1. Introduction
Prostate cancer is the diagnosed malignancy in man in the world, and remains a major risk of cancer-related mortality, following lung and colorectal cancer (Siegel et al., 2024). In the past decade, more than two million men in the United States have been diagnosed with this disease, with incidence rates have also rising in Asian populations (Panaiyadiyan and Kumar, 2024; Siegel et al., 2024). Current therapeutic options, including androgen deprivation, hormone therapy, immunotherapy, radiopharmaceuticals, and cytotoxic agents, have shown significant efficacy (Guinney et al., 2017; Sonpavde and Kantoff, 2012; Tanimoto et al., 2010). However, these treatments often lead to adverse effects, including damage to healthy tissues and the development of drug resistance (Frederiks et al., 2015; Karavelioglu et al., 2016), ultimately allowing unresponsive tumors to progress to a metastatic stage. Increasing evidence suggests that natural bioactive compounds, including plant-derived extracts and phytochemicals, offer promising alternatives due to their lower toxicity and potential biocompatibility in body (Kelloff et al., 2000; Lozano-Casabianca et al., 2022). As a result, there is growing interest in studying dietary natural products with cancer-preventive properties, as elucidating their molecular mechanisms could aid in the development of more effective cancer treatments.
Pyrogallol (PG), a naturally occurring catechin compound (benzene-1,2,3-triol), is a polyphenol widely found in plants such as sorghum (Djanaguiraman et al., 2018), green tea (Liu et al., 2022), and gooseberry (Ptak et al., 1986). In recent years, several studies have demonstrated the biological properties of PG, with a particular focus on its cytotoxic effects against cancer cells. It has been shown to induce to cell death in various malignancies, including lymphoma (Saeki et al., 2000), human lung cancer cells (Han et al., 2009a; Han et al., 2009b), human gastric cells (Park et al., 2008), and human breast cancer cells (Nemec et al., 2016). Beyond its anticancer properties, PG has demonstrated antimicrobial activity (Lima et al., 2016), anti-inflammatory effects in bronchial epithelial cells (Nicolis et al., 2008), and inhibition of vascular smooth muscle cell migration in carotid ligation models (Ma et al., 2016). Although PG has shown promising effects, the specific molecular pathways underlying its impact on human prostate cancer cells are not yet well understood.
Cell cycle regulation is crucial for cancer progression, enabling tumor cells to transition from a quiescent (G0) state to active proliferation and metastasis while maintaining genomic stability (Kim, 2021; Rahman et al., 2021). Notably, abnormalities in cyclin-dependent kinase (CDK) regulation across different phases in cell cycle led to increased efforts to identify small-molecule inhibitors that specifically target these kinases. Five key CDKs govern cell cycle progression: CDK4, CDK6, and CDK2 in G1; CDK2 in S; and CDK1 in G2 and M phases. Each phase requires specific cyclins to complex with CDKs, facilitating precise cell cycle transitions (Chen et al., 2017; Roh et al., 2016; Wang et al., 2017). At the same time, apoptosis, which is a key progress in the cell death, plays a vital role in removing damaged or abnormal cells from the body. Apoptotic events are characterized by several factors (e.g., DNA fragmentation, chromatin condensation, and increasing the apoptotic bodies (Walker et al., 2016). Links between apoptosis and cell cycle regulation have been established, with tumor suppressor proteins such as p53 acting as key regulators (Pucci et al., 2000). Since several proteins involved in cell death also regulate proliferative signaling, compounds that can induce the cell cycle arrests and trigger apoptosis to apoptosis hold great promise as potential cancer therapeutics.
This study examines the potential effects of PG in prostate cancer cells derived from human (PC-3) and a C57BL/6 nude mouse xenograft model, focusing on its underlying mechanisms. In particular, we investigated how PG induces the apoptosis signaling and cell cycle arrest for assessing its potential as a prostate cancer therapeutic agent. Gaining a deeper understanding of the molecular mechanisms behind PG’s effects could contribute to the development of new prostate cancer treatments derived from natural products.
2. Materials and methods
Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin, were obtained from Gibco BRL Co. (Gaithersburg, MD, USA). The pan-caspase inhibitor (z-VAD-fmk) was sourced from R&D Systems (Minneapolis, MN, USA). Antibodies targeting cyclin-dependent kinase 2 (CDK2), CDK4, Cyclin D1, p53, p21, Bax, Bcl-2, cytochrome C, caspase-3, PARP, and α-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Extracellular-signal-regulated kinase (ERK), p-ERK, c-Jun N-terminal kinase (JNK), p-JNK, p38, p-p38, and caspase-8 were purchased from Cell Signaling Technologies (Beverly, MA, USA). Chemiluminescence detection kit for analyzing western blotting assay was obtained from Amersham Life Science (Amersham, Buckinghamshire, UK). Pyrogallol (PG) and other chemicals were purchased from Sigma-Aldrich Co. Ltd., St. Louis, MO, USA For all experiments, PG was dissolved in distilled water at a concentration of 100 mM for storage at 4°C before use in experimental procedures.
All cancer cell lines derived from human [i.e., LNCaP-FGC (lymph node metastasis), PC-3 (bone metastasis), and RWPE-1 (normal epithelial cells)], were acquired at American Type Culture Collection (ATCC, Rockville, MD, USA). In addition, primary cell (RC-58T/h/SA#4) was obtained from the Center for Prostate Disease Research (CPDR). LNCaP, PC-3, and RC-58T/h/SA#4 cells were cultured in DMEM with 10% FBS and 1% penicillin-streptomycin (Gibco, USA). RWPE-1 cells were maintained in Keratinocyte-SFM with 10% FBS and 1% penicillin-streptomycin. Each cell was incubated at 37°C in 5% CO2.
This experiment was assessed via sulforhodamine B (SRB) assay (Sigma-Aldrich Co.), according to previous study (Kim et al., 2016). Each cell was seeded (2.5×104 cells/well) in 48-well plates and treated with PG at different concentrations for 24 h. After treatment, the cells fixed by treating 10% trichloroacetic acid at 4°C for 1 h. Plates were then stained with 0.4% (w/v) SRB reagent. The stained cells were removed using 1% acetic acid and then dissolved using Tris (10 mM). The dissolved solution was detected at 540 nm with a microplate reader (Molecular Devices, Inc., San Jose, CA, USA). IC50 values for PG were represented using GraphPad Prism 5 software (GraphPad Software Co., San Diego, CA, USA). For investigating the role of caspase inhibition, we used z-VAD-fmk as a caspase inhibitor.
Cell cycle progression and apoptosis were analyzed as following the protocol provided by Merck Millipore Co. (Burlington, MA, USA). PC-3 cells were seeded into six-well plates at the recommended density and treated with PG for 24 h. For cell cycle analysis, cells were detached using trypsin-EDTA and fixed at −20°C for 3 h. Following fixation, a staining reagent (200 μL) was added to the cells and incubated for 30 min at room temperature. To assess apoptosis, cells stained by using Annexin V staining solution and incubated during 30 min at room temperature. Flow cytometry was performed on 5,000 gated events per sample, and cell cycle distribution was analyzed based on fluorescence intensity.
The changes in nuclear fragmentation and chromatin condensation were observed using bis-Benzimide (Hoechst 33258) chemicals under fluorescence microscopy. Cells were seeded (1×105 cells/mL) in six-well plates and exposed to PG for 24 h. After treatment, cells were harvested and reacted with bis-Benzimide (5 μg/mL) during 20 min at room temperature. An aliquot of the cell suspension was placed on a glass slide and observed via fluorescence microscope (Olympus Optical Co. Ltd., Japan)
For the apoptosis pathway investigation, cells were seeded in 100 mm dishes and treated with various concentrations of PG for 24 h. In contrast, to assess the activation of mitogen-activated protein kinase (MAPK) pathways, cells were similarly seeded in 100 mm dishes and exposed to PG at the same concentration range for 30 min. Cells or tumor tissues collected from mice were lysed by using lysis buffer (49 mM Tris-HCl, 149 mM NaCl, 1 mM EDTA, 49 mM NaF, 29 mM Na4P2O7, 1 mM PMSF, and 2 μg/mL aprotinin) during 1 h at 4°C. The protein concentration was calculated via bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology Co., Waltham, MA, USA). Equal amounts of protein (5 μg) were separated on 10-12% SDS-PAGE gels and transferred onto nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin (BSA) and then incubated with primary antibodies (1:1000) overnight at 4°C. In the next day, each membrane was washed and incubated with secondary antibodies (1:2000) during at 4°C. The bands of protein were detected using an ECL kit (Santa Cruz, CA, USA), and band intensities were measured with Image StudioTM Lite software (LI-COR Inc., NE, USA), with normalization to α-tubulin levels.
All animal procedures were conducted following the guidelines approved by Dong-A University’s Institutional Animal Care and Use Committee (DIACUC-17-15, approved in Sep. 2017). Male C57BL/6 athymic mice (five weeks old) were maintained under pathogen-free conditions. Mice were fed ad libitum access to an AIN-93G diet and sterilized water. Each mouse (Eight mice per each group) randomly divided as follows: (1) Control group by treating distilled water (p.o.); (2) 2.5 mg/kg/day of PG-treated group (p.o.); (3) 5 mg/kg/day PG treated group (p.o.). PG administration started on day 6, once tumors reached 20-25 mm2 volumes. The treatments of PG were freshly prepared daily and administered via oral gavage (p.o.) for 2 weeks. Tumor volume was measured daily using calipers and calculated as follows: length/2 × width2. Also, collected tumor from mice were measured the expression of protein levels in Bax, Bcl-2, PARP, and Caspase-3.
All data are represented as the mean±standard error (SE) based on a minimum of three independent experiments (n=3-8). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test for post-hoc comparisons. Differences were considered statistically significant at *p<0.05, **p<0.01, and ***p<0.001.
3. Results and discussion
Pyrogallol (PG; C6H6O3) is a naturally occurring phenolic compound present in various plants, such as sorghum, green tea, gooseberries, and hardwood species. Previous studies have demonstrated PG’s ability to inhibit the proliferation of several cancer cell types (Arjsri et al., 2023; Han et al., 2009a; Park et al., 2008; Saeki et al., 2000); however, its precise mechanisms in prostate cancer remain unclear. Many plant-derived compounds have been explored for their potential in cancer treatment due to their lower toxicity in normal cells and ability to selectively target malignant cells (Lee et al., 2012; Naeem et al., 2022). Therefore, we assessed the anti-cancer activity of PG in prostate cancer cells and investigated its underlying mechanisms.
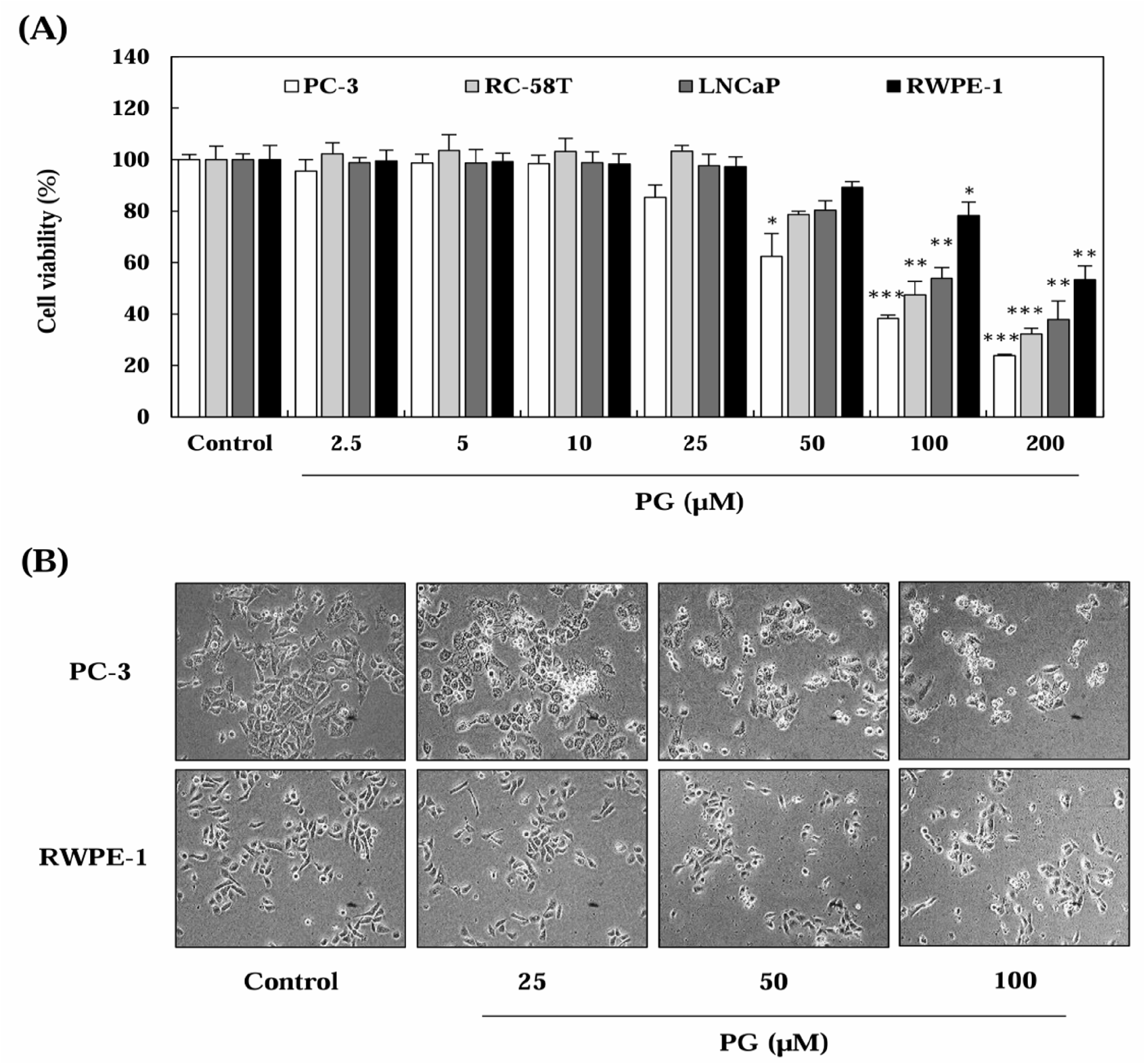
To determine the effect of PG on prostate cancer cell proliferation, SRB assays were performed after treating cells with PG at varying concentrations (2.5-200 μM) for 24 h (Fig. 1). As illustrated in Fig. 1A and 1B, PG treatment significantly reduced cell viability in a dose-dependent manner in prostate cancer cells, whereas RWPE-1 prostate epithelial cells exhibited no significant decline in proliferation. The IC50 values of PG were calculated as 55.53 μM for PC-3, 65.03 μM for RC-58T, and 71.78 μM for LNCaP, while RWPE-1 cells had an IC50 exceeding 200 μM (Fig. 1C). Interestingly, our results showed that PG exerted prostate cancer-specific cytotoxic effects in PC-3 cells, a castration-resistant prostate cancer cell line, when compared to normal prostate epithelial cells (RWPE-1). These findings suggest that PG preferentially inhibits the proliferation of prostate cancer cells while exhibiting minimal cytotoxicity toward normal epithelial cells, indicating its selective anti-cancer potential.
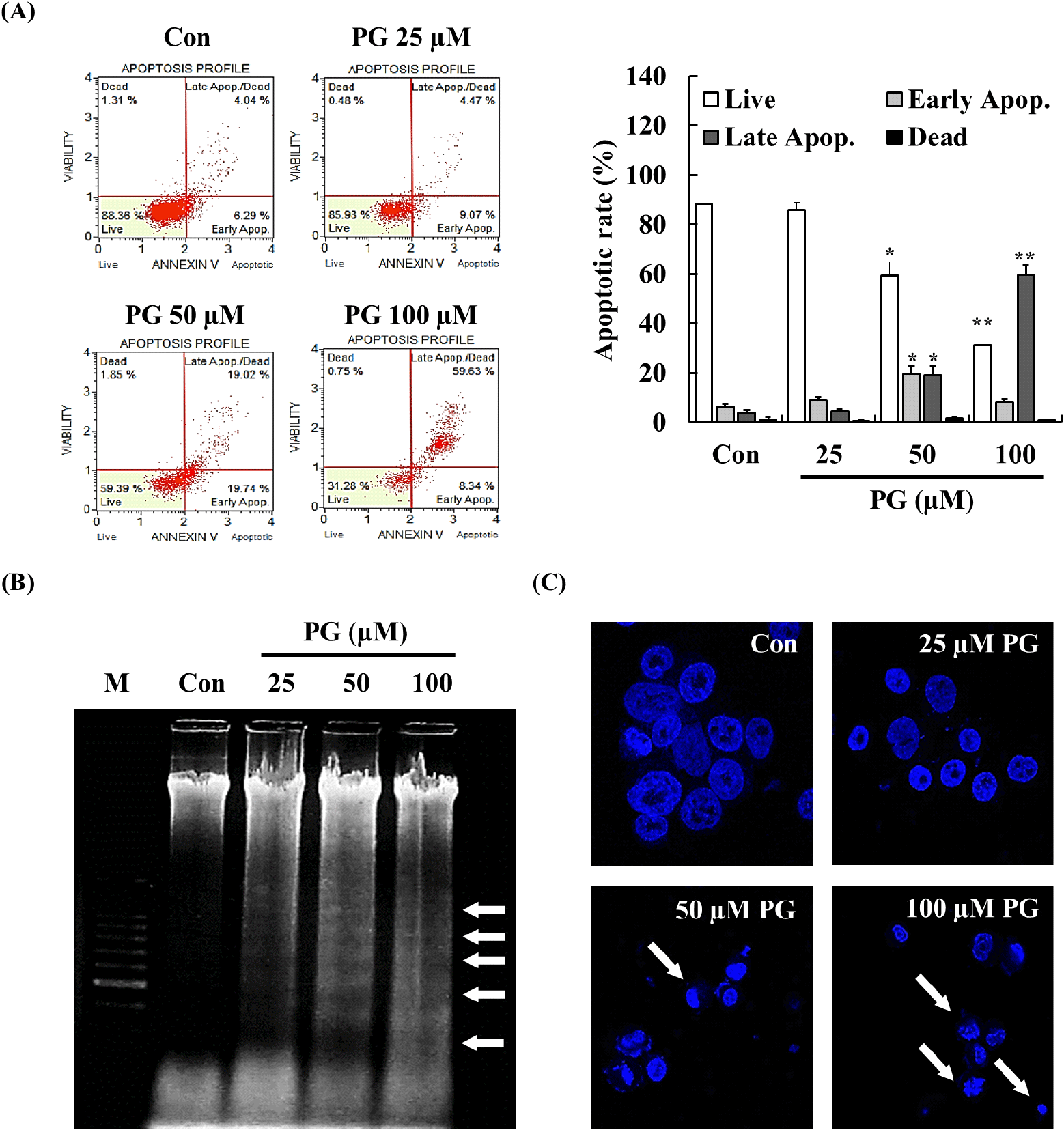
Apoptosis involves cellular changes such as chromatin condensation, apoptotic body formation, DNA fragmentation, and protein degradation (Elmore, 2007). Both apoptosis induction and cell cycle arrest are considered essential mechanisms in cancer treatment, as they contribute to inhibiting uncontrolled cell proliferation (Lin et al., 2013; Naeem et al., 2022; Preya et al., 2017; Semprebon et al., 2015). To investigate whether the anti-proliferative effects of PG were associated with apoptosis, annexin V staining, DNA fragmentation analysis, and Hoechst staining were performed. As shown in Fig. 2A, treatment with PG for 24 h significantly increased the late apoptotic cell population in a dose-dependent manner. Cells undergoing apoptosis exhibited characteristic morphological changes, including membrane blebbing, cytoplasmic condensation, nuclear fragmentation, and chromatin condensation (Erekat, 2022). Additionally, DNA fragmentation and chromatin condensation were detected in PC-3 cells following treatment with 50-100 μM PG for 24 h (Fig. 2B and 2C), providing further evidence of apoptosis induced by PG.
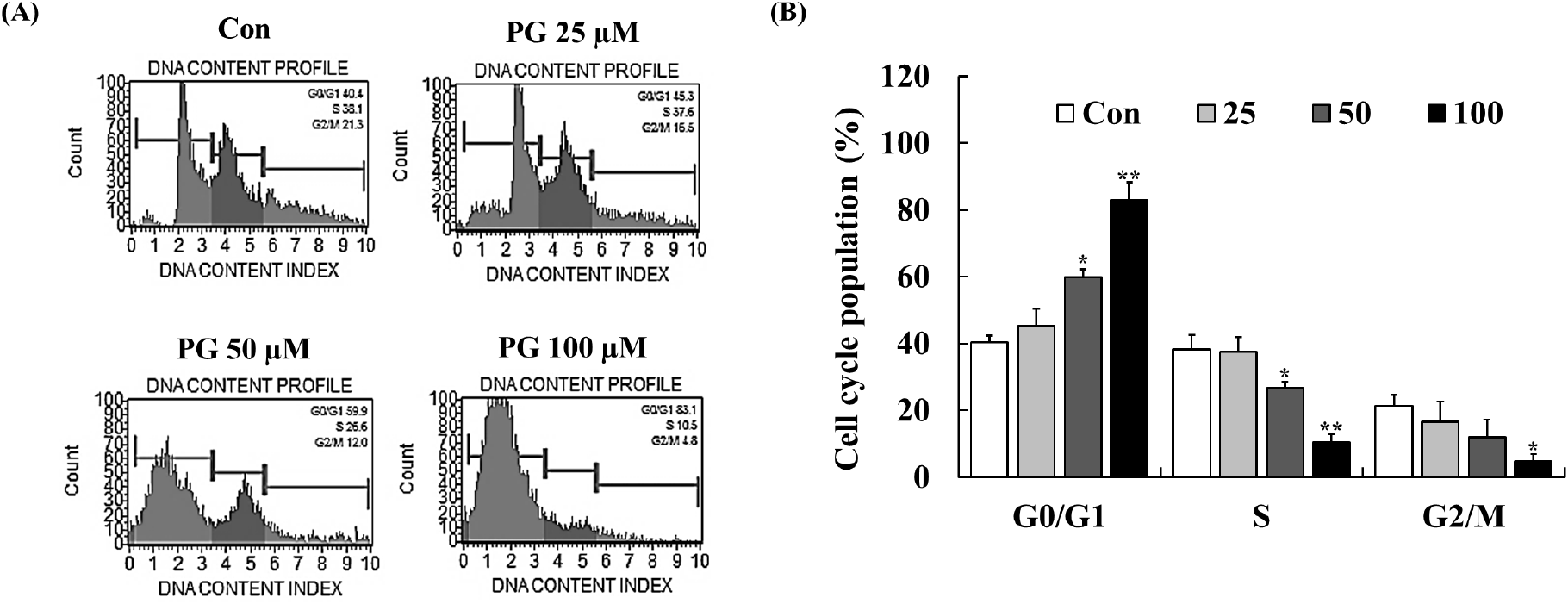
Cell cycle regulation plays a crucial role in maintaining cellular homeostasis, and disruptions in this process contribute to cancer progression (Vermeulen et al., 2003). CDKs, cyclins, p21, and p53 are key regulators of cell cycle checkpoints at G1/S and G2/M phases, and inhibiting these transitions can prevent cancer cell proliferation (Kastan and Bartek, 2004). PG has previously been reported to induce cell cycle arrest in lung and cervical cancer cells (Han et al., 2009b; Kim et al., 2007; Yang et al., 2009). To examine the impact of PG on cell cycle progression, flow cytometry analysis was conducted following 24 h of treatment. As shown in Fig. 3, PG treatment led to a dose-dependent accumulation of PC-3 cells in the G0/G1 phase, accompanied by a corresponding decrease in the proportion of cells in the S and G2/M phases. These results indicate that PG inhibits prostate cancer cell proliferation by inducing G0/G1 cell cycle arrest.
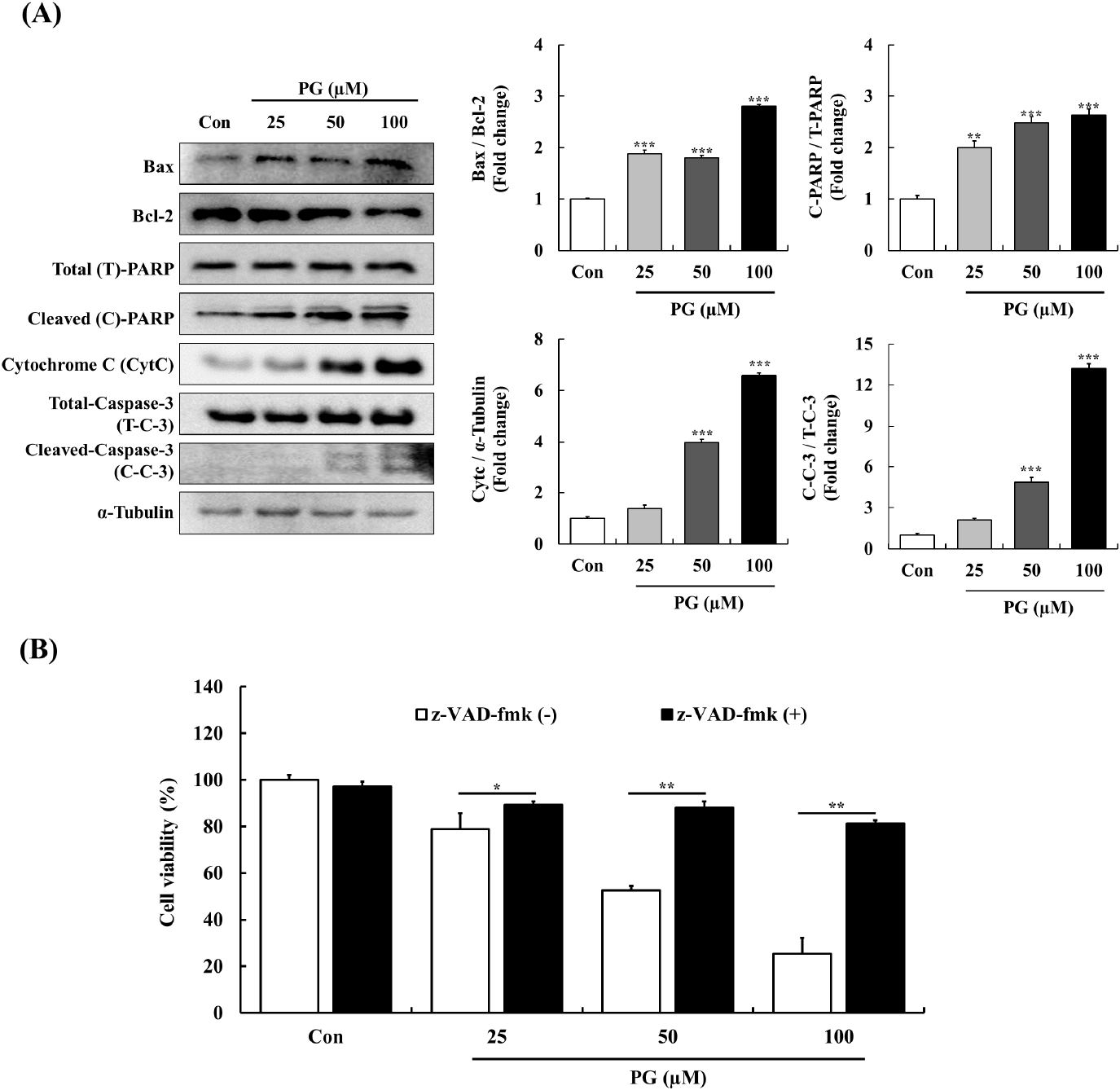
The Bcl-2 family of proteins regulates mitochondrial apoptosis by modulating cytochrome C release and PARP cleavage in response to cellular stress (Chaitanya and Babu, 2009). The caspase cascade, which includes intrinsic and extrinsic pathways, plays a pivotal role in apoptosis regulation (Hengartner, 2000). Previous research has linked PG-induced apoptosis in epithelial cells to caspase-3 activation (He et al., 2012) and demonstrated its apoptotic effects in lung cancer cells through caspase and PARP cleavage (Han et al., 2009a).
To examine the apoptotic mechanism of PG, the expression of key apoptosis-related proteins was analyzed. In PC-3 cells, PG treatment resulted in an upregulation of the pro-apoptotic protein Bax and a downregulation of the anti-apoptotic protein Bcl-2. Additionally, PG-treated cells exhibited higher levels of cytochrome C and cleaved-PARP, while cleaved caspase-3 expression was significantly reduced compared to untreated controls. Moreover, as shown in Fig. 4B, pre-treatment with the pan-caspase inhibitor z-VAD-fmk completely abolished PG-induced apoptosis in a dose-dependent manner. These findings confirm that PG-induced apoptosis in PC-3 cells is caspase-dependent.
MAPKs pathways, including p-ERK, p38, and JNK, regulate key cellular functions such as survival, apoptosis, and differentiation (Rodríguez-Berriguete et al., 2012). Generally, elevated JNK and p38 signaling are linked with cell death and tumor suppression, whereas increased ERK signaling promotes cell survival and tumor progression (Rodríguez-Berriguete et al., 2010; Yap et al., 2008). To further investigate the effects of PG, we evaluated its impact on MAPK phosphorylation after a 30-min exposure (Fig. 5A). PG treatment resulted in a concentration-dependent increase in JNK expression, while phosphorylated ERK and p38 levels were significantly reduced. Previous studies have demonstrated that the JNK cascade is pivotal in apoptosis through the regulation of Bcl-2 family proteins, with several anti-prostate cancer agents downregulating Bcl-2 via JNK signaling in PC-3 cells. Our findings suggest that PG promotes the degradation of Bcl-2 and activates caspase-3 through JNK pathway regulation, which leads to cell cycle arrest. Additionally, the expressed levels of p38 significantly decreased by treating PG. Elevated p38 activation is associated with pro-apoptotic effects for survival signal, implied that PG may not be primarily mediated through p38 signaling.
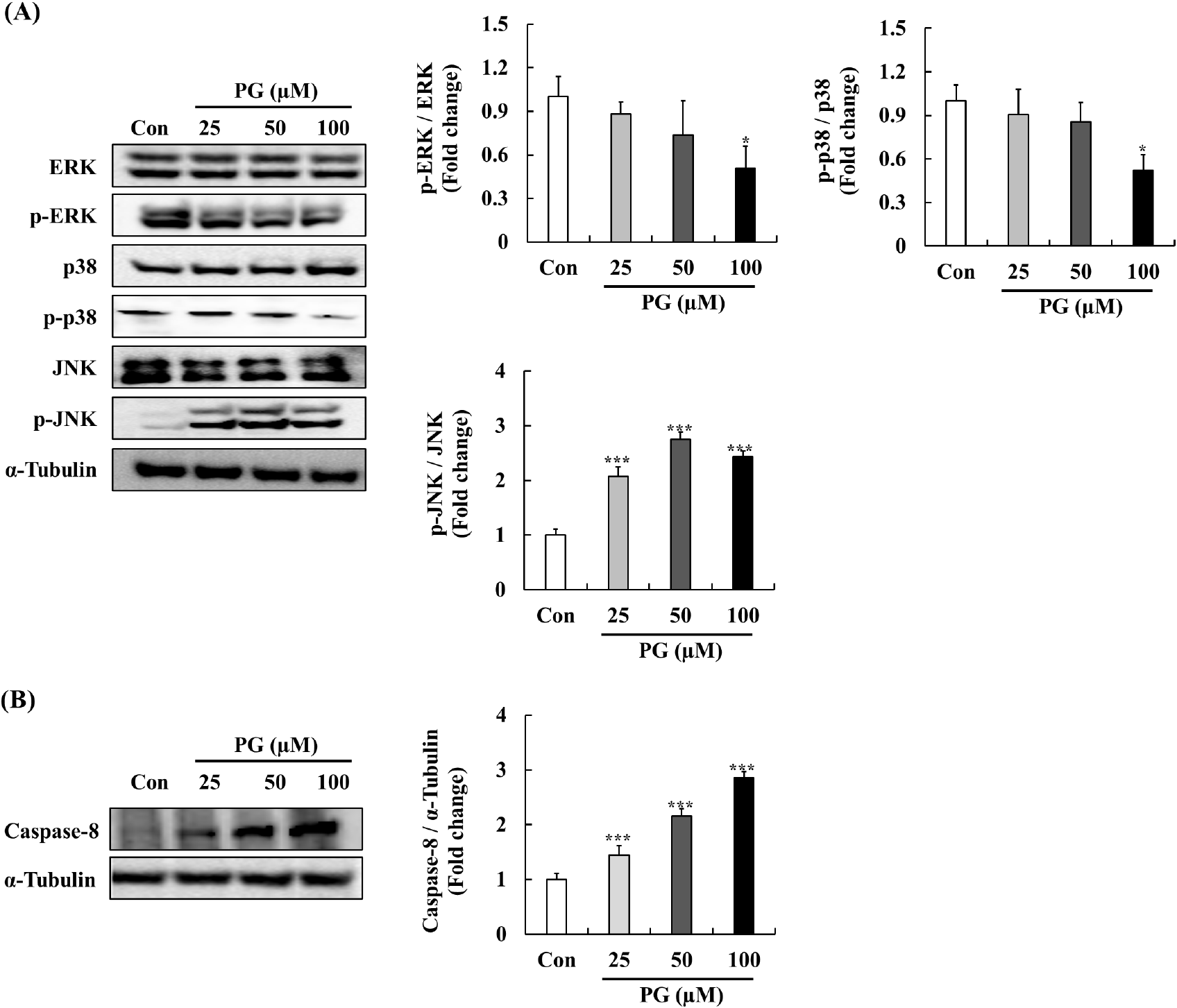
Elevated ERK signaling is frequently observed in various tumors, including prostate cancer, and is associated with apoptosis pathway (Bos, 1989). Previous studies has demonstrated that phosphorylated ERK levels associate with tumor grade and relapse in primary prostate cancer (Jull et al., 2001). Furthermore, phytochemicals such as formononetin have been shown to inhibit PC-3 cell proliferation by inactivating ERK signaling, which leads to increased expression of Bax protein (Ye et al., 2012). Our findings suggest that PG may exert its anti-cancer effects on human prostate cancer cells through modulation of the ERK-Bax pathway. In addition, PG treatment significantly elevated the expression levels of caspase-8 as a key extrinsic apoptotic marker critical for initiating programmed cell death. The activation of JNK signaling is linked to the regulation of capase-8-mediated apoptosis (Xu and Hu, 2020). As shown in Fig. 5B, the treatment of PG at various concentrations significantly increased caspase-8 expression, suggesting that PG may be play a potential role in modulating the JNK-Caspase-8 pathways.
To further examine PG’s anti-tumor potential, its effects were tested in a xenograft prostate cancer model using athymic mice. PC-3 xenografts were established, and mice were administered PG (2.5-5 mg/kg/day) via oral gavage for 14 days. PG treatment significantly reduced tumor weight and volume without causing significant changes in body weight (Fig. 6A). As illustrated in Fig. 6B, control mice had an average tumor weight of 3.13±1.24 g, whereas PG-treated mice exhibited a significantly lower average tumor weight of 0.49±0.4 g. Similarly, PG-treated mice showed a substantial reduction in tumor volume compared to the control group (Fig. 6C). Furthermore, we assessed the expression levels of apoptosis markers including Bax, Bcl-2, cleaved PARP, and caspase-3 in tumor tissues. As shown in Fig. 6D, the PG-treated groups exhibited expression levels of Bax, Bcl-2, cleaved PARP, and caspase-3 that were consistent with our in vitro findings. These results support the potential of PG as an effective anti-prostate cancer candidate.
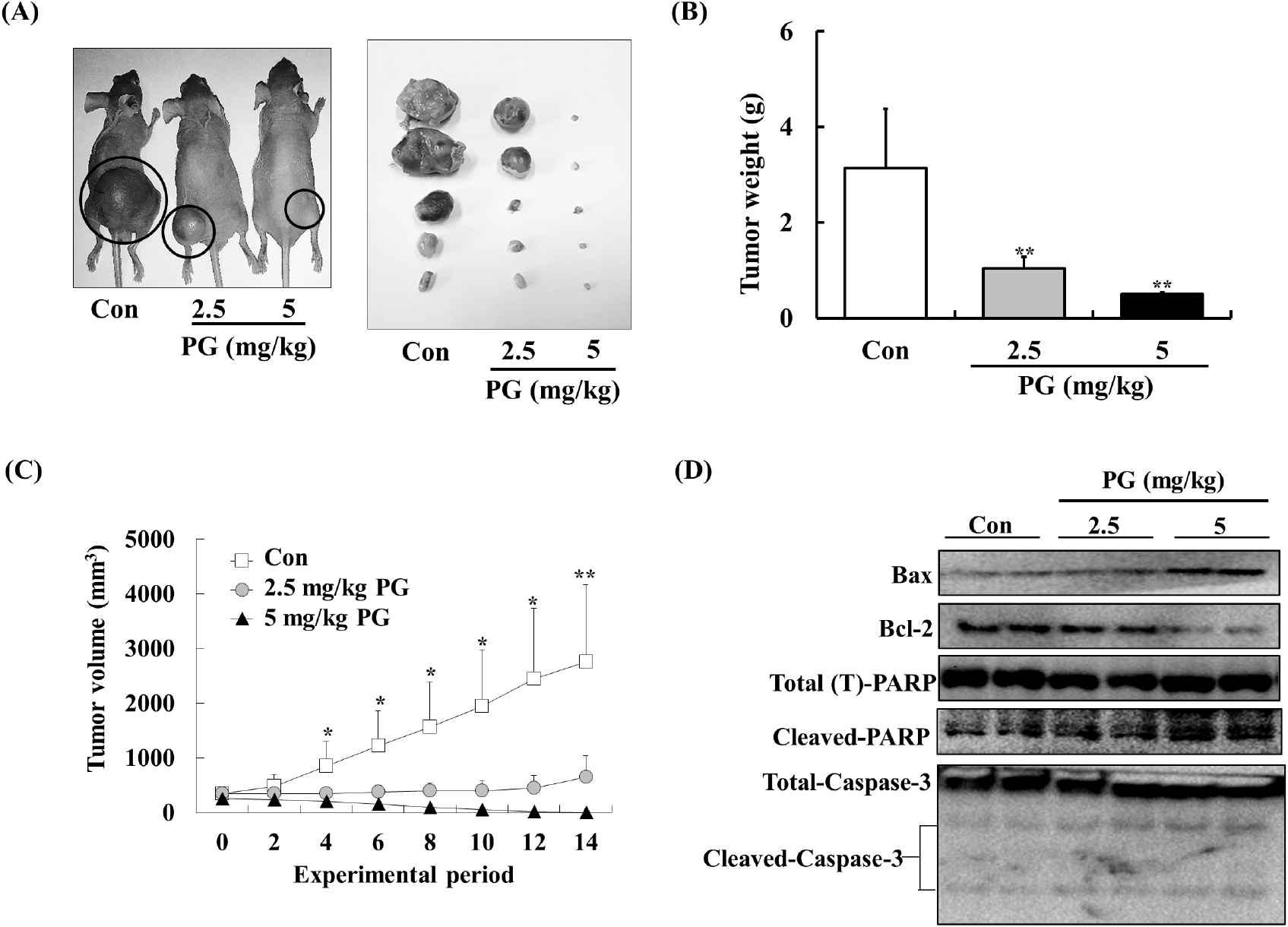
Previous studies have reported an oral LD50 for PG of approximately 800-1,270 mg/kg (Berndt et al., 1991). Consequently, we anticipated that daily administration of PG at doses of 2.5 and 5 mg/kg would effectively suppress prostate tumor growth in vivo without inducing any toxic effects. Although numerous therapeutic agents (e.g., abiraterone acetate, enzalutamide, apalutamide, etc.) are currently employed in the management of prostate cancer (Crawford et al., 2018), the potential for synergistic interactions between these drugs and pyrogallol has not been previously explored. Thus, we propose that future study should be investigated whether combining PG with established prostate cancer therapies may enhance anti-prostate cancer effect via synergistic mechanisms.
4. Conclusions
This study offers new insights into the anti-cancer effects of PG in prostate cancer, both in vitro and in vivo. Our results indicate that PG induces apoptosis in PC-3 prostate cancer cells through mitochondrial-mediated pathways while also causing cell cycle arrest at the G0/G1 phase. Additionally, PG treatment exhibits caspase-3 activation and MAPKs signaling, reinforcing its role in apoptotic regulation. In a xenograft mouse model, PG administration significantly reduced tumor growth and increased apoptosis markers such as Bax/Bcl-2, cleaved PARP, and caspase-3, suggesting its potential as a therapeutic agent. These findings highlight PG as a promising candidate for the treatment and prevention of prostate cancer. Further studies are required to evaluate its clinical applications and its potential synergy with existing therapies.
