1. Introduction
Dyslipidemia is a disorder that includes hypercholesterolemia, hyperlipidemia, and hyperglycemia, resulting from various abnormalities in lipid metabolism, leading to disrupted lipid homeostasis in the bloodstream. It is primarily characterized by an excess of cholesterol, LDL (low-density lipoprotein) cholesterol, triglycerides, and low HDL (high-density lipoprotein) cholesterol levels in blood vessels, contributing to impaired circulation. This condition is known to cause inflammation, vascular leakage, atherosclerosis, and cardiovascular diseases. One of the primary forms of dyslipidemia, familial hypercholesterolemia (FH), affects approximately 1 in 250-500 individuals worldwide. When including secondary dyslipidemia, which can be induced by alcohol consumption, smoking, obesity, and diabetes, the global prevalence of dyslipidemia is estimated to range between 20% and 80% (Pappan et al., 2024). With the continued growth of the drug market, newly developed dyslipidemia treatments have been shown to improve cardiovascular and metabolic indicators in individuals. However, numerous studies have reported various adverse effects, including hepatic dysfunction, diabetes, malignancy, myalgia, and rhabdomyolysis (Cheon and Jo, 2022; Ghusn et al., 2024; Liu et al., 2024; Ramkumar et al., 2016). Therefore, research has continuously been performed on improving dyslipidemia through natural ingredients and functional foods, which are associated with relatively fewer safety concerns.
Dietary fat and cholesterol are absorbed by small intestinal cells and subsequently transformed into chylomicrons, which are then released into the bloodstream. Chylomicrons, including esterified and non-esterified forms, transport fatty acids and cholesterols into adipose and liver tissues via lipoproteins through interactions with the LDL-receptor, remnant receptors, and LDL receptor-related protein 1 (LRP1). To deliver fats to peripheral tissues, the liver secretes very low-density lipoprotein (VLDL), which contains a single molecule of APOB100, along with APOC3 and APOE. Meanwhile, HDL containing APOA1 is also secreted by the liver and small intestinal cells to transport accumulated LDL back to the liver for metabolism (Bays et al., 2024; Burks et al., 2025). In addition, excessive cholesterol levels can be diminished by the synthesis and secretion of bile acids, mostly in the liver. Recent findings have demonstrated that patients with obesity may store more than 50% of free cholesterol in adipocytes and/or adipose tissue, which is significantly higher than the approximately 25% stored in normal-weight individuals (Chung and Parks, 2016; Murphy and Yvan-Charvet, 2015). Thus, inhibiting the absorption of cholesterol and fatty acids in the small intestine, suppressing chylomicron synthesis, or reducing lipid and cholesterol synthesis in the liver can lead to decreased triglyceride and LDL levels in tissues and bloodstream, thereby contributing to the prevention of obesity (Heck et al., 2000; Jasińska et al., 2007).
Policosanol is a combination of long-chain sugar alcohols, including triacontanol, hexacosanol, tetracosanol, heptacosanol, and octacosanol, which are purified from sugarcane (Saccharum officinarum), grain sorghum, and corn (Leguizamón et al., 2009). Evidence from in vitro studies suggests that policosanol may inhibit hepatic cholesterol synthesis at a step preceding mevalonate production (Banerjee et al., 2011; Singh et al., 2006). Animal and clinical studies have also indicated that LDL catabolism and improvement of lipid profile may be enhanced through receptor-mediated mechanisms (Cho et al., 2023a; Cho et al., 2023b; Kim et al., 2023; Nam et al., 2019). Additionally, policosanol exhibits other beneficial properties, including effects on smooth muscle cell proliferation, platelet aggregation, and LDL peroxidation (Gouni-Berthold et al., 2022). It also interferes with the progression of the NF-κB and MAPK signaling pathways involved in inflammation. The cholesterol-lowering effect of policosanol is attributed to inhibiting cholesterol synthesis in the liver via the indirect inactivation of HMG-CoA reductase (Romero-Martinez et al., 2021). Currently, policosanol produced from Cuban sugarcane wax alcohol has been approved by the Ministry of Food and Drug Safety (MFDS) in South Korea for its cholesterol-lowering and blood pressure-improving effects. While the cholesterol-lowering effects of policosanol have been documented in previous studies, research on its underlying mechanisms remains relatively limited, and comparative studies with pharmaceutical drugs have not been reported.
Sugarcane is an essential agricultural crop utilized for bioenergy, sugar production, and animal feed. In 2021, Brazil, India, and China have accounted for more than 65% of global sugarcane production, underscoring their central role in the world’s sugarcane industry (Suguna and Murugesan, 2021). However, most studies have focused exclusively on Cuban policosanol, despite its effectiveness may vary depending on the extraction solvent, source, and country of origin. Moreover, some policosanol derived from sugarcane are often obtained using organic solvents such as benzene and hexane, which are unsuitable for food applications and raise safety concerns (Díaz de Los Ríos et al., 2022). Given its potential applications, further research is needed to determine whether policosanol from other sources and extracted through safer processes also exhibit lipid profile-improving effects.
Therefore, in this study, we investigated the effects of policosanol (Reduchole22®) derived from sugarcane EtOH extract on lipid metabolism and the expression of related factors in hepatocytes and intestinal cells, which play key roles in lipid absorption and metabolism. The results were compared with those of atorvastatin, a widely used hyperlipidemia drug, to evaluate the potential of policosanol as a functional food ingredient for hypercholesterolemia prevention.
2. Materials and methods
Policosanol (Reduchole22®) from Saccharum officinarum was provided from Ingex Botanicals (Karnataka, India). Atorvastatin was obtained from Cipla Ltd (Mumbai, India). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dulbecco’s Phosphate-Buffered Saline (DPBS), Trypsin-EDTA and Antibiotic solutions was purchased from Hi-Media (Thane, Maharashtra, India). Minimum essential medium eagle (MEM) and fetal bovine serum (FBS) was procured from Gibco (Waltham, MA, USA). Lipid Quantification Kit (#101420245, Cell Biolabs, San Diego, CA, USA), Cholesterol Quantification Kit (#8L11K06030, Sigma-Aldrich, Bangalore, Karnataka, India), Human HMG-CoAR ELISA Kit (#WA1600T25063, Elabscience®, Houston, TX, USA) OxiSelect™ Human Oxidized LDL ELISA Kit (#17022241, Cell Biolabs, San Diego, CA, USA) were purchased for the analysis of selected biomarkers. RNA Isoplus (Takara, New Delhi, India), iScript cDNA Synthesis kit (#1708891, Bio-Rad, Hercules, CA, USA), SsoAdvanced Universal SYBR-Green Super Mix (#1725270, Bio-Rad, Hercules, CA, USA) were procured of molecular biology grade. Primers for the selected gene of interest were purchased from Eurofins Scientific (Bangalore, India).
HepG2 (Human Liver Carcinoma) and Caco-2 (Human Colon Carcinoma) cells were procured from NCCS, Pune, India. Cells were cultured in MEM supplemented with 10% heat-inactivated FBS, along with antibiotic solution comprising of penicillin (100 IU/mL), streptomycin (100 μg/mL) and amphotericin B (5 μg/mL) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks and all experiments were carried out in 96 micro-titer and 6 well plates.
Policosanol (Reduchole22®) from Saccharum officinarum was provided from Ingex Botanicals (Karnataka, India) and 10 mg of policosanol (Reduchole22®) was weighed and dissolved in culture medium supplemented with 2% inactivated FBS to obtain a stock solution of 10 mg/mL. Further dilutions were prepared from the obtained stock solution for in vitro analysis.
The cell culture monolayer was trypsinized and the cell count was adjusted to 100,000 cells/mL using MEM containing 10% FBS. To each well of 96 well micro-titer plates, 0.1 mL of the diluted cell suspension was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off, the monolayer was washed once with DPBS and different test concentrations were added in the micro-titer plates. The untreated cells were maintained as cell control. The plates were then incubated at 37°C for 24 h in 5% CO2 atmosphere, microscopic examination was carried out and observations were noted. After 24 h, the test solutions were discarded and 100 μL of MTT diluted with DPBS was added to each well. The plate was incubated for 3 h at 37°C in 5% CO2 atmosphere. The supernatant was removed and 100 μL of DMSO was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a micro plate reader at a wavelength of 570 nm and the percentage cell viability was calculated.
The cell culture monolayer was trypsinized and the cell count was adjusted to 100,000 cells/mL using MEM containing 10% FBS. Cells were seeded in 6 well plates and after attaining confluence, the cells were treated with different non-toxic concentrations of policosanol (Reduchole22®), Standard (Atorvastatin) and untreated cells were maintained. The treated cultures were incubated for 24 h at 37°C in 5% CO2 atmosphere. After the incubation period, the supernatant was aspirated from wells and cultures were washed twice with DPBS. Then 1,500 μL DPBS was added and the cells were harvested by scraping and cell suspension was centrifuged at 2,500 rpm for 5 min. The cell pellets were collected to process PCR analysis.
The treated cells were subjected to cell lysis by treating with Tri-extract reagent for 5 min at RT followed by addition of chloroform to isolate the total RNA. Further, the samples were centrifuged at 4,000 RPM for 5 min to separate protein, DNA and RNA into three distinct layers. From the centrifuged tube the upper layer consisting of RNA was collected into fresh tubes, equal volume of isopropanol was added and incubated at 20°C for 10 min. After the incubation period the tubes were centrifuged at 12,000 RPM for 20 min to separate the pellet, supernatant was discarded. For the obtained pellet 0.5 mL of 75% ethanol was added and suspended, these tubes were centrifuged at 12,000 RPM for 20 min. Supernatant was discarded, the obtained pellet was kept for air drying and nuclease free water was added to the pellet. Then RNA quantification was done by using TECAN SPARK and the ratio 260/280 was determined. The isolated RNA was used for cDNA synthesis, by priming with oligo dT primers followed by reverse transcriptase enzyme treatment according to kit manufacturer’s protocol. The synthesized cDNA was taken up for qPCR for the amplification of gene of our interest such as SREBP, LDL-receptor, ABCA1, ACAT, ApoB, ApoA1, and CYP7A1 were determined in HepG2 Cells and HMG-CoA reductase, and ACAT2 in Caco-2 cells. The house keeping gene GAPDH was used as internal control. Table 1 indicates the list of primer sequences.
The mRNA expression levels of selected genes were determined using CFX Opus 96 Real time PCR system (BIO-RAD). 20 μL of the reaction mixture containing cDNA and specifically designed primers were subjected to PCR for amplification of genes. GAPDH as housekeeping gene was co-amplified in each reaction as an internal control.
After subjecting to PCR, initial 2 min incubation step at 50°C followed by 30 min of reverse transcription at 60°C. The 40 cycles of two step PCR reactions consist of 5 min period at 95°C followed by 35 cycles of denaturation at 95°C for 30 sec, annealing Tm for 30 sec and extension at 72°C for 45 sec. This was followed by final extension at 72°C for 10 min. Gene specific primers were designed for target genes as well as GAPDH using NCBI primer blast and were procured from Eurofins, India.
The qRT-PCR results were further quantified by plotting fluorescent signal intensities against the number of PCR cycles on a semi-algorithmic scale. A threshold cycle (CT) was designated as the amplification cycle at which the first significant increase in fluorescence occurred. The relative gene expression of target genes in comparison to the reference gene GAPDH was calculated using the 2−ΔΔCT method. Statistical significance is compared between cell control versus treatment groups.
Caco-2 cells were treated with non-toxic concentrations of policosanol, Atorvastatin as standard and untreated cells as negative control. The plates were incubated for 24 h at 37°C in 5% CO2 atmosphere. The cell pellets were collected by scraping to estimate cellular Cholesterol levels using a cholesterol quantification kit.
The HepG2 cells were treated with 250-500 μg/mL of policosanol and 10-20 μM of atorvastatin for a comprehensive 24 h, and H2O2 was stimulated to induce LDL oxidation. The plates were maintained in a CO2 incubator for 24 h at 37°C in a 5% CO2 atmosphere. Cell pellets were collected using scraping to measure cellular oxidized LDL levels using the Oxidized LDL ELISA Kit method.
The Caco-2 cells treated with policosanol and respective control cell pellets were collected to measure Human HMG-CoA (3-Hydroxy-3-Methylglutaryl Coenzyme A) Reductase activity by using Human HMG-CoA Reductase ELISA Kit method.
The Caco-2 cells treated with policosanol and respective control cell pellets were collected to estimate the lipid content by sulfo-phospho-vanillin method using Lipid quantification kit.
Statistical analysis was carried out using Graph-Pad Prism Software, version 5.01 (Northside, San Diego, CA, USA). All values were expressed as Mean±SD. The significant difference in control and policosanol and the atorvastatin-treated group was tested using one-way ANOVA with Dunnett’s test.
3. Results and discussion
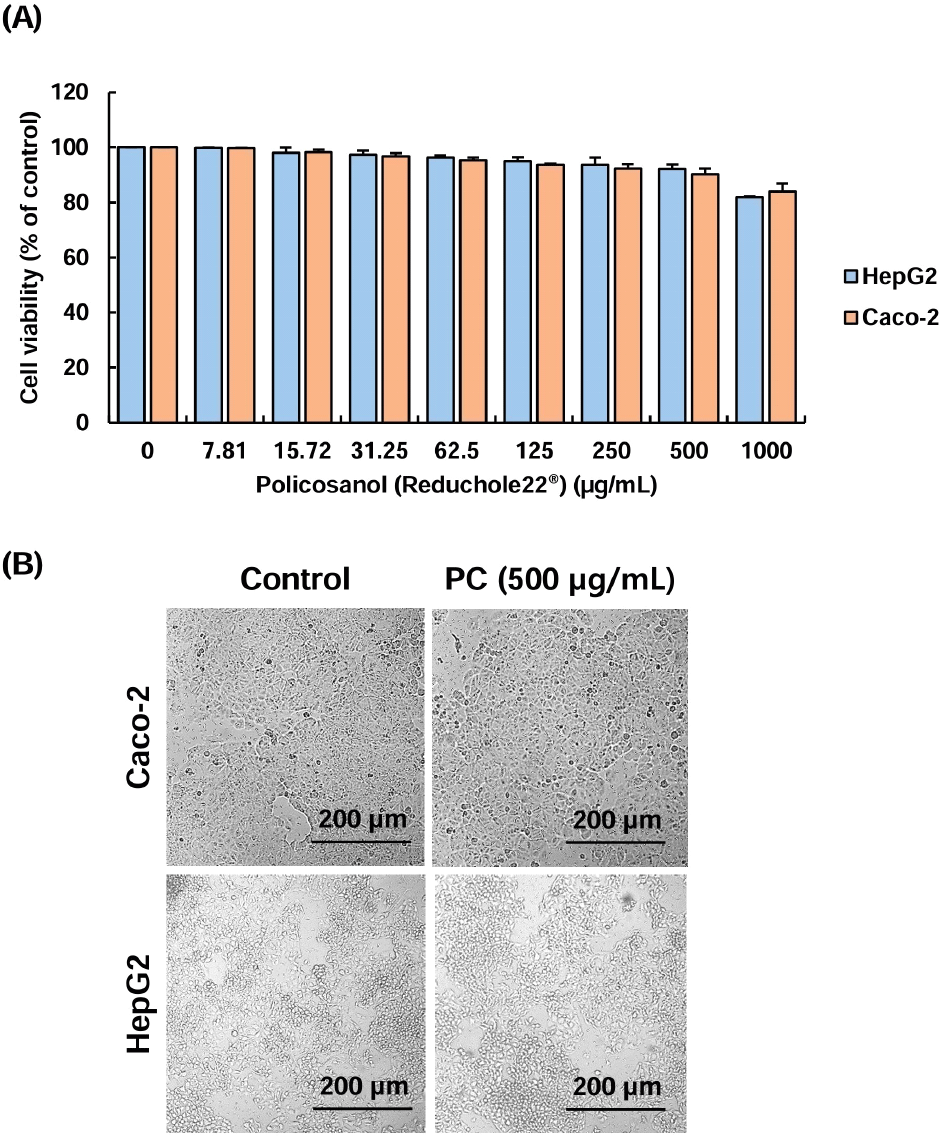
To evaluate the effects of policosanol on the viability of HepG2 hepatocytes and Caco-2 intestinal cells, an MTT assay was performed (Fig. 1). Cells prepared in a confluent condition in a 96-well plate were treated with policosanol at concentrations ranging from 0 to 1,000 μg/mL in culture medium for 24 h. HepG2 and Caco-2 cells maintained their viability above 90% at concentrations up to 500 μg/mL of policosanol. However, cell viability in HepG2 and Caco-2 cells decreased by 16.02-18.11% compared to control group at the concentration of 1,000 μg/mL (Fig. 1A). We also confirmed that treatment with 500 μg/mL of PC resulted in minimal morphological changes in the cells compared to the control group (Fig. 1B). This result was similar to findings from Korean oat (Avena sativa L.)- and barley sprout-derived policosanols, which showed low cytotoxicity in HepG2 cells at a concentration range from 200-500 μg/mL (Lee et al., 2022; Seo et al., 2013). Therefore, we selected 250-500 μg/mL of policosanol as the concentration for the cholesterol-lowering experiment in both cell types.
Hypercholesterolemia is a condition characterized by excessive cholesterol accumulation in the bloodstream due to impaired cholesterol metabolism in the liver. This can lead to atherosclerosis, angina pectoris, and myocardial infarction. One of the major causes of hyperlipidemia is the disruption of the balance between LDL and HDL cholesterol produced in the liver. The regulation of hyperlipidemia and hypercholesterolemia can be considerably affected by the expression of genes related to cholesterol and lipoprotein metabolism (Hajighasemi et al., 2019).
The effects of policosanol at concentrations of 250-500 μg/mL on the expression of cholesterol metabolism-related genes in HepG2 cells were examined using qRT-PCR, and the results are indicated in Fig. 2. Compared to the control cells, the expression levels of SREBP, ACAT, and APOB were significantly reduced upon treatment with 500 μg/mL of policosanol. This effect was comparable to that observed with 10-20 μM atorvastatin, a widely used hyperlipidemia drug. Furthermore, LDL-receptor, ABCA1, and APOA1 expression was significantly increased following treatment with 500 μg/mL of policosanol, showing a similar trend to atorvastatin. Interestingly, CYP7A1, which was markedly upregulated by 20 μM atorvastatin, exhibited an increasing trend with policosanol treatment as well, although the difference was not statistically significant (Fig. 2).
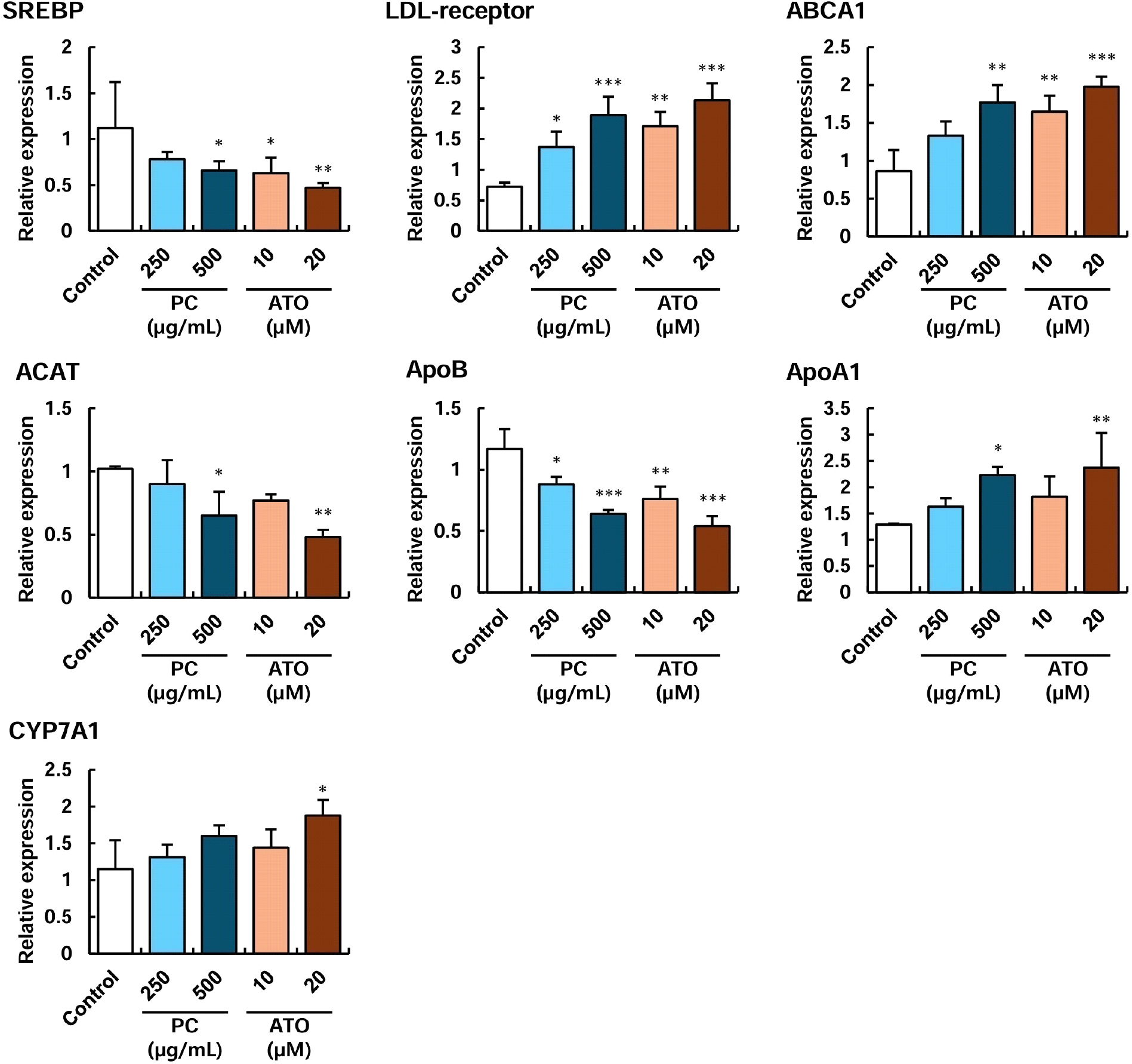
In hepatocytes, cholesterol synthesis and accumulation are promoted by SREBP and ACAT proteins, which can contribute to the development of fatty liver disease. Additionally, excessive LDL cholesterol levels induce increased synthesis of APOB lipoproteins, exacerbating lipid profiles in the bloodstream (Hajighasemi et al., 2019). Circulating LDL cholesterol can be oxidized by reactive oxygen species and accumulated in blood vessels, eventually leading to atherosclerosis. In contrast, an increase in LDL-receptor expression in hepatocytes facilitates the clearance of excess circulating LDL cholesterol, thereby improving vascular conditions (Hajighasemi et al., 2019; Srivastava, 2023). On the other hand, HDL cholesterol, which is promoted by APOA1 and ABCA1 proteins, plays a pivotal role in scavenging excess cholesterol from the bloodstream (Kosmas et al., 2018). Thus, maintaining a proper balance between these HDL and LDL lipoproteins is essential for cardiovascular health. Once internalized into hepatocytes via HDL or LDL, cholesterol can be converted into bile acids by CYP7A1 for excretion or stored for energy metabolism. In this study, atorvastatin, used as a drug control, was found to reduce LDL synthesis and cholesterol accumulation by suppressing the expression of SREBP, ACAT, and APOB while simultaneously enhancing the expression of LDL-receptor, ABCA1, APOA1, and CYP7A1 to promote HDL formation and cholesterol excretion. Similarly, treatment with 500 μg/mL of policosanol yielded comparable results to atorvastatin, except for CYP7A1 expression, which did not show a statistically significant increase (Fig. 2). Lee et al. (2015) reported that policosanol extracts from barley sprout at a concentration of 200 μg/mL increased the HDL/LDL ratio in HepG2 cells while downregulating SREBP, ACAT2, and HMG-CoA reductase, thereby improving cholesterol metabolism. Additionally, Lee et al. (2022) demonstrated that policosanol extracted from Korean oat at 100 μg/mL enhanced AMPK expression in HepG2 cells, suggesting its potential to inhibit fatty acid synthesis.
Overall, this study’s findings indicate that policosanol treatment modulates the expression of genes involved in cholesterol metabolism in hepatocytes, contributing to the improvement of lipid profiles.
HMG-CoA reductase and ACAT proteins play key roles in the cholesterol biosynthesis process in intestinal cells. In particular, HMG-CoA reductase is a primary target for statin-based drugs used to treat hyperlipidemia. Additionally, inhibition of HMG-CoA reductase activity has been shown to suppress the transport of cholesterol to the basolateral compartment in the Caco-2 intestinal cell infiltration model, indicating its close association with cholesterol absorption (Arantes et al., 2016; Levy et al., 1995). The effects of policosanol on the expression of genes related to cholesterol absorption and metabolism in Caco-2 intestinal cells were examined. The results are presented in Fig. 3. Compared to the control group, treatment with 250-500 μg/mL of policosanol significantly reduced the expression of HMG-CoA reductase and ACAT2 genes, showing no significant difference compared to the positive control, atorvastatin (Fig. 3). Furthermore, the measurement of HMG-CoA reductase activity in Caco-2 cells confirmed that both 250-500 μg/mL of policosanol and atorvastatin exerted similar inhibitory effects.
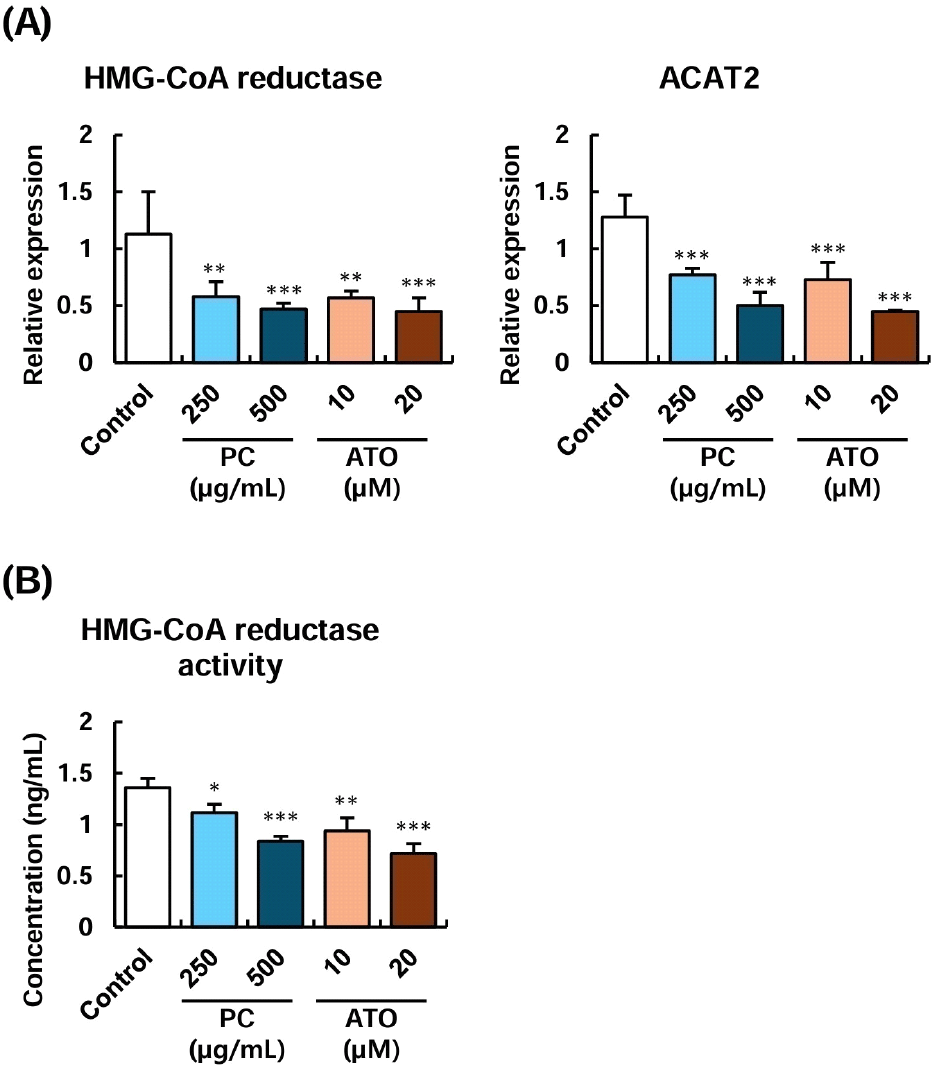
Previous studies have reported that administering 10-20 mg/kg of policosanol for 12 weeks in a hypercholesterolemic rat model reduced levels of HMG-CoA reductase and ACAT in liver tissues (Nam et al., 2019). Additionally, treatment with policosanol-containing mixed extracts has decreased the expression of HMG-CoA reductase and ACAT proteins in HepG2 hepatocytes (Amante et al., 2022). These findings collectively suggest that policosanol can modulate cholesterol metabolism by reducing the expression and activity of genes involved in cholesterol synthesis in both hepatocytes and intestinal cells, where cholesterol metabolism is most active.
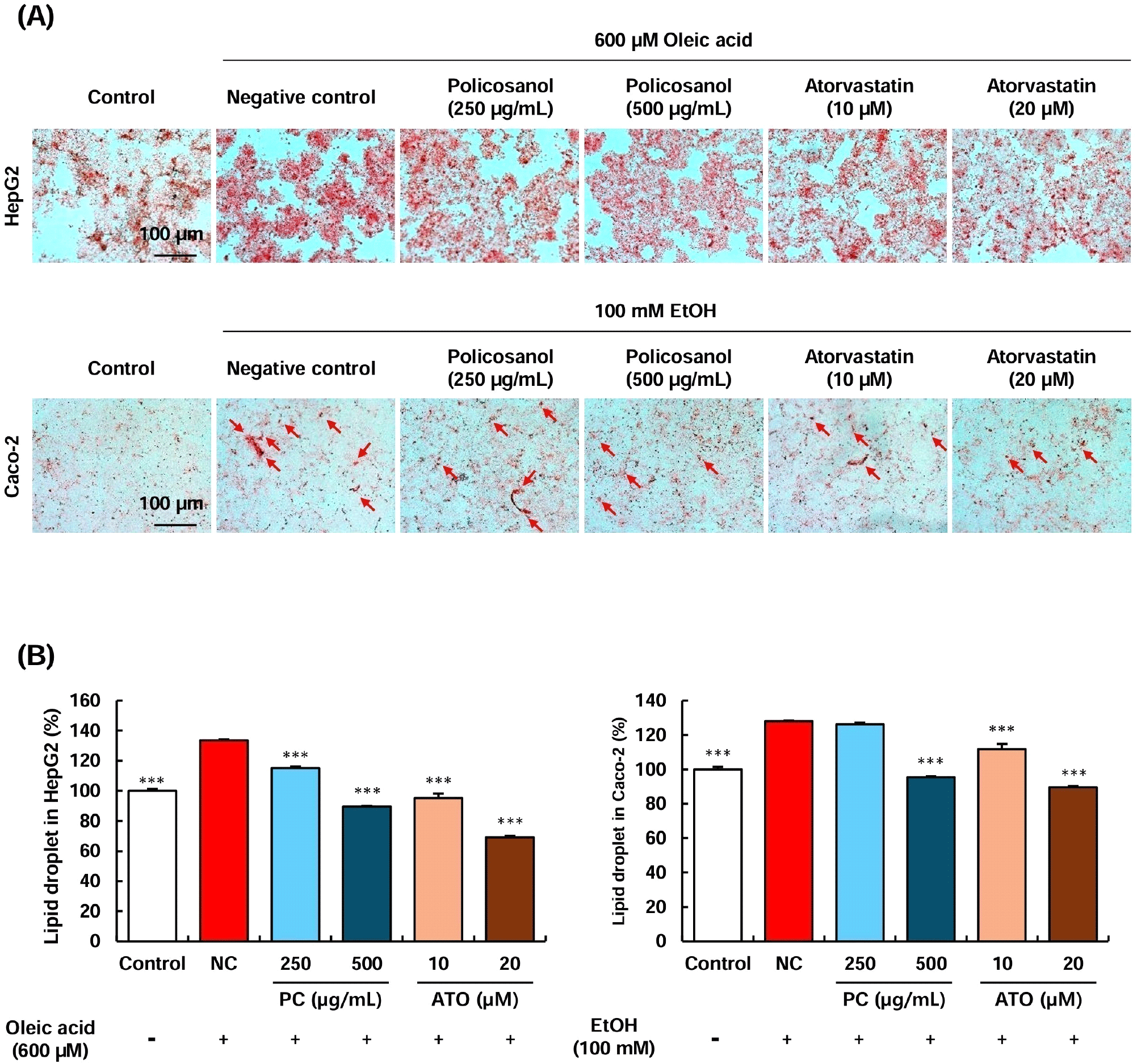
Oxidized LDL cholesterol is a well-known marker closely associated with atherosclerosis. In hepatocytes, LDL cholesterol oxidation occurs through the generation of superoxide anions, which has been reported to influence the secretion of APOB and APOA1, ultimately leading to a decrease in the HDL/LDL ratio (Bourdon et al., 2006; Pico et al., 1996). Since policosanol treatment regulated the expression of genes involved in cholesterol metabolism in HepG2 cells, we further investigated its effect on inhibiting LDL cholesterol oxidation under oxidative stress conditions. Compared to the control group (not treated by H2O2), the negative control (NC) group, which received H2O2 treatment, showed a significant elevation in oxidized LDL cholesterol levels. However, treatment with 250-500 μg/mL of policosanol and 10-20 μM of atorvastatin significantly inhibited LDL cholesterol oxidation compared to the NC group (Fig. 4). Furthermore, treatment with 250-500 μg/mL of policosanol and 10-20 μM of atorvastatin markedly suppressed oleic acid-induced lipid accumulation in HepG2 cells (Supplementary Fig. S1). A previous study reported that direct treatment of LDL cholesterol with policosanol did not suppress oxidation in vitro (Ng et al., 2005). However, human clinical trials in which participants were administered 10-20 mg of policosanol demonstrated that the experimental group that consumed policosanol exhibited a significant reduction in LDL peroxidation levels (Gouni-Berthold and Berthold, 2002; Menéndez et al., 2000).
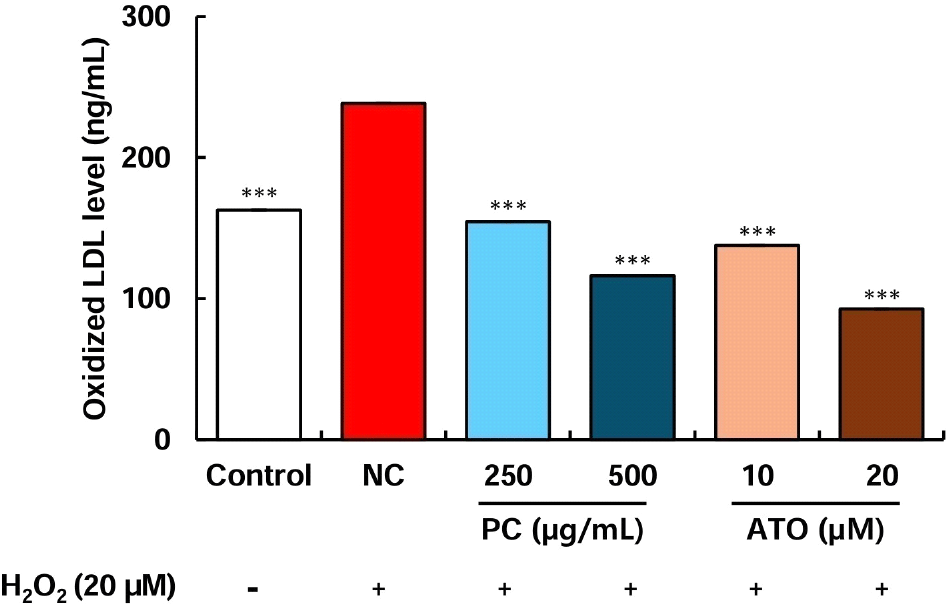
Although policosanol does not exert a direct antioxidant effect to inhibit LDL cholesterol oxidation, these findings suggest that it may indirectly suppress LDL cholesterol oxidation by regulating the expression of cholesterol metabolism-related genes, as observed in preclinical and clinical studies.
The Caco-2 intestinal cell line is widely used as an in vitro model to evaluate cholesterol transport, fatty acid accumulation, and lipoprotein production, which are key processes in lipid metabolism (Levy et al., 1995). Within the endoplasmic reticulum of intestinal cells, accumulated cholesterol and triacylglycerols are transferred for VLDL synthesis, a process facilitated by APOB lipoproteins (Ho and Pal, 2005; Ros, 2000). Previous studies have demonstrated a significant correlation between cholesterol accumulation and APOB lipoprotein upregulation in Caco-2 cells (Ho and Pal, 2005). Based on these findings, the present study further examined the intracellular cholesterol content and total lipid content in Caco-2 cells (Fig. 5). The intracellular cholesterol levels were significantly reduced upon treatment with 500 μg/mL of policosanol and 20 μM of atorvastatin compared to the control group. However, treatment with 250 μg/mL of policosanol and 10 μM of atorvastatin exhibited a decreasing trend without statistical significance (Fig. 5A). A similar tendency was observed for total lipid content. Treatment with 500 μg/mL of policosanol and 20 μM of atorvastatin significantly reduced total lipid content. Furthermore, treatment with 500 μg/mL of policosanol and 10-20 μM of atorvastatin markedly attenuated oleic acid-induced lipid accumulation in Caco-2 cells (Supplementary Fig. S1).
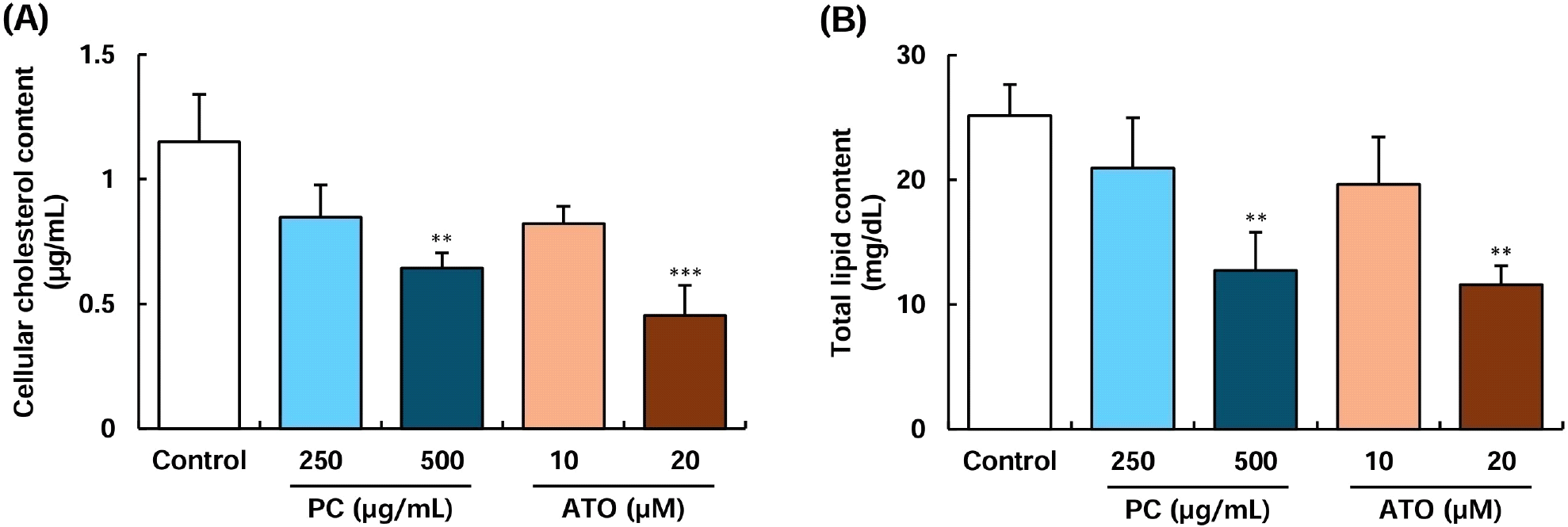
In contrast, lower concentrations did not show a substantial effect (Fig. 5B). Caco-2 cells, along with hepatocytes, actively synthesize and secrete lipoproteins such as VLDL, HDL, and chylomicrons, playing a critical role in lipid metabolism and systemic lipid homeostasis (Spalinger et al., 1998). Qin et al. (2023) reported that curcumin considerably reduced TRPA1 channel activity in Caco-2 cells, thereby blocking cholesterol uptake. Arantes et al. (2016) also demonstrated that caffeoylquinic acid extracted from Vernonia condensate leaves inhibited both cholesterol accumulation and efflux in Caco-2 cells. Furthermore, administration of 20 mg/kg of policosanol for 12 weeks alongside a high-cholesterol diet significantly increased fecal excretion of cholesterol and bile acids in rats (Nam et al., 2019). These findings indicate that policosanol may improve blood lipid profiles by inhibiting cholesterol metabolism and absorption in intestinal cells. However, further animal studies are required to elucidate the precise mechanisms underlying its cholesterol absorption-inhibitory effects.
4. Conclusions
This study demonstrated that policosanol (Reduchole22®) extracted from sugarcane using ethanol inhibits intracellular cholesterol and lipid accumulation and oxidation in human hepatocytes and enterocytes by regulating genes involved in cholesterol and lipoprotein metabolism. These findings revealed that policosanol (Reduchole22®) regulates cholesterol metabolism through similar mechanisms to atorvastatin, a widely used antihyperlipidemic drug. Therefore, the present study suggests that policosanol could serve as a functional food ingredient for hyperlipidemia prevention, providing a beneficial alternative in the food industry.