1. Introduction
Annonaceous acetogenins (ACGs) are a lipophilic molecule constituted by 35 or 37 carbons which is originated from long chain fatty acids derived from the polyketide pathway (Alali et al., 1999). So far, more than 500 ACGs have been discovered from 51 species in 13 genera which mostly appeared in tropical or sub-tropical regions of the world (Leboeuf et al., 1980). Chemically ACGs have one to three tetrahydrofuran (THF) rings and contain a long aliphatic chain with a methyl-substituted α, β-unsaturated γ-lactone (Ohta et al., 2022). Depending on the chemical structure, ACGs are classified in several types and demonstrated a wide range of biological activities including antimicrobial, antiparasitic, pesticidal, and immunosuppressive effect (Neske et al., 2020). Also, it has been observed that these phytochemicals have selective toxicity to diverse types of tumor cells and powerful inhibitory effects against the mitochondrial respiratory chain complex I (Qayed et al., 2015; Tormo et al., 2003). More recently, ACGs have also been reported to overcome resistance in multidrug resistant (MDR) cancers (Manoharan et al., 2024). Because of these health benefits, commercial development has been reached as a material of pesticides and dietary supplement. Furthermore, commercial product containing twig and stem extracts of pawpaw is sold over the world as treatments for cancer because of containing ACGs (Bravo-Alfaro et al., 2024).
Asimina triloba (L.) Dunal., commonly known as the pawpaw, is a small tree distributed mainly in eastern part of the united states and also has been planted in East Asian countries such as Japan, China, and Korea (Nam et al., 2024). The pawpaw is member of the Annonaceae family, approximately 2,300 species and 130 genera, only the genus Asimina grows in the temperate climate zone and all other genera grow in the tropical region (Callaway, 1992). Members of the Annonaceae family, such as soursop (Annona muricata), custard apple (Annona squamosa), and cherimoya (Annona cherimola), has considerable amounts of ACGs (Pomper and Layne, 2005). Also, pawpaw revealed the presence of various bioactive compounds including phenolic compounds and alkaloids (Avula et al., 2018; Nam et al., 2017a; Nam et al., 2019). Due to these natural compounds, the pawpaw possesses high biological activities such as antioxidant, anticancer, and pesticidal effect (Nam et al., 2018; Rupprecht et al., 1986).
Previous investigations provided over 40 other ACGs which have been isolation and identified from different parts of North American pawpaw. The stem bark has trilobacin and asimicin isomers including asimin, asiminacin, and asiminecin (Zhao et al., 1992; Zhao et al., 1994). Pawpaw twigs possess asimicin, bullatacin, and trilobacin, which show a variation in concentration with seasonal changes (Gu et al., 1999). Recently, the seeds of this plant have not only ACGs contained in stem bark mentioned above but also squamostatin and bullatanocin (Avula et al., 2018). As such, the variety and contents of acetogenins contained in pawpaw have been extensively studied. However, most of these studies have been focused on pawpaw trees cultivated in the USA, with limited research available on those grown in Korea. Notably, existing studies have primarily concentrated on the fruit (Nam et al., 2018), and no comprehensive analysis has been conducted on the acetogenin content across different parts or in relation to various extraction solvents.
Therefore, the aim of this study was to evaluate the amount of ACGs contained in each part (root, twig, and leaf) of pawpaw tree grown in Korea and to confirm the antiproliferative activity of the related ACGs on AGS and HeLa cells.
2. Materials and methods
Reference standards for various acetogenin compounds, including annonacin, asimin, annomontacin, aromin, cis-annonacin, annomuricin, muricatacin, and bullatanocin, were sourced from ChemNorm Biotech Co. (Wuhan, China). Cell culture reagents such as Dulbecco’s Modified Eagle Medium (DMEM), RPMI-1640, fetal bovine serum (FBS), a streptomycin-penicillin antibiotic mixture, phosphate-buffered saline (PBS), and trypsin-EDTA were obtained from Gibco (Rockville, MD, USA). Dimethyl sulfoxide (DMSO) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents and solvents utilized were of analytical grade.
Pawpaw were harvested from a farm located in Okcheon, Korea (36.31°N, 127.57°E). The species-level identification was conducted by Dr. Otto Jahn of the Agriculture Research Service, United States Department of Agriculture (USDA). A representative voucher specimen was submitted to the herbarium managed by the U.S. National Plant Germplasm System. Following collection, the pawpaw tree was divided into individual parts, rinsed thoroughly with water, chopped into small segments, and subjected to lyophilization using a freeze dryer (Model LP100, Ilshin Lab Co., Daejeon, Korea). The resulting dried materials were pulverized into a fine powder using a Robot Coupe Blixer (Robot Coupe USA, Jackson, MS, USA), and stored at −70°C in a deep freezer until further extraction.
Powdered pawpaw samples were subjected to extraction using either 80% methanol or distilled water and labeled accordingly: methanol extracts of root (PRM), twig (PTM), and leaf (PLM); water extracts of root (PRW), twig (PTW), and leaf (PLW). Twenty grams of each powdered sample were mixed with 200 mL of the respective solvent (80% methanol or distilled water) and left to extract at ambient temperature for 24 h with continuous shaking at 120 rpm (BS-21, Jeio Tech Company, Daejeon, Korea). After incubation, the mixtures were centrifuged at 11,000 ×g for 40 min, and the supernatants were filtered through Whatman No.1 filter paper to obtain clear solutions. The collected filtrates were then concentrated under reduced pressure using a rotary evaporator (R-210, Buchi, Flawil, Switzerland) and subsequently freeze-dried to obtain the final extracts. Extraction yield was determined using the following formula:
Quantitative analysis of acetogenin compounds was carried out using a 2695 Alliance high-performance liquid chromatography (HPLC) system (Waters, Milford, MA, USA) coupled with a photodiode array (PDA) detector set to 280 nm for compound detection. Chromatographic separation was achieved on a Welchrom C18 analytical column (4.6×250 mm, 5 μm; Welch Materials, MD, USA), maintained at 30°C. The injection volume was set at 10 μL, and the mobile phase was delivered at a constant flow rate of 1.0 mL/min. The binary mobile phase consisted of solvent A (methanol:acetonitrile:0.5% acetic acid in acetonitrile:tetrahydrofuran=10:7:880:103) and solvent B (methanol:acetonitrile:0.5% acetic acid in water: tetrahydrofuran=10:7:880:103). Gradient elution was programmed as follows: from 0 to 40 min, the composition was changed from 100% to 15% solvent B, followed by a decrease from 15% to 5% B over the next 20 min (40-60 min). Standard acetogenin solutions were prepared in 50% methanol at concentrations of 0, 125, 250, and 500 ppm. Quantification of acetogenins in the extracts were accomplished by comparing retention times and peak areas with those of the corresponding authentic standards.
The human carcinoma cell lines AGS (gastric adenocarcinoma, KCLB No. 21739) and HeLa (cervical carcinoma, KCLB No. 10002) were obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin-streptomycin. Cultures were maintained at 37°C in a humidified incubator with 5% CO2. The medium was replaced every 2-3 days.
The antiproliferative activity of acetogenins were evaluated using the MTT assay. Cells were seeded into 96-well plates at a density of 2×103 cells/well and incubated for 24 h at 37°C in a 5% CO2 humidified atmosphere. Subsequently, cells were exposed to various concentrations of acetogenins (6.25, 12.5, 25, 50, 100, 200, and 400 μM) dissolved in dimethyl sulfoxide (DMSO). After 24 h of treatment, MTT solution (5 mg/mL in PBS) was added to each well, and plates were incubated in the dark for 3 h. Following incubation, the medium was discarded, and DMSO was added to dissolve the resulting formazan crystals. The absorbance was measured at 570 nm using a microplate reader (Infinite M200, Tecan, Männedorf, Switzerland) after gentle shaking.
3. Results and discussion
The solvent extraction has been widely used for the extraction of plant tissue, generally, solvent mixtures containing a polar and a non-polar component are more efficient than pure solvents for extracting bioactive compounds (Yang et al., 2017). In this study, 80% methanol and distilled water were used as extraction solvent.
The extraction yields of six pawpaw extracts (PRM, PTM, PLM, PRW, PTW, and PLW) are described in Table 1. Among the 80% methanol extracts, PLM was the highest accounted for 15.38% followed by PRM (9.28%) and PTM (3.97%). The results of distilled water extract also indicated that the highest extraction yield was obtained from leaf (12.03%) but showed no significant difference with PRW (11.50%) sample (p>0.05). In addition, PTW presented the lowest extraction yield (4.90%).
| Solvent | Plant parts | Extraction yield (%) |
|---|---|---|
| 80% Methanol | Roots | 9.28±1.981)b2) |
| Twigs | 3.97±1.10c | |
| Leaves | 15.38±2.84a | |
| Distilled water | Roots | 11.50±0.49b |
| Twigs | 4.90±0.13c | |
| Leaves | 12.03±0.84b |
A similar trend was observed, showing lower extraction yield from twigs compared to other parts of Annona muricata L., which showed the following order: leaves > roots > twigs (Nam et al., 2017b). Essama et al. (2015) reported that the yield acquired from ethanol extracts of A. muricata L. were ranged from 3.82% (stems) to 5.46% (leaves), which lower than pawpaw extracts. The yield of water extract obtained from A. squamosa L. leaves was higher than that of 80% methanol extract, 10.34% and 5.76%, respectively (El-Chaghaby et al., 2014). These studies exhibited that the extraction yield depends on the method used in the plant species and type of solvents. The method used in the extraction yield and the phytochemicals that have biological activities (Celep et al., 2019).
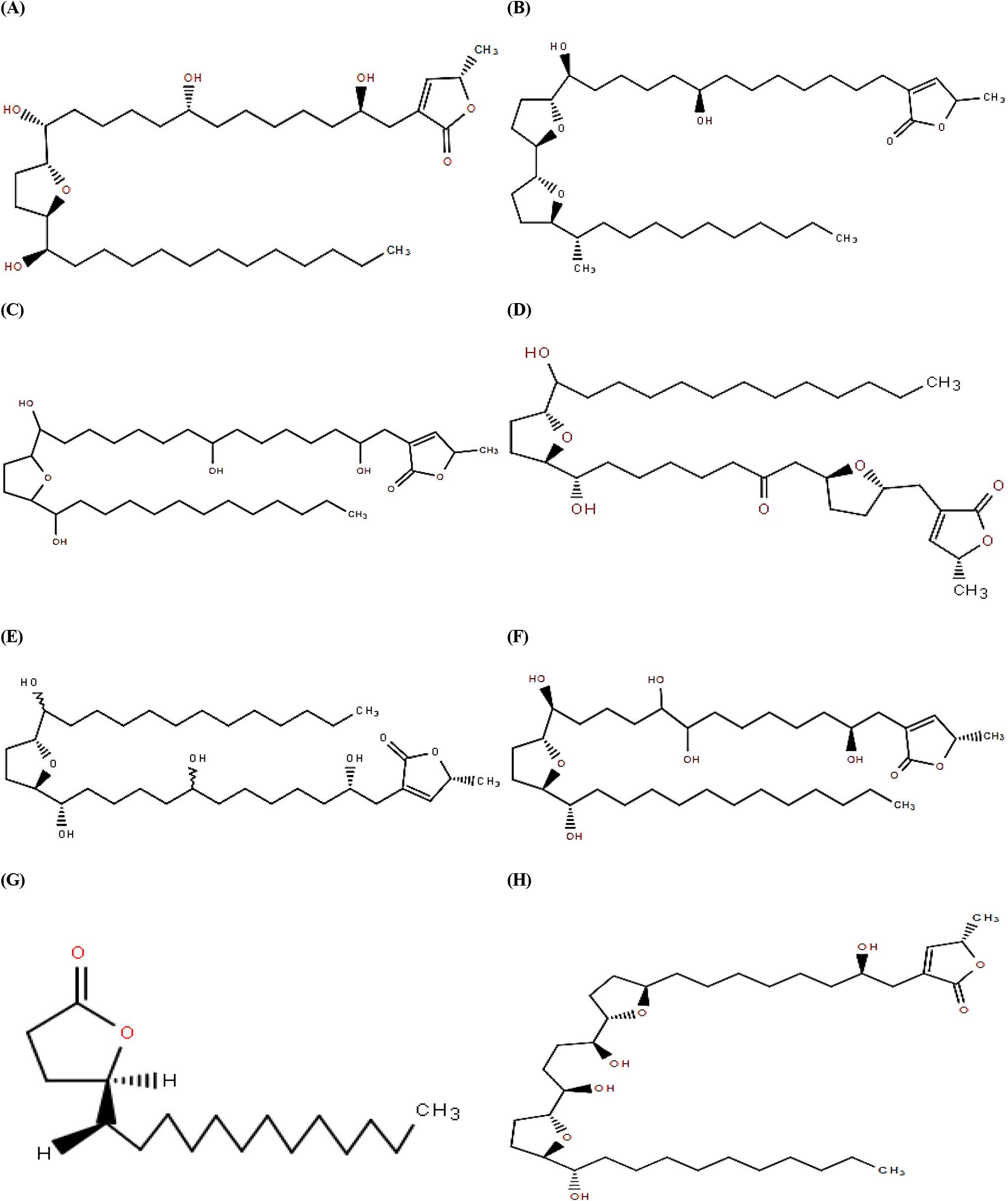
ACGs associated with pawpaw including annonacin, asimin, annonmontacin, aromin, cis-annonacin, annonmuricin, muricatacin, and bullatanocin are listed in Fig. 1. For the purpose of a detailed evaluation of acetogenin compounds in pawpaw extracts, HPLC-DAD analysis was conducted and chromatograms are illustrated in Fig. 2 and Fig. 3. Only six of the eight compounds were quantified in pawpaw extracts, and their content given in Table 2.
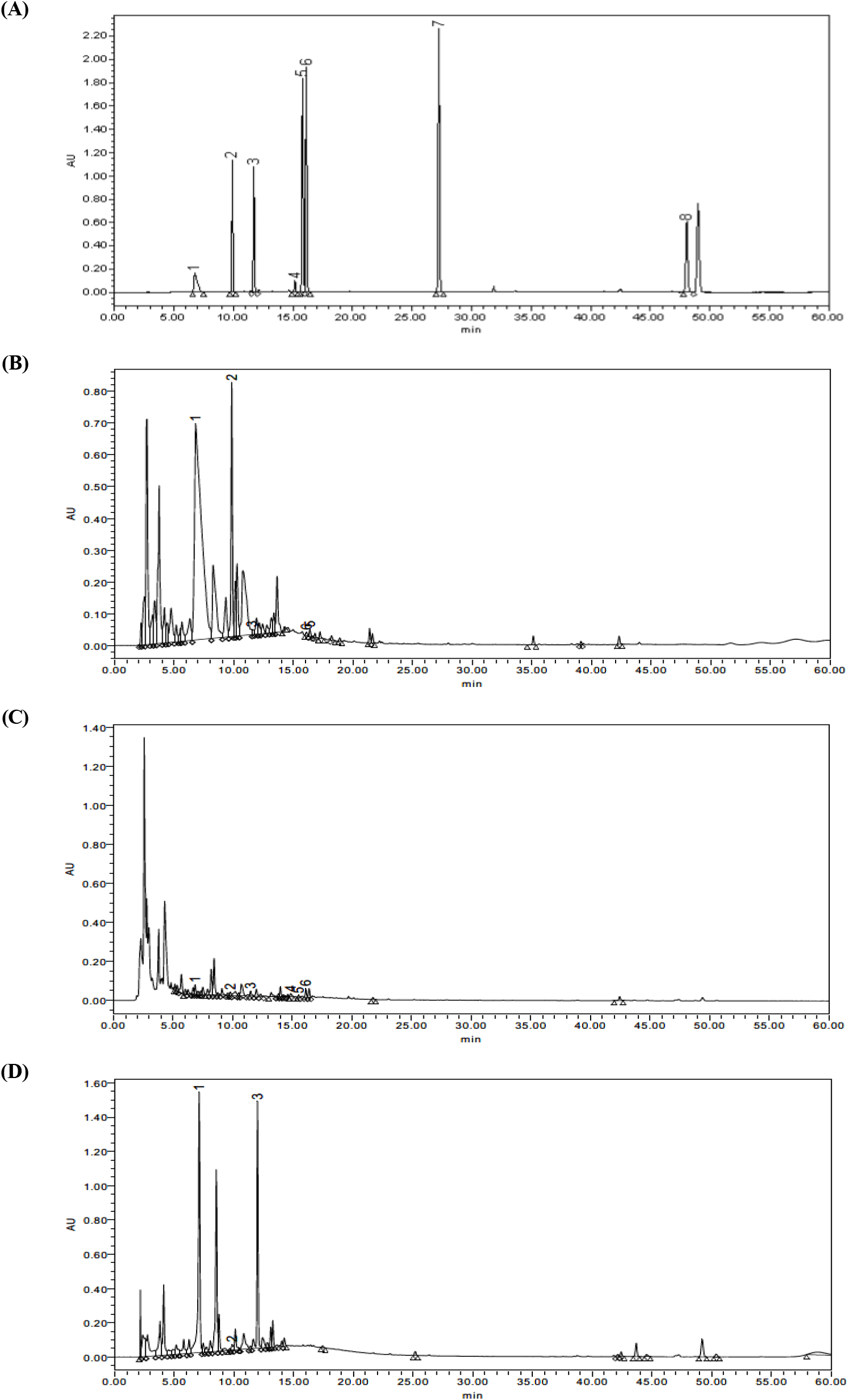
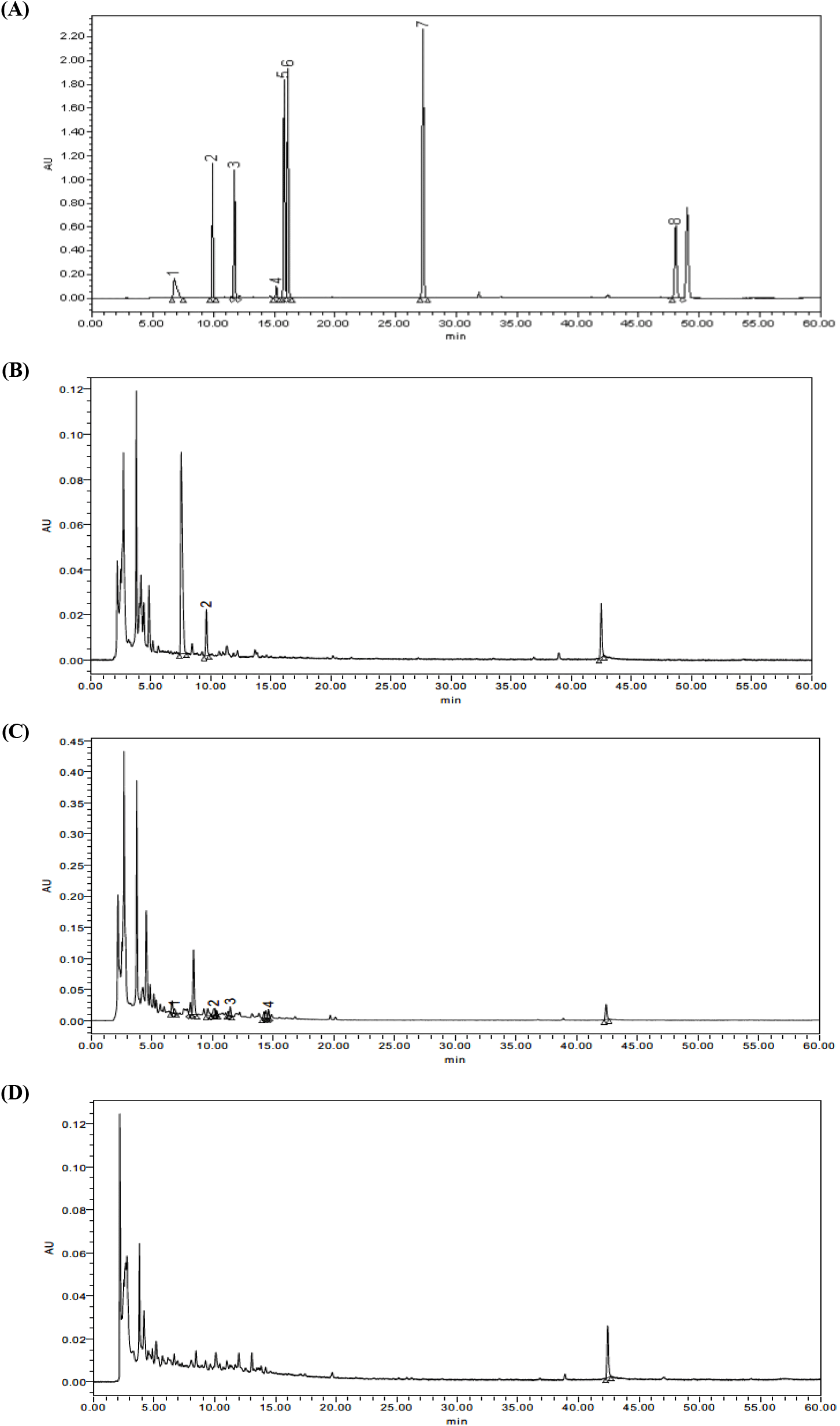
Generally, a significant variation was observed regarding the content of acetogenins depending on the plant parts and extraction solvent (p<0.05), and the acetogenin content of 80% methanol extract was clearly higher than that of distilled water extract. Methanol is polar but has hydrophobic methyl groups, so it can dissolve more hydrophobic substances than distilled water. Acetogenin is an amphipathic molecule that has both polarity and nonpolarity, however, it is not very polar substance. Accordingly, it is thought that a larger amount of acetogenin was confirmed in the methanol extract than in the water extract.
In 80% methanol extracts, the amount of identified acetogenins varied widely ranged from 21.31 mg/100 g (annomuricin, PRM) to 10,108.34 mg/100 g (annonacin, PRM). The most abundant acetogenin in twigs was aromin (217.70 mg/100 g), but relatively lower than that in root. Annomontacin was determined as the main component in leaf extract with concentrations of 1,707.98 mg/100 g. For distilled water extracts, the highest amount of acetogenin compounds was recorded in twigs which was aromin at the level of 201.28 mg/100 g. Among the studied components, asimin was determined in all pawpaw extracts except PLW and cis-annonacin was detected only in PTM. Muricatacin and bullatanocin were not found in neither of samples.
A remarkable difference was observed between extracts made from roots, twigs, and leaves. More precisely, the concentration of annonacin in PRM was about 56 times higher than its concentration in leaves. This can be explained that plants presumably due to their physiological significance and interaction of the individual plant parts with the environment (Brooker, 2006). Namely, harvest time and cultivation conditions such as moisture, temperature, and fertilizer could be account for the notable difference in acetogenins content. Gu et al. (1999) reported that different levels of acetogenin compounds including asimicin, bullatacin, and trilobacin accumulated in twigs of pawpaw in dependence on seasonal changes. Also, another study performed by Avula et al. (2018) reported that motrilin and squamocin were identified in pawpaw twig cultivated in the North America as well as annonacin and annomontacin which detectable in this study.
Previous studies of annonaceae species focused on isolation of ACGs from plant because the potential of pawpaw extracts to inhibit the cancer cell growth could be partly explained by the presence of ACGs such as annonacin, aromin, and annomontacin (Alfonso et al., 1996; Roduan et al., 2019). Therefore, the inhibition activity of different concentrations of ACGs, which were abundant in pawpaw tree cultivated in Korea, were evaluated.
The results of three acetogenins (annonacin, aromin, and cis-annonacin) among the six acetogenins detected in pawpaw that showed antiproliferative activity in AGS and HeLa cells are shown in Fig. 4. For AGS cells (Fig. 4A), both annonacin and aromin exhibited potent antiproliferative activity in a dose-dependent manner and 100 μM of annonacin inhibited approximately 53% (p<0.001) of cell proliferation. At 400 μM of acetogenins, all acetogenins presented significant inhibition of cancer cells and annonacin showed the highest antiproliferative effect with a cell viability of 5.14% (p<0.001) compared with control. However, cis-annonacin showed relatively weak inhibition of cell proliferation compared with the other samples.
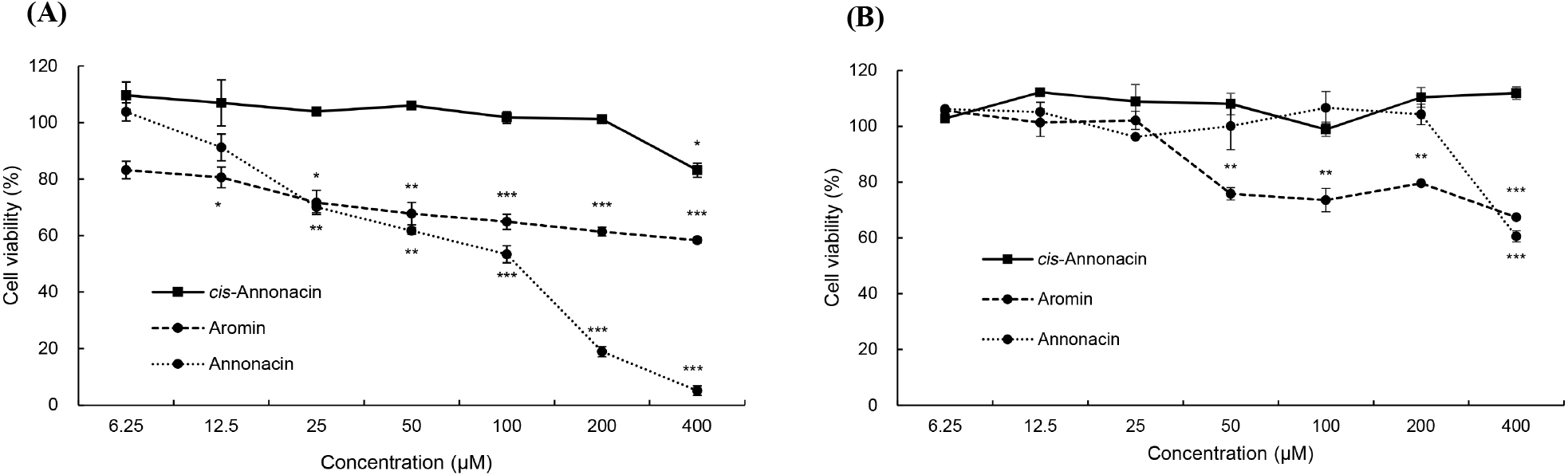
The inhibition of HeLa cell proliferation treated with different concentrations of acetogenins and the percentage cell viability was calculated as shown in Fig. 4B. It was observed that 50 μM concentration of aromin reduces the cell viability 75.78% (p<0.01) compared with control and it was further reduced to approximately 67% (p<0.001) when treated with the aromin at a concentration of 400 μM. Annonacin also exhibited a significant decrease in the number of viable HeLa cells compared to the control group at the highest concentration (p<0.001), while any concentration of cis-annonacin showed no effect on HeLa cell viability. These results indicated that annonacin and aromin presented more active against AGS than against the HeLa cell line.
To date, little is known about the cytotoxicity of cis-annonacin, however Oberilies et al. (1997) demonstrated that structures with cis and trans configurations in the THF ring have a very important effect on biological activity. Briefly, in the case of the trans form, the cis-form bullatacin showed up to 250 times more potent antiproliferative activity against MCF-7 cells (human breast cancer cells), and it was confirmed that asimicin and trilobacin had lower activity because they contained the cis form. Accordingly, it is thought that the difference in activity between annonacin and cis-annonacin occurred. However, since the antiproliferative activity of acetogenin varies not only depending on the three-dimensional configuration of the THF ring but also the length of the carbon chain, the position of the lactone, and the number of hydroxyl groups, more detailed studies are needed in the future.
The antiproliferative effects of acetogenins have been carried out on diverse cancer cells with significant positive results (Nam et al., 2018). Annonacin has been reported to be abundant in the bark and seeds of Asimina triloba, which inhibits growth in MCF-7 (breast carcinoma), A-549 (lung carcinoma), and HT-29 (colon adenocarcinoma) (Ko et al., 2011; Zhao et al., 1992). Aromin displayed an antiproliferative effect in MCF-7 and Hep G2 (liver carcinoma) cell lines with the effective dose 50% between 30 and 130 μM (de Pedro et al., 2013). Moreover, annomontacin isolated from the fruits of Annona glabra has cytotoxicity in Hep G2 cells (Chen et al., 2004). Sun et al. (2017) reported that annonacin from graviola showed strong growth inhibition on PC-3 (prostate carcinoma) cells which cell viability was reduced by 96.9% at 20 μg/mL. According to the references, it is clear that the isolated acetogenins could inhibit the proliferation of cancer cells and the antiproliferative activity is closely related to the chemical structure of ACGs depending on a number of hydroxyl groups (Sun et al., 2017).
4. Conclusions
This study was performed to evaluate the annonaceous acetogenins (ACGs) of extracts from different parts (roots, twigs, and leaves) of pawpaw (Asimina triloba [L.] Dunal) tree, and the antiproliferative activities of the major ACGs from pawpaw extracts against human gastric carcinoma (AGS) and human cervical cancer (HeLa) cells were investigated. The findings suggest that pawpaw trees cultivated in Korea may serve as a promising source of bioactive acetogenins with antitumor properties. However, further research is needed to purify the active compounds and explore their mechanisms of action using additional cell models.
