1. Introduction
The skin is constantly exposed to ultraviolet rays and air, leading to the generation of reactive oxygen species (ROS). ROS include radical species such as superoxide anion radical (O2 · −) and hydroxyl radical ( · OH), as well as non-radical species like singlet oxygen (1O2) and hydrogen peroxide (H2O2). Additionally, ROS can react with biomolecules to form peroxyl radicals (ROO · ) and alkoxyl radicals (RO · ), which contribute to oxidative stress (Fisher et al., 2002; Imokawa, 2009). This oxidative stress accelerates wrinkle formation and melanin accumulation, further contributing to skin aging (Rhee, 2006; Ozben, 2007). To mitigate ROS-induced skin damage, the skin is equipped with an antioxidant defense system, comprising enzymatic antioxidants such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSHPx), as well as non-enzymatic antioxidants like vitamins C and E. However, excessive exposure to ultraviolet rays can overwhelm this defense system, leading to oxidative stress and subsequent skin deterioration. Accordingly, extensive research has focused on developing strategies to mitigate skin aging by both inhibiting excessive ROS production and enhancing ROS scavenging mechanisms (Rinnerthaler et al., 2015).
Houttuynia cordata, a perennial herb belonging to the Saururaceae family, is native to Korea, China, Japan, and Vietnam and is characterized by a distinctive fishy odor (Lee et al., 2000). Previous studies have highlighted various physiological activities of H. cordata extract, including antioxidant activity (Park et al., 2020), antibacterial effects (Russo et al., 2012), immune hypersensitivity reduction (Li et al., 2005), and skin barrier enhancement (Wang and Park, 2020). Given its bioactive potential, H. cordata has been investigated for applications in functional food and skincare. However, despite these promising properties, further research is needed to enhance its efficacy and broaden its potential applications.
Fermentation is gaining increasing attention as a promising approach to enhance the bioactivity of natural products. This process, mediated by beneficial microorganisms such as lactic acid bacteria, alters the chemical composition of plant extracts, leading to the production of bioactive metabolites with improved antioxidant and anti-aging properties (Whang et al., 2021). Compared to other methods, fermentation is not only cost-effective but also scalable for industrial applications, making it an attractive strategy for developing functional ingredients (Ha et al., 2010). Specifically, lactic acid fermentation generates lactic acid and various metabolic byproducts, which have been reported to boost immune function, improve gut health (Jeong et al., 2023), and enhance antioxidant activity (Park and Kang, 2020). Furthermore, interactions between natural plant components and fermentative microorganisms can yield synergistic effects, further amplifying bioactive properties (Jeon et al., 2005; Kong et al., 2008).
Given these potential benefits, this study aims to investigate the feasibility of using H. cordata as a functional cosmetic and health ingredient by fermenting it with lactic acid bacteria. We sought to assess the antioxidant and anti-wrinkle effects of fermented H. cordata extract (FEEH), thereby providing insights into its applicability as a natural agent for skin health improvement. Through this study, we aim to provide a scientific basis for developing sustainable and effective skincare solutions, thereby bridging the gap between natural bioactive compounds and their commercial applications.
2. Materials and methods
The strain used in the experiment, Lactobacillus plantarum KCTC 3108, was derived from kimchi and provided by the Korean Collection for Type Cultures (KCTC), Korea Research Institute of Bioscience and Biotechnology. Lactobacillus plantarum KCTC 3108 was cultured on MRS agar at 30°C for 24 h. The human dermal fibroblast cell line CCD-986sk was purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). The cells were cultured in DMEM medium containing 5% fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37°C in a 5% CO2 atmosphere.
Houttuynia cordata used as an ingredient in this study was harvested in 2023, originating in Korea, and its leaves were used for fermentation. Lactobacillus plantarum KCTC 3108 was cultured in MRS broth medium (DifcoTM Lactobacilli MRS broth, BD, Franklin Lakes, NJ, USA) at 30°C and 120 rpm. The leaves of Houttuynia cordata were cut into approximately 1 cm pieces, and the cultured Lactobacillus plantarum KCTC 3108 was inoculated into them. Houttuynia cordata was pasteurized at 65°C for 10 min using an autoclave (LAC-5060SD; Labtech, Daejeon, Korea). Afterward, 0.1 N NaOH was added to adjust the pH of the Houttuynia cordata homogenate to 6.8. Meanwhile, lactic acid bacteria were incubated in MRS broth for 24 h, and then 3% (v/w) of the culture medium was inoculated into the Houttuynia cordata homogenate. The fermentation was carried out at 30°C for 7 d.
Houttuynia cordata ethanol extract (EEH) and lactic acid bacteria-fermented Houttuynia cordata ethanol extract (FEEH) were prepared by extracting the leaves and fermented product using 70% ethanol in a round-bottom flask equipped with a vertical reflux cooler. The extracts were filtered through filter paper (Whatman No. 2, Maidstone, England) and concentrated using a rotary evaporator (Laborota 4000-efficient, Heidolph Instruments GmbH & Co., KG, Germany). Afterward, the concentrated extracts were freeze-dried for 72 h using a freeze dryer (Eyela, Tokyo Rikakikai Co., Tokyo, Japan) and then powdered for use in experiments. The extraction yield was calculated based on the dry weight of the sample.
The total polyphenol content was measured using a slightly modified version of the method by Folin and Denis (1912). The total polyphenol content was expressed as mg gallic acid equivalents (GAE)/g dry weight. The flavonoid content was measured using a modified version of the method by Moreno et al. (2000). The flavonoid content was expressed as mg rutin equivalents (RE)/g dry weight.
The antioxidant activity was measured using ABST, FRAP and SOD assay. The 2,2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS, Sigma-Aldrich, St. Louis, MO, USA) radical scavenging activity was measured by modifying the method of Roberta et al. (1999). A 7 mM ABTS+ solution and a 2.45 mM potassium persulfate solution were mixed in a 1:1 ratio and left to stand in the dark for 12 h. Then, the solution was diluted with 5 mM potassium phosphate buffer (pH 7.4) until the absorbance reached 0.7 at 413 nm. The samples were prepared by dissolving them in distilled water to final concentrations of 50, 100, 200, 300, 400, and 500 μg/mL. A 40 μL sample and 4 mL of the diluted ABTS+ solution were reacted for 1 min, and the absorbance was measured at 413 nm using a spectrophotometer. L-ascorbic acid (AA) was used as the positive control.
The ferric reducing antioxidant power (FRAP) value was measured using the method of Benzie and Strain (1996). The measurement was repeated three times, and the average value was used.
The SOD-like activity was performed based on the method of Marklund et al. (1974). The SOD-like activity was expressed as a percentage (%) of the difference in absorbance between test and control group.
The elastase inhibition rate was measured based on the method of Cannell et al. (1988). The elastase inhibition rate was expressed as the reduction in absorbance of the sample solution with the addition compared to the blank.
Cytotoxicity was evaluated using the MTT solution, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, Sigma-Aldrich). The skin fibroblast cell line CCD-986sk was used for the cytotoxicity evaluation, and the cells were seeded at a density of 1.3×105 cells/mL in a 96-well plate and incubated for 24 h. After 24 h, the medium was removed, and EEH and FEEH were added at various concentrations (200 μL each). The cells were then incubated for another 24 h. After 24 h, the supernatant was removed, and 200 μg/mL of MTT solution was added. The cells were incubated at 37°C for 3 h, protected from light. After incubation, the MTT solution was removed, and the cells were washed twice with PBS. Then, 200 μL of DMSO was added, and the reaction was allowed to proceed for 30 min. The absorbance was measured at 570 nm using a microplate reader (Infinite M200 Pro, Tecan, Männedorf, Switzerland) to evaluate the cytotoxicity.
Experiments were conducted using CCD-986sk cells, and collagen production was measured using the Procollagen Type I C-peptide (PIP) EIA Kit (Takara, Otsu, Japan). In the plate provided by the kit, 100 μL of antibody-POD conjugate solution was added, and 20 μL of EEH and FEEH, which had been pre-treated with the cells and cultured for 24 h, were added to each well. After the addition, the plate was incubated at 37°C for 3 h in the dark. The culture medium was then removed, and each well was washed four times with 400 μL of washing buffer. After washing, substrate solution was added to each well, and the plate was incubated at room temperature for 15 min. After incubation, 100 μL of 1 N H2SO4 was added as a stop solution, and the plate was shaken for about 1 min. The absorbance was measured at 450 nm using a microplate reader, and the collagen synthesis was quantified using a standard curve based on the kit’s standard.
Data analysis of the measurements between groups in the experimental results (with three replications, n=3) was performed using the IBM SPSS Statistics program (25, IBM Corp., Armonk, NY, USA). The significance of differences between experimental groups was tested using analysis of variance (ANOVA) at p<0.05, and post hoc analysis was conducted using Duncan’s multiple range test.
3. Results and discussion
The extraction yield was 8.39% for EEH and 7.96% for FEEH. The results of measuring the total polyphenol content of EEH and FEEH are shown in Table 1. The total polyphenol content of EEH was 55.38 mg GAE/g, while FEEH was significantly higher at 74.09 mg GAE/g, indicating that the total polyphenol content increased in the fermented extract using lactic acid bacteria. Ha et al. (2010) reported that the bark of Berberis koreana Palibin by lactic acid fermentation showed a significant increase compared to non-fermented bark of Berberis koreana Palibin, which is similar to the findings of this study. Phenolic compounds are common secondary metabolites in plants, characterized by phenolic hydroxyl groups binding to macromolecules and possessing antioxidant activity (Jeong et al., 2007). According to Rodriguez et al. (2009), fermentation using lactic acid bacteria breaks down macromolecules like tannins, producing antioxidants such as gallic acid and pyrogallol. It is believed that the polyphenolic compounds in Houttuynia cordata were also broken down into smaller molecules due to microbial fermentation, resulting in an increase in total polyphenol content.
| Sample1) | Extraction yield (%) | Total polyphenol contents (mg GAE/g) | Total flavonoid contents (mg QE/g) |
|---|---|---|---|
| EEH | 8.39±0.632) | 55.38±0.30 | 19.04±0.58 |
| FEEH | 7.96±0.41 | 74.09±3.71***3) | 25.06±0.08*** |
Meanwhile, the flavonoid content measurement showed that EEH contained 19.04 mg QE/g, while FEEH exhibited a significantly higher flavonoid content of 25.06 mg QE/g (p<0.05), indicating that the fermentation of Houttuynia cordata extract using lactic acid bacteria led to a significant increase in flavonoid content. This phenomenon aligns with previous findings, where the hydrolysis of macromolecular proteins during lactic acid bacteria fermentation results in the generation of soluble low-molecular-weight peptides or their conversion into low-molecular-weight compounds (Kim et al., 2021). Additionally, Moktan et al. (2008) reported that the increase in polyphenolic content during food fermentation is attributed to the enzymatic breakdown of macromolecules by microbial enzymes, facilitating the release of soluble bioactive components. Similarly, in this study, the increase in polyphenol and flavonoid content may be attributed to the enzymatic hydrolysis of bound phenolic compounds and the enhanced solubilization of bioactive constituents induced by lactic acid bacteria fermentation.
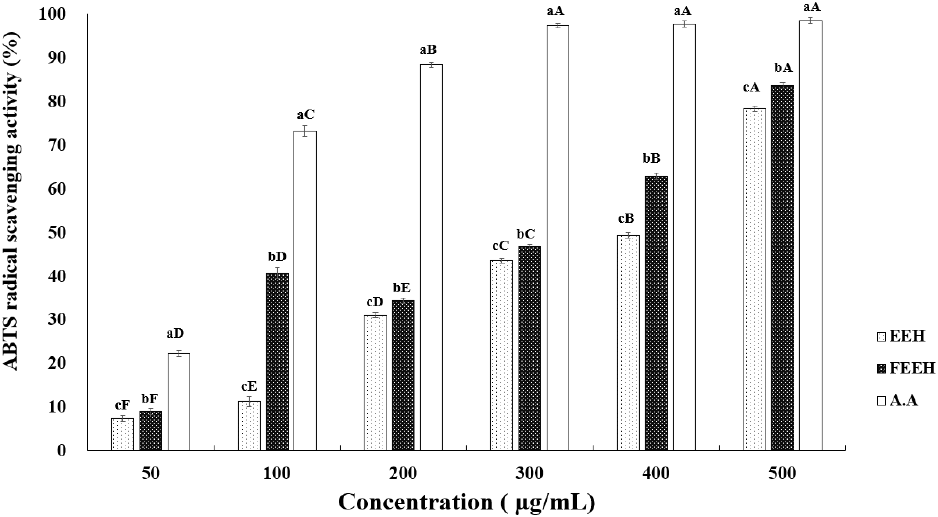
The results of measuring the ABTS free radical scavenging activity of EEH and FEEH are shown in Fig. 1. The positive control group, ascorbic acid, showed an ABTS free radical scavenging activity of 98.41% at a concentration of 500 μg/mL, whereas EEH showed 78.28% and FEEH showed 84.54% at the same concentration, indicating a significant increase in the fermented extract. At a concentration of 100 μg/mL, FEEH showed higher ABTS free radical scavenging activity than EEH, but no significant differences were observed at other concentrations. Park et al. (2009) reported that the ABTS free radical scavenging activity of fermented Codonopsis lanceolata was significantly higher than that of non-fermented Codonopsis lanceolata, showing a similar trend to the results of this study. These results are consistent with the findings of Kim et al. (2012), which indicated that antioxidant activity is significantly influenced by specific components among various polyphenols and that the content of polyphenols and flavonoids is proportional to the radical scavenging activity. Rinnerthaler et al. (2015) reported that high antioxidant activity reduces reactive oxygen species, which affects anti-wrinkle effects on the skin. Based on this, an experiment was conducted to evaluate the anti-wrinkle efficacy through the collagen production ability, which maintains skin elasticity.
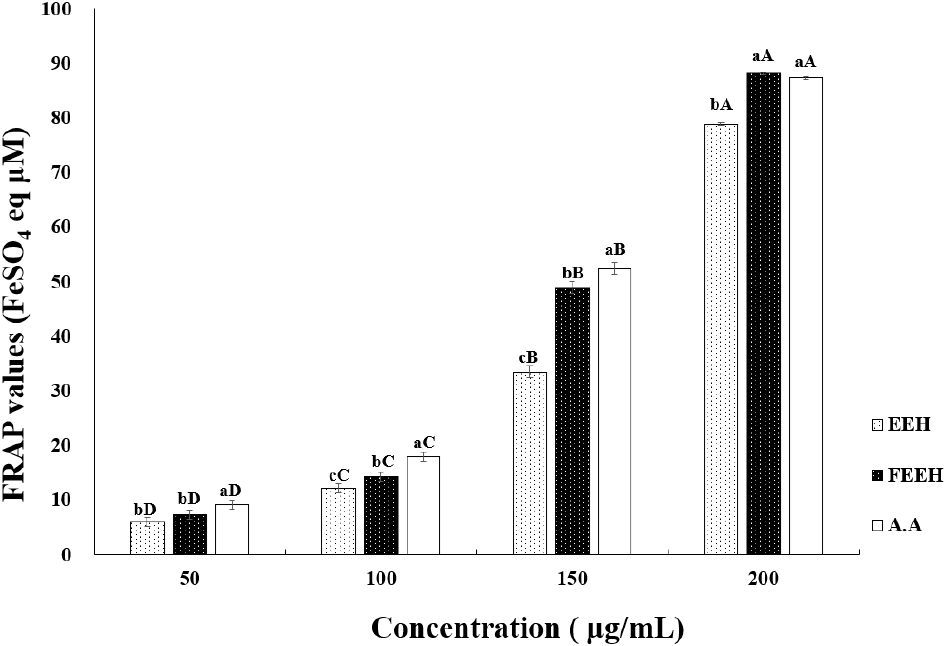
The results of measuring the FRAP activity of EEH and FEEH are shown on Fig. 2. The FRAP activity measurement results confirmed that the FRAP activity of Houttuynia cordata ethanol extract fermented using lactic acid bacteria was significantly higher at concentrations of 100, 150, and 200 μg/mL compared to the non-fermented Houttuynia cordata extract. According to the study by Kim and Yook (2024) on the physiological activities of fermented Mentha piperita extracts, it was reported that the FRAP activity increases when peppermint is fermented using lactic acid bacteria, showing a similar trend to this study. Generally, the antioxidant capacity of polyphenolic compounds is mainly due to their reducing power, and it has been reported that the higher the content, the greater the antioxidant activity (Holasova et al., 2002). The results of the total polyphenol and flavonoid content measured earlier seem to influence the FRAP activity results.
The results of measuring the SOD-like activity of EEH and FEEH are shown in Table 2. The SOD-like activity of EEH and FEEH was 16.96% and 24.25%, respectively, with FEEH showing a significantly higher increase compared to EEH. SOD is an enzyme that reacts with the highly harmful superoxide anion radical to produce hydrogen peroxide, acting as a representative inhibitor of reactive oxygen species in the body and providing defense against oxidative stress (Choi et al., 2010). SOD-like active substances are not enzymes, but are mainly low-molecular-weight phytochemicals that perform roles similar to SOD by inhibiting the reactivity of superoxide, thus protecting the body from superoxide and being used to evaluate typical antioxidant activities (Baehner et al., 1975). Choi et al. (2010) reported a SOD-like activity of 12.66% in ethanol extracts of bindweed leaves, indicating that the SOD activity of Houttuynia cordata extracts used as antioxidant experimental samples in this study shows relatively high SOD-like activity.
| Sample1) | SOD activity inhibition rate (%)2) | Elastase inhibition activity (%) |
|---|---|---|
| EEH | 16.96±0.803)c4) | 12.20±0.76c |
| FEEH | 24.25±0.81b | 25.61±1.10b |
| A.A. | 72.14±0.20a | 77.43±0.34a |
The elastase inhibition rate, which affects the formation of skin wrinkles by inhibiting elastin that maintains skin elasticity, was measured and the results are shown in Table 2. When the sample was treated at a concentration of 0.5 mg/mL, the positive control group, ascorbic acid, showed an inhibition rate of 77.43%, EEH showed an inhibition rate of 12.20%, and FEEH showed an inhibition rate of 25.61%. Although FEEH showed a higher inhibition rate compared to EEH, it was still significantly lower than the positive control, ascorbic acid. Future studies may consider testing additional concentrations to better understand the dose-response relationship. Elastase activity increases in abnormal tissues, leading to tissue destruction, which causes skin wrinkles and loss of elasticity, and affects the degradation of elastin, a structural protein that maintains skin elasticity in the dermis (DeWitt et al., 1981). In this study, the elastase inhibition rate of fermented Houttuynia cordata extract using lactic acid bacteria was significantly higher than that of non-fermented Houttuynia cordata extract. Kim et al. (2015) studied the anti-wrinkle effect of Agastache rugosa Kentz through the fermentation process of lactic acid Lactiplantibacillus plantarum, reporting that the elastase inhibition activity of 70% ethanol extract at 0.3 mg/mL showed an inhibition rate of 20.3%, which significantly increased through the fermentation process. This study showed a higher elastase inhibition rate compared to previous studies, confirming its effect on wrinkle improvement. These results indicate that the fermentation process enhances the elastase inhibition rate, and fermented Houttuynia cordata extract has superior elastase inhibition activity compared to non-fermented Houttuynia cordata extract. Additionally, Kim et al. (2014) reported that Liriope platyphylla extracts, a traditional herbal medicine material, increased SOD activity and were effective in improving skin wrinkles caused by photoaging. Based on these previous studies, it is believed that the high SOD-like activity of Houttuynia cordata extract and fermented Houttuynia cordata extract in this study influenced the elastase inhibition rate used for skin wrinkle improvement.
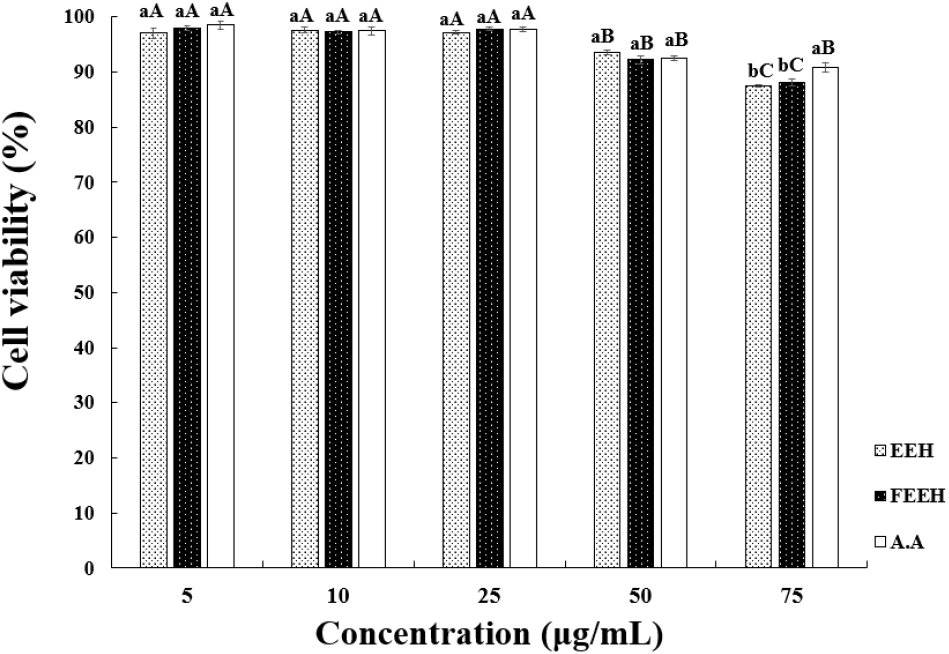
To measure the cytotoxicity of EEH and FEEH, CCD-986sk fibroblast cells were used to determine cell viability, as shown in Fig. 3. In this study, at a concentration of 75 μg/mL, EEH showed a high cell viability of 87.39%, FEEH showed 88.10%, and vitamin C showed 90.84%. Although there was a significant difference compared to the control group, there was no significant difference between Houttuynia cordata extract and fermented Houttuynia cordata extract. Therefore, EEH and FEEH did not affect CCD-986sk cell damage at the given concentration, and it is presumed that the amount of EEH and FEEH released outside the cells was low. Thus, both samples showed low cytotoxicity and did not affect cell viability. Subsequent experiments were conducted at concentrations where no cytotoxicity was observed.
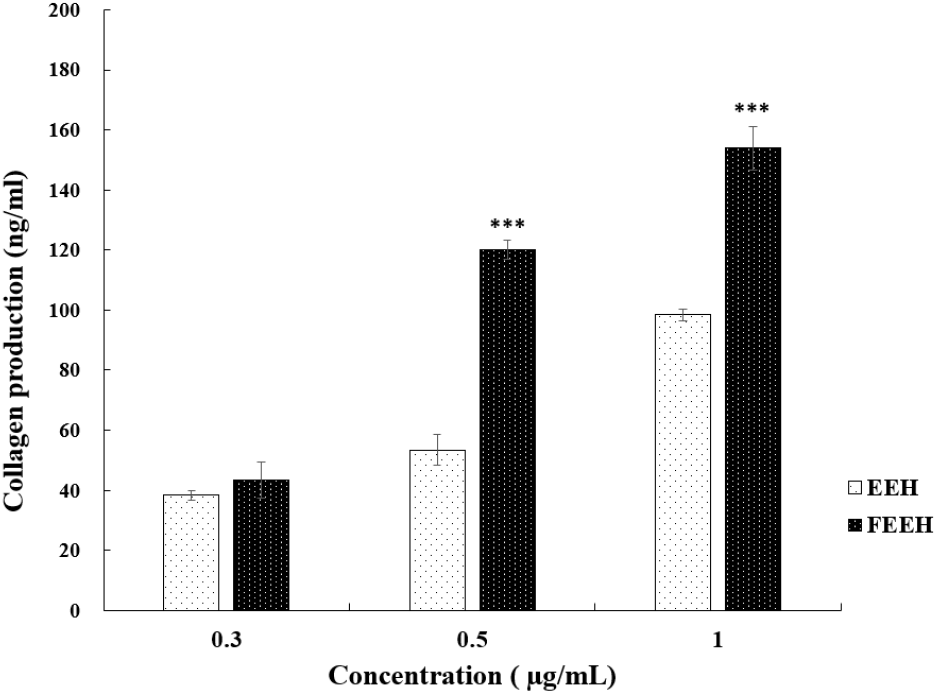
To confirm the wrinkle-improving efficacy of EEH and FEEH, the amount of collagen, a protein that maintains skin elasticity, was measured and shown in Fig. 4. At a concentration of 0.3 mg/mL, the collagen production of EEH and FEEH was similar, but it significantly increased as the concentration increased. At a concentration of 0.5 mg/mL, EEH produced 98.47 ng/mL of collagen, while FEEH produced 153.93 ng/mL, showing a significant increase compared to EEH. These results indicate that the fermentation process helped increase collagen production, and the increase in collagen production was more pronounced compared to the increase in antioxidant activity observed in the ABTS results. Collagen accounts for 70-80% of the total weight of the skin and forms most of the organic substances such as tendons, bones, and teeth. It is degraded by various harmful environmental stresses and external factors like UV radiation, leading to reduced skin elasticity and causing wrinkles and sagging. Kim et al. (2010) also reported that the collagen biosynthesis ability of extracts cultured with the mycelium of Grifola frondosa significantly increased compared to before culturing. In this study, a significant difference in collagen production was observed between Houttuynia cordata extract and fermented Houttuynia cordata extract, suggesting that these extracts have wrinkle-improving effects. This confirms that higher antioxidant activity, as determined by the DPPH and ABTS assay, correlates with wrinkle-improving efficacy, and that fermented Houttuynia cordata extract has relatively superior wrinkle-improving activity.
4. Conclusions
In conclusion, the fermentation of Houttuynia cordata with lactic acid bacteria could enhance physiological activity and be utilized as a natural antioxidant and anti-wrinkle ingredient. These findings confirm the potential of FEEH as a functional health food ingredient. For the practical application and commercialization of FEEH as a cosmetic ingredient, further studies on its stability, cost-effectiveness, and preservation methods are required. Additionally, to ensure its safety and efficacy, comprehensive evaluations through animal and human clinical trials should be conducted. These aspects are essential for establishing FEEH as a viable and sustainable component in the cosmetic industry.
