani et al., 2024).
Linoleic acid ethyl ester, which has been reported for its significant health benefits in reducing total low-density lipoprotein (LDL) cholesterol (Huang et al., 2010), was detected in both untreated and fermented CAS samples. Trisocane, a straight-chain alkane hydrocarbon found in various plant extracts, exhibits antimicrobial activity and antioxidant properties (Baltacı et al., 2022). This compound was detected only in the blanched CAS sample. Additionally, hentriacontane, which has been reported to possess pharmacological effects such as anti-inflammatory, anti-tumour, and antimicrobial activities (Hamed et al., 2025), was also found exclusively in the blanched CAS sample. In contrast, the autoclaved CAS sample contained gingerol, a major bioactive compound in ginger (Dalsasso et al., 2022). Gingerol has been associated with numerous potential health benefits, including anticancer, antioxidant, antimicrobial, and anti-inflammatory effects (Zhang et al., 2021).
4. Multivariate analysis of the influence of processing on proximate, mineral, and antioxidant properties of CAS flour
Multivariate analysis using principal component analysis is a veritable tool to establish the relationship between dependent and independent variables (Osunrinade et al., 2023). Presented in Table 5 is the summary of principal component analysis showing the percentage variance contributed by each principal component. The first two components accounted for over 50% of the variance, while the first four components described 80% of the total variation in the influence of processing methods on the proximate, mineral, and antioxidant properties of CAS flour.
| Number | Eigenvalue | Variance (%) |
|---|---|---|
| 1 | 4.646288 | 29.039 |
| 2 | 3.520447 | 22.003 |
| 3 | 2.91173 | 18.198 |
| 4 | 1.722683 | 10.767 |
| 5 | 1.511708 | 9.448 |
| 6 | 1.161904 | 7.262 |
| 7 | 0.525241 | 3.283 |
Visibly from Fig. 1, the biplot indicated that all the antioxidants’ properties were positively correlated with fermented, germinated, autoclaved and untreated CAS flour as they were all on the positive side of the first principal component. Interestingly, the first principal component had the crude fibre showing a positive correlation with all the antioxidant properties evaluated. The positive correlation of the crude fibre to antioxidant properties could be attributed to the high percentage of antioxidant potential in the bran and aleurone layers of grains, which contribute significantly to the fibre content.
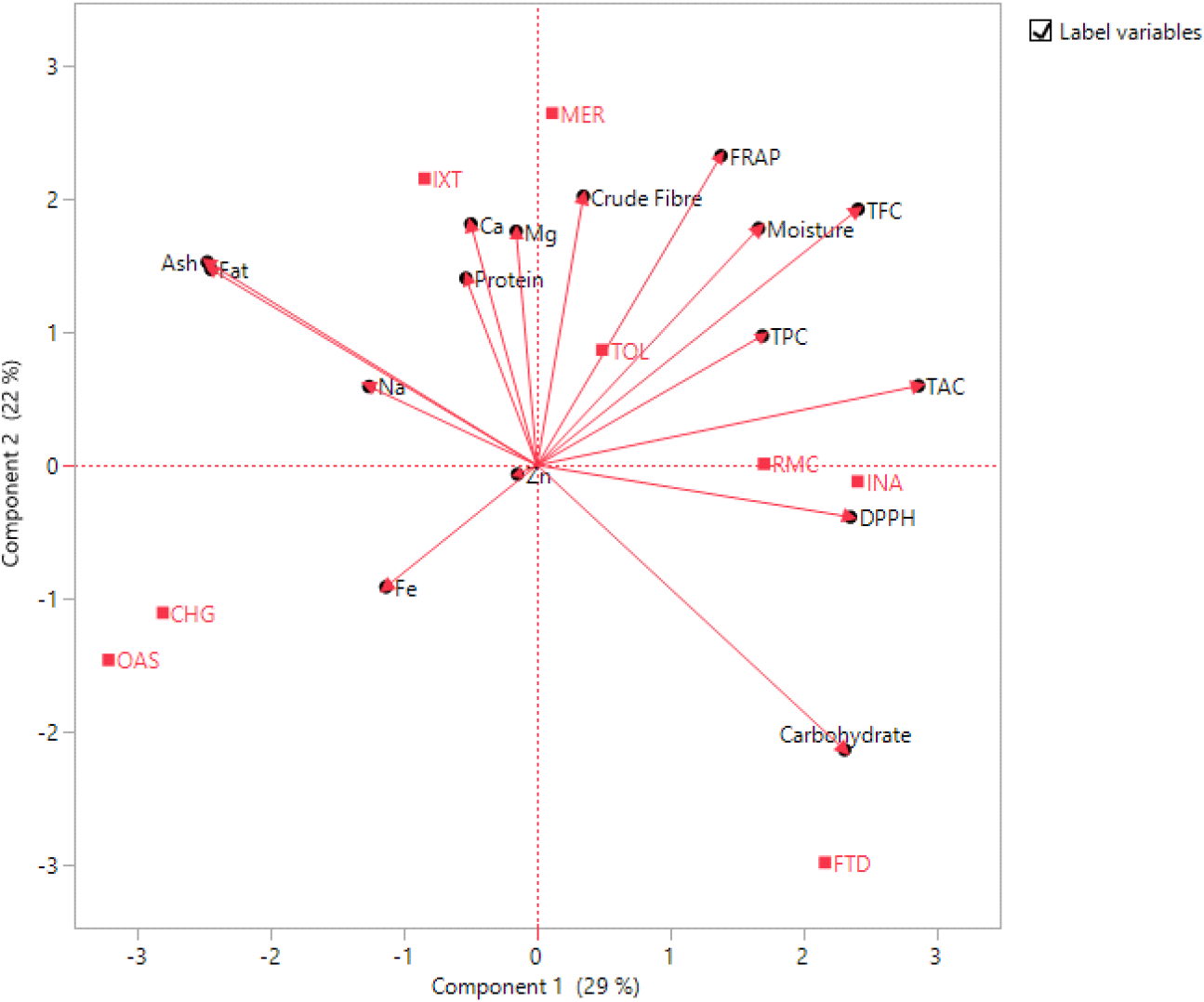
The biplot (Fig. 1) established the influence of nixtamalization on grains by having ash, calcium, magnesium, sodium, and protein on the positive side of the second principal component, which also has the nixtamalized sample (IXT). Being on the same side of these parameters is an indication of correlation, which indicates the influence of nixtamalization on the mineral and protein content of the CAS flour. However, the negative side of the second principal component was iron, showing a correlation with roasted and blanched CAS flour. As such, roasting and blanching are the two-unit operations that improve the quantity of iron detectable in CAS flour.
5. Conclusions
Processing methods used in this study significantly influenced the nutritional and bioactive components of Celosia argentea seed flour. The protein content of CAS was significantly improved by fermentation and germination, while N-free extract content was reduced. All the processing methods increased the fat content except defatting. Nixtamalized CAS flour had the highest values of calcium, magnesium, and sodium, while the blanched sample had the highest iron content. Nixtamalization increased the total antioxidant capacity of CAS flour, while the total flavonoid content, total phenolic content, and ferric-reducing antioxidant power were significantly increased by fermentation and germination. GC-MS analysis detected 58 bioactive compounds, which have been reported to possess antioxidant, anti-inflammatory, antimicrobial, and antifungal properties. As such, processed CAS has the potential as a viable pseudocereal for the production of nutrient-dense and bioactive-rich cereal-based products.
