1. Introduction
Sugar beet (Beta vulgaris L.), recognized as a significant source of sucrose, is extensively cultivated across various regions globally, including Europe, Asia, and North America. The root of the sugar beet serves as a rich reservoir of minerals, vitamins, and bioactive compounds (Koprivica et al., 2014; Mikołajczyk-Bator et al., 2016), which confer numerous health-promoting effects, such as the inhibition of neoplastic cell proliferation (Valli et al., 2012), and the mitigation of oxidative damage (Filipčev et al., 2010; Mohdaly et al., 2010). Notwithstanding, sugar beet is infrequently utilized for direct human consumption and is predominantly processed commercially for sugar extraction, a procedure that incurs substantial costs and results in the degradation of health-promoting compounds. Consequently, the incorporation of clarified sugar beet juice in the formulation of food products as a viable alternative to refined beet sugar may represent a prudent strategy to avert the loss of bioactive and nutritional constituents.
The conventional treatment process involves a complex multi-step process such as preliming, main liming, first carbonation and second carbonation. Lime has shown very significant performance in beet juice treatment but also has some shortcomings. Some impurities, including saponins, phenolic compounds, proteins, and lipids, are not separated by the conventional treatment process and can affect the treatment performance. The presence of various dissolved and suspended compounds in RSBJ and the incomplete adequacy of conventional methods to separate all of them highlight the need for optimal use and combination of clarifying agents to achieve the best performance (Arjeh et al., 2019).
Raw sugar beet juice (RSBJ) represents an intermediate product within the sugar beet processing sector, derived through either diffusion or pressing methodology. Given the diverse array of compounds, whether dissolved or suspended, present in RSBJ and the potential for selectively eliminating various types of compounds through specialized processes, a range of fining agents is necessitated to achieve the clarification of raw juices. Among the most prevalent adsorbents employed across numerous applications, fields, and processes, particularly in the food industry, are bentonite, silica sol, gelatin, and activated carbon. The discrepancies in the nature of ionic charges between the juice constituents and the fining agents facilitate processes of neutralization and flocculation, thereby enabling their removal from the juice matrix. Bentonite, classified as a clay-based mineral within the montmorillonite subgroup, exhibits an affinity for binding with positively charged impurities such as proteins (Alper et al., 2011). Furthermore, bentonite demonstrates the capability to adsorb heavy metal ions, pesticides, and darker compounds from the juices (Koyuncu et al., 2007). Research has also indicated a synergistic effect between bentonite and quartamin, enhancing the removal efficiency of color and turbidity, achieving a notable reduction of up to 33%. Silica sol serves as a generic designation for colloidal suspensions of silicon dioxide (SiO2). Characterized by a negative charge, silica sols electrostatically associate with positively charged compounds. Their predominant application lies in the clarification process, acting in conjunction with positively charged gelatin during the fining of juices. Gelatin, a protein-derived clarifying agent, precipitates negatively charged particles, including polyphenols and decomposed pectin, which would otherwise contribute to turbidity and floc formation during storage. It is typically utilized in conjunction with both bentonite and silica sol. Gelatin exhibits a preferential adsorption behavior towards larger molecules containing a higher number of phenolic groups and a greater potential for hydrogen bonding (Arjeh et al., 2019). Activated carbon is characterized as a carbonaceous and highly porous material, frequently utilized in conjunction with an additional fining agent. Its extensive internal surface area, exceeding 2,500 m2/g, renders activated carbon exceptionally adept at adsorbing organic compounds from both liquid and gaseous phases (Balcerek et al., 2017).
Laboratory researchers have investigated the kinetics of the sugar beet juice production process by pressing and have described the potential and limitations of its application in production. The amount of pectin and protein compounds in the pressed juice is higher and, as a result, the purity of the juice prepared by this method is lower compared to the diffusion method in the actual production process. This difference can be explained by the existence of different mechanisms for the preparation of raw diffused juice (RDJ) and raw pressed juice (RPJ). During the formation of RDJ, dissolved substances (mainly sugars) are introduced into the water. In this case, the solubility of sugars and other water-soluble substances and their diffusion rate through the protoplasm of sugar beet cells can affect the quality of the juice. In the RPJ, since the sugar beet cells are randomly deformed, broken, and crushed under the applied pressure, the vacuole shells are almost completely destroyed. However, the destruction mechanism is different in the RDJ. The resulting RPJ contains all the ingredients found in fruit juice, which leads to a decrease in its quality, and this has also been reported in related research (Klyuchnikov et al., 2020; Ovsyannikov et al., 2021).
The wide variety of non-enzymatic fining agents has provided the juice industry with many options to tailor the fining process to meet consumer needs and enhance the sensory characteristics of the juice. Environmental impacts, waste management, increased productivity, health and safety concerns, quality control, regulatory standards, alternative methods, consumer perception, increased production, and cost-effectiveness are factors that can guide future research on the application of fining agents. Combined use and optimization of fining agent consumption can address the challenges facing the industry and provide innovative solutions to improve the efficiency of fining processes (Ahamad et al., 2023).
Little research has been conducted on the clarification of RSBJ by various refining agents and the feasibility of direct application of sugar beet juice in food formulations. Therefore, the main purpose of this study was to compare the RPJ and in the RDJ and their effects on the quality indicators of raw beet juice. Also, the efficiency of bentonite, silica sol, gelatin, and activated carbon, individually and in combination, and their synergistic effects in removing impurities from RSBJ have been considered as an alternative method, focusing on the possibility of producing clear sugar beet juice for direct use in the food industry without the need to separate sugar crystals.
2. Materials and methods
Freshly harvested sugar beet roots were systematically acquired from the Piranshahr sugar factory located in Iran. The fining agents employed for the clarification process included bentonite (Na-Ca Bentonite ERBSLÖH, Geisenheim, Germany), 15% silica sol (Baykisol 15%), gelatin (type A; 100 bloom, Erbigel, Germany), and activated carbon (CS-2000, Gostar Ghoumes CO, Semnan, Iran), all of which were sourced from Azar Kam Co, Urmia, Iran. All other chemical materials utilized were of analytical grade.
In order to obtain the raw juice (RDJ), the roots underwent a washing process and were subsequently chopped into slices utilizing a slicing machine. The diffusion process of the slices was then conducted in a batch diffuser at a temperature of 73°C for a duration of 80 min, with the process draft maintained at 115%. Following this, the resulting juice was filtered through cheesecloth. For the refined juice (RPJ), the washed sugar beet was subjected to crushing, enclosed in cheesecloth, and subsequently pressed utilizing a laboratory-type press. The extracted juice was then filtered through cheesecloth.
A flow diagram illustrating the treatment process for the raw sugar beet juice (RSBJ) is depicted in Fig. 1. For each experimental trial, 200 mL of the raw juice was subjected to various refining treatments. The RPJ and RDJ underwent refinement at 50°C and 70°C for 100 min with the application of fining agents, respectively. The bentonite was pre-swelled and dissolved in distilled water for 10-12 h prior to the experiment at 50°C. The addition of fining agents to the beet juice samples was conducted in accordance with preliminary experimental findings. The pH of the juices was adjusted to the desired level (4.5) using citric acid. Upon completion of the clarification process, juice samples were passed through a microfilter (45 µm) to remove the formed floc and were pasteurized at 90°C for 5 min (Vladisavljević et al., 2013). The specifics of the treatments are detailed as follows:
First, it should be noted that 10% (w/v), 2% (w/v), and 15% (w/v) aqueous solutions were added to the juices as the initial solution of bentonite, gelatin, and silica sol, respectively. Also, activated carbon and gelatin were added to the juice first before other clarifiers in combination treatments containing them. The exact amount of different ratios of clarifying agents in each treatment is presented in Table 1.
Process 1: Raw juices (RDJ and RPJ) were treated with 1, 2, and 3 g/L of bentonite at the desired condition for 100 min. Also, bentonite was pre-swelled and dissolved in distilled water 10-12 h before the experiment at 50°C.
Process 2: Raw juices (RDJ and RPJ) were treated with 1, 2.5, and 4 g/L of silica sol at the desired condition for 100 min. Aqueous solution of 15% (w/v) silica sol was used for treatments.
Process 3: Raw juices (RDJ and RPJ) were clarified by 2 g/L of bentonite and 0.01, 0.03, and 0.05 g/L of gelatin at the desired condition for 100 min.
Process 4: Raw juices (RDJ and RPJ) were clarified by 2.5 g/L of silica sol and 0.01, 0.03, and 0.05 g/L of gelatin at the desired condition for 100 min.
Process 5: Raw juices (RDJ and RPJ) were clarified by 2 g/L of bentonite and 0.01, 0.03, and 0.05 g/L of activated carbon at the desired condition for 100 min.
Process 6: Raw juices (RDJ and RPJ) were clarified by 2.5 g/L of silica sol and 0.01, 0.03, and 0.05 g/L of activated carbon at the desired condition for 100 min.
The turbidity was measured by a turbidimeter (Box 389, Hach Company, Loveland, CO, USA), and results were reported as Nephelometric Turbidity Units (NTU).
The color of the sample was measured by using a UV-2100 spectrophotometer (SCINCO, Seoul, Korea) at a wavelength of 420 nm according to recommendations of ICUMSA (International Commission for Uniform Methods of Sugar Analysis) (De Whalley, 2013). Results were expressed in ICUMSA units (IU) and calculated as follows:
Where A is the absorbance at 420 nm, °Brix is the concentration of soluble solids in the sample (%) measured by a refractometer (DR-Al ATAGO, Kobe, Japan), L is the cell thickness (path length of light) in cm, and ρ is the density of the sample in g/cm3.
Total ash was estimated by the conductimetry method. 5 g of juice samples were weighted and poured into a 100 mL volumetric flask. Then, the flask was filled up to the mark with deionized (DI) water and thoroughly mixed. The conductivity was determined at 20°C using a conductivity meter (Model LF 538, WTW, Weilheim, Germany). The total ash was calculated as follows (Jahed et al., 2014):
where AC is the total ash (%), FA is the correction factor, AS is the electrical conduction for juice samples (μs), Aw is the electrical conduction for DI water in use (μs), m is the mass of juice sample (g) and Bx° is the Brix (%).
The total concentration of colloids and polymers within the sample was ascertained by boiling 5 mL of the sample in conjunction with 50 mL of 96% ethanol in a flask for 15 min, followed by cooling, filtering (using ashless filter paper), washing the residue with 90% ethanol (100 mL), and drying the filter until a constant weight was achieved (Cherniavskaia et al., 1995). The total concentration of precipitated colloids and polymers was determined via the weight method and expressed in grams per liter (g/L).
The concentration of proteins was determined according to the dye-binding method described by Bradford (1976). The details of analysis are available in Technical Bulletin for Bradford Reagent (B 6916, Sigma-Aldrich, St. Louis, MO, USA). Results were expressed in mg of bovine serum albumin/L of the sample.
The experimental design was a completely randomized design with three replications. Statistical analyses were performed using Minitab software (version 17, Minitab Inc., State College, PA, USA). Tukey’s test was used to compare means and significant differences between treatments at a significance level of α=0.05.
3. Results and discussion
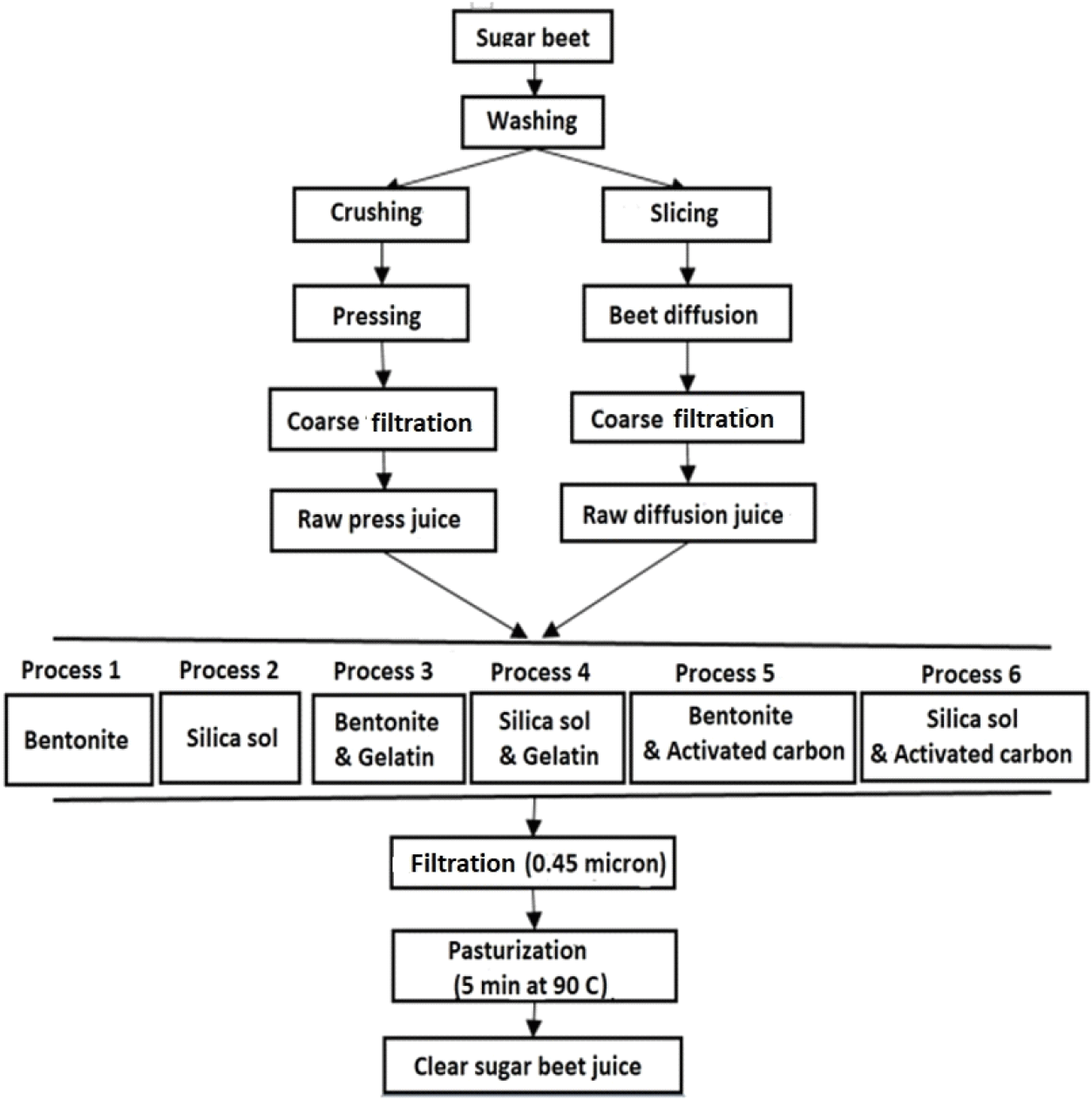
In the juice production step, some undesirable beet macromolecules pass into the juice and cause a turbid state (Downing, 2012). In the present study, the ability of adsorbents in the turbidity-caused compounds removal was studied (Fig. 2). The initial turbidity of control samples (RDJ and RPJ) was 582 and 1,691 NTU, respectively, significantly decreased after clarification (p<0.05). The results also showed that bentonite was more effective in the elimination of particles (dissolved or suspended) than silica sol. A significant decrease in turbidity by bentonite fining was previously reported by Jahed et al. (2014) for RSBJ (p<0.05). Reducing the turbidity of juice by adding bentonite and silica sol is mainly due to its ability to absorb proteins and other positive charge impurities (Jahed et al., 2014). Bentonite can also remove heavy metal ions, pesticides, and dark compounds from juices by adsorption. In contrast, Laksameethanasana et al. (2012) reported that there was no significant difference in turbidity of sugarcane juice with different concentrations (1-3%) of bentonite. Similar to the decreasing effect of P3 (2.45 and 21.74 for RDJ and RPJ, respectively) and P4 (7.71 and 29.44 for RDJ and RPJ, respectively) treatments on turbidity, there were significant decreases (p<0.05) in turbidity of raw juices after combined treatment with active carbon (P5 and P6). Similar results have been reported by Laksameethanasana et al. (2012). They showed that the clarification with active carbon led to a reduction in turbidity. This was related to the ability of activated carbon to adsorb organic compounds, which rose as surface area increased. As shown in Fig. 2, the combined treatments containing bentonite had lower turbidity levels (P3 and P5), and notably, the lowest turbidity levels for all levels were recorded for P5 treatment (1.34 and 10.62 for RDJ and RPJ, respectively). In contrast, the highest turbidity levels were recorded for P1 (7.71 and 37.62 for RDJ and RPJ, respectively) and P2 treatments (19.3 and 47 for RDJ and RPJ, respectively), which are related to the individual effects of the clarifier agents. From these results, it can be inferred that the synergistic effect of gelatin and bentonite showed greater efficiency in precipitating impurities. Other researchers have also reported similar results and acclaimed that the main mechanism of action in the fruit juice purification process may also be the result of the anionic and cationic charges of bentonite and gelatin (Heshmati et al., 2020; Jafari et al., 2024). The combination of gelatin and bentonite has shown better performance in precipitating turbid substances from raw fruit juice due to the aforementioned charge interaction between them, thereby helping to clarify. The synergistic effects of the combination of gelatin and bentonite cause precipitation of a wider range of impurities and allow for significant juice clarity through sedimentation and increased filtration performance (Jalali et al., 2014).
Bentonite and silica sol are negatively charged, while gelatin is positively charged at the 4.5 pH of raw juices (RDJ and RPJ). Therefore, during clarification, positively charged gelatin and negatively charged adsorbent interact with each other, and the interaction leads to flocculation. Once the flocs are formed between the adsorbent, turbidity-causing compounds are also removed from raw juices with flocs (Türkyılmaz et al., 2012). Similar results have been noticed by Meyer et al. (2001) and Gökmen and Çetinkaya (2007) using silica sol-gelatin and bentonite-gelatin treatment, respectively. They stated that bentonite/silica sol-gelatin treatment had a significantly positive impact on raw juice turbidity by removing particles suspended or dissolved in juice (p<0.05).
Also, Comparing the obtained values for the turbidity of RPJ and RDJ revealed, that in general, in all tested samples, including the control sample and treatments P1 to P6, the turbidity of the RDJ method was significantly lower than RPJ (p<0.05). In the RPJ, since the sugar beet cells are randomly deformed, broken, and crushed under the applied pressure, the vacuole shells are almost completely destroyed. It seems that the RPJ contains all the ingredients found in fruit juice, which leads to a decrease in its quality.
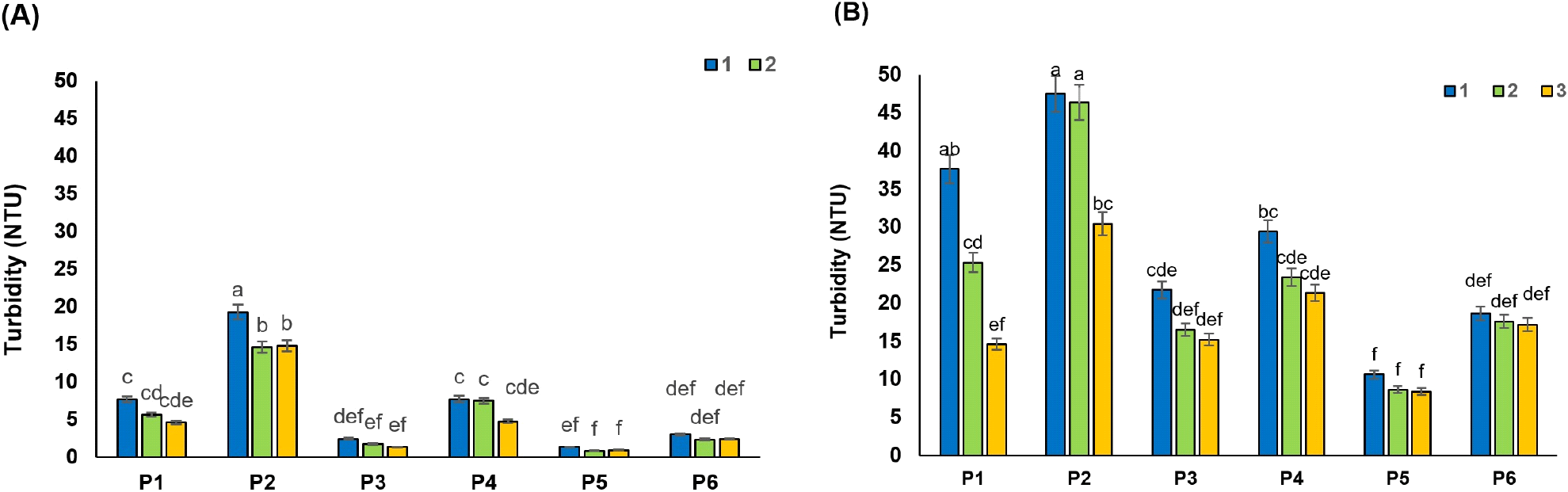
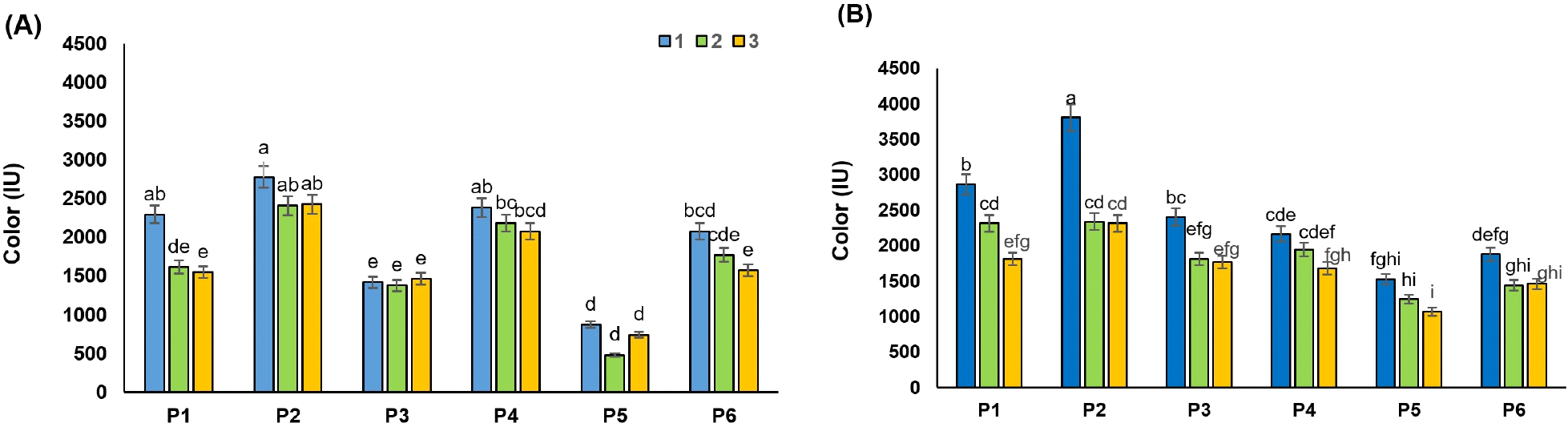
The colorants present in the RSBJ are melanins and melanoidins. The melanins result from the browning enzymatic oxidation of phenolics or the reaction between phenolic acids with amino acids, while melanoidins result from the “Maillard reaction” of amino acids with reducing sugars (Arajshirvani and Hojjatoleslami, 2017; Godshall et al., 1991). Various parameters can affect the color of sugar beet juice. Research has shown that the color of RSBJ is mostly the result of the alkaline decomposition of inverted sugars, in which melanoidins are formed. In contrast, the natural color of RSBJ is mainly due to plant color pigments, which can sometimes go through all the processing steps and enter the product. Simple sugars, especially glucose and fructose, can play a role as the main precursors in the formation of color. Heating simple sugars under acidic or basic conditions lead to polymerization reactions, ultimately forming colored compounds (Arajshirvani and Hojjatoleslami, 2017). The results showed that all the applied treatments had a significant effect on the color of the juice samples (RDJ and RPJ) (p<0.05). The initial color of the control samples for RDJ and RPJ was 6,234 and 5,993 IU, respectively, which decreased significantly after clarification (p<0.05). These values reached their lowest values of 481 and 1071 for RDJ and RPJ, respectively, in treatment P5, which is a combination of bentonite and activated carbon. The initial color of RDJ was higher than RPJ, which might be due to the formation of colorant product in high temperature applied for the diffusion process. Fig. 3A and 3B show the change in color of raw juices from various processing treatments. There was a higher decrease in the color of juices by bentonite treatment (P1) compared to silica sol treatment (P2) in most cases. Reducing the color is related to the surface adsorption of colorants by the bentonite. Adsorption of colorants by bentonite depends on many factors, including molecular size and concentration of colorant, the effect of van der Waals forces, the chain length of colorant, and the entropy effect (Erdogǎn et al., 1996). For example, smaller molecules have a greater surface area-to-volume ratio, and therefore greater surface adsorption. Bentonite has a surface adsorption property due to its heterogeneous surface. Acid-activated bentonites have the highest anionic dye adsorption capacity. The adsorption mechanism of bentonite can be explained by the electrostatic attraction between the clay surface and the dye molecule (Fernandes et al., 2020).
In addition, Türkyılmaz et al. (2012) stated that color reduction might be the result of the interactions between bentonite, protein, and polyphenols. As known, differences in the nature of ionic charges of bentonite and the proteins induce the irreversible absorption of proteins by clay platelets, and it prevents the juice from becoming hazy. However, bentonite also indirectly adsorbs polyphenol colorants, which interact with proteins by hydrogen bonding (Türkyılmaz et al., 2012). Downing (2012) illustrated that flocs formed between bentonite, protein, and polyphenols could adsorb and remove the colorant from the juice during settling.
As shown in Fig. 3B, the percentage of colorant removal by a combination of gelatin with bentonite and silica sol (P3 and P4) was clearly higher than in P1 and P2 treatment for RPJ juices (p<0.05). A decrease in the colorant content of fruit juice treated with a combination of gelatin and bentonite/silica sol (Hatamikia et al., 2013) and a combination of gelatin and bentonite (Gökmen et al., 2001) have been reported. Combined treatments with activated carbon (P5 and P6) were found to be more effective than combined gelatin treatments on the colorant reduction of raw juices, as shown in Fig. 3. Similar results were reported by Gökmen et al. (2001) as such that a sharp fall in the color of the apple juices was noted after the addition of the activated carbon. The ability of powder-activated carbon to adsorb organic substances (colorant) is essentially related to its textural and surface properties and rises with increasing surface areas (Laksameethanasana et al., 2012). The hydrophobic and apolar nature of the activated carbon is essential to remove the substances responsible for color (Mudoga et al., 2008).
Ash refers to water-soluble salts of mineral and non-mineral compounds, often determined in raw juice by conductivity. High ash levels do not have a significant effect on the storage of raw juice but are important to sugar producers because high ash levels reduce yield and increase molasses. After clarification of raw juices (RDJ and RPJ), the changes in juice ash were evaluated (Fig. 4A and 4B). Bentonite alone (P2) recorded the lowest effect in ash reduction, with values of 0.66 and 0.88 for RDG and RPJ, respectively. Although no significant difference was observed between the different treatments on ash, in general, combined treatments of clarifying agents have shown better effects (p>0.05). The higher content of ash in juices may also occur due to the lack of recovery of added adsorbents. Consistent with our results, Jahed et al. (2014) reported that the ash content of the RSBJ was slightly decreased by increasing the concentration of bentonite (1-5 g/L). Also, Türkyılmaz et al. (2012) stated that when bentonite bonds to the positive charge protein, the metals are replaced by proteins and released from bentonite into juice.
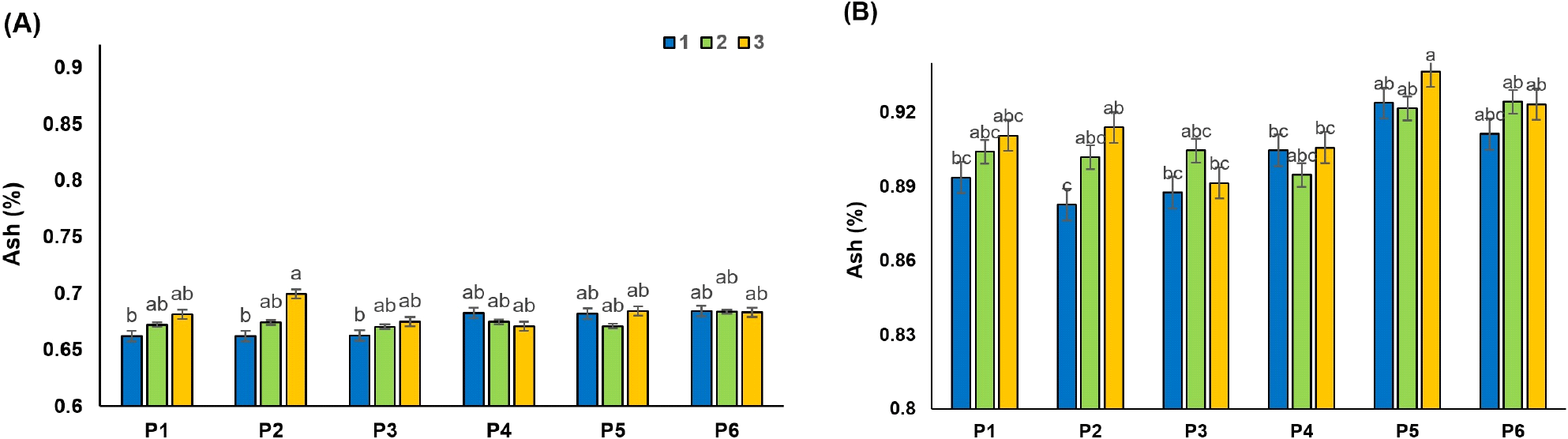
The results also showed that combining gelatin with bentonite or silica sol resulted in the lowest ash content in the samples, although these effects were not statistically significant (p>0.05) (Fig. 4). Hatamikia et al. (2013) found similar results in grape juice, in which no significant effect (p<0.05) was observed in the ash content of the samples clarified with bentonite, silica sol, gelatin, and their combination. The combination of activated carbon with bentonite or silica sol (P5 and P6) also recorded a higher ash content compared to the control juice. Al‐Farsi (2003) indicated that the higher ash content was due to the addition of carbon, which could not be completely removed by the filtration process. The ash results in the RPJ and RDJ showed that, in general, in all the samples tested (P1 to P6), these values were significantly higher in the RPJ method than in the RDJ (p<0.05). These different results could be the result of different mechanisms in the two methods for cell breakage and disintegration during juice production.
Colloids consist of solid particles, including water-insoluble cell wall polysaccharides, which are mainly cellulose, hemicellulose, and water-insoluble pectin polymers. Pectins are structurally composed of (1→4)-α-D-galacturonic acid (Wan et al., 2019). Colloids in the RSBJ have different surface charges and include high-molecular substances such as coloring materials, dextran, pectin, decaying beet particles, and microorganisms (Asadi, 2006). The results presented in Table 2 show that both bentonite and silica sol significantly reduced the colloidal content of the juices in a concentration-dependent manner. For example, high levels of bentonite (3 g/L) were able to reduce the amount of colloids in the RDG and RPG samples from 1.61 and 2.8 in the control sample to 0.92 and 1.38, respectively (p<0.05). Also, high levels of silica sol (4 g/L) were able to increase the amount of colloids in the RDG and RPG samples from 1.61 and 2.8 in the control sample to 1.3 and 1.77, respectively. Meanwhile, bentonite produced juices with lower colloid content compared to silica sol (p<0.05). Table 2 clearly shows that all treatments used were able to reduce the amount of colloids compared to the control sample (p<0.05). Among them, treatment P3 with the highest levels of gelatin (0.05 g/L gelatin and 2 g/L bentonite) had the greatest effect on the amount of colloids, reaching their amount to 0.49 and 1.06 in RDG and RPG juices, respectively. One of the principal mechanisms of these clarifying agents, such as bentonite and silica sol, is based on electrical charge and electrostatic bonding and has been explained to the same extent in similar studies. In addition to electrostatic attraction, bentonite has high surface adsorption due to its porous structure, which can absorb many impurities, including colloids (Babahoum and Ould Hamou, 2021).
Positively charged colloids and proteins are the most substances that adsorb onto the negatively charged surface of bentonite and silica sol (Xifang et al., 2007). The study of Lambri et al. (2012) illustrated that a preliminary treatment of musts with bentonite reduced colloidal substances, which confirms our results. Also, Marchal and Jeandet (2009) stated that enhancing the membrane filterability after bentonite fining was related to a reduction in the colloidal particle number in juices.
P3 and P4 treatments showed that by increasing gelatin, the concentration of colloids was significantly decreased (p<0.05). Raw fruit juices are rich in negatively charged colloids, which are mainly composed of pectic substances and some proteins. In these colloidal particles, there is a protein nucleus with a positive surface charge coated with negatively charged pectin molecules. This negative charge causes the pectin molecules to repel one another. Reducing electrostatic repulsion between cloud particles, which causes these particles to aggregate into larger particles. These larger particles eventually settle out. However, to improve the process, flocculating agents (fnings) such as gelatin, tannin or bentonite can be added. (Kashyap et al., 2001). These negatively charged particles and phenolic compounds present in raw juices are proposed as the main compounds adsorbed by gelatin (Benítez and Lozano, 2007).
Comparing the effects of P5 and P6 treatments on the concentration of colloids, it can also be concluded that there is a significant difference (p<0.05). This indicates that the synergistic effect of activated carbon with bentonite had a greater reduction effect on the concentration of hydrocolloids compared to silica sol. Activated carbon has advantages such as high surface area, good adsorption capacity, porous structure, and thermal stability. Activated carbon is a porous carbon compound that is often used in combination with other adsorbents (Asadi and Rostami, 2021). One of the characteristics of activated carbon is the non-polarity of its surface, which leads to its tendency to combine with non-polar organic molecules (Arjeh et al., 2019). Experiments have shown that the intensity of surface adsorption gradually increases in alcohols, ketones, and aromatics, respectively, and finally reaches its maximum in fatty substances. The efficiency of activated carbon fining depends on many factors, including the amount of activated carbon used, the temperature of the juice, and contact time. A comparison of the two extraction methods (RPJ and RDJ) and their statistical significance in Table 2, which is given with the letters (A and B) in each row, shows that in most of the tested samples (control sample and treatments P1 to P6), the concentration of colloids in the RDJ method was significantly lower than that in the RPJ method (p<0.05). The reason for the difference in the concentration of colloids in RPJ and RDJ can probably be explained by different mechanisms in breaking cells and releasing substances into the juice.
Results showed that the total protein content of RDJ and RPJ decreased significantly at all of the applied treatments compared to the initial protein content (p<0.05; Table 2). In the P1 and P2 treatments, there was a general trend showing a decrease in the protein content of samples as the concentration of bentonite and silica sol increased (p<0.05). Bentonite and silica sol had negative charges and bonded to the positive charge impurities such as proteins. A similar result was reported by Hatamikia et al. (2013). They showed that protein removal efficiency increased by increasing bentonite and silica sol concentration. Proteins are haze-active compounds that react with polyphenols in juices and induce haze and turbidity during storage. Therefore, reducing the amount of proteins to an acceptable level is essential. However, Kim et al. (2014) reported that bentonite and silica sol treatments had no significant effect on the protein content of the grape wine in the treated range (1-20 g/L).
T3 and T4 showed that gelatin had the lowest effect on the protein removal of raw juices and led to the significantly high protein content of samples compared to control samples (p<0.05). This likely occurred due to the competition between positively charged proteins (those naturally present in the raw juices) and gelatin to combine with negatively charged bentonite and silica sol (Benítez and Lozano, 2007; Hatamikia et al., 2013). Gelatin is the first one added to the juice and plays its role in the precipitation of negatively charged particles, including pectic compounds, etc. Then bentonite or silica sol with a negative charge is added to the juice and one of their purposes is to absorb protein. However, in the meantime, if there is excess gelatin in the juice in free form, it can act as a protein competitor and react with bentonite and silica sol. In other words, bentonite can also absorb and precipitate excess gelatin. In general, it can be stated that the lowest protein content in RDG and RPG juice samples (57.06 and 77.65, respectively) was recorded for treatment P5, which contains activated carbon and bentonite. The highest protein content was observed in treatment P4, which is related to the combination of gelatin and silica sol.
Unlike gelatin, a combination of activated carbon with bentonite and silica sol had a significant decrease in the protein content of juices, indicating the synergic effect between the adsorbents (p<0.05). The effect of P5 and P6 treatments on protein concentration also indicates that the synergistic effect of activated carbon with bentonite had greater reducing effects on protein concentration compared to silica sol. Kim et al. (2014) found similar results in grape wine, in which the treatment of activated carbon caused a significant decrease in protein content in a concentration-dependent manner (p<0.05). Proteins are a key factor for floc formation in acidic beverages (Hassanzadeh et al., 2022; Morton and Murray, 2001; Sastre Siladji et al., 2020). Therefore, elimination of them in the purification step by activated carbon helps the sugar manufacturer to improve the quality of sugar and prevent floc formation.
By looking at Table 1 and comparing RPJ and RDJ, it can be seen that in most of the tested samples, including the control sample and treatments P1 to P6, the protein content of the RDJ method was significantly lower than that of the RPJ method (p<0.05). Different mechanisms in cell breakage and release of substances into the juice can explain the difference in the protein content between RPJ and RDJ. A general observation of Table 2 and a comparison of RPJ and RDJ indicate that in most of the tested samples, including the control sample and treatments P1 to P6, the protein content of the RDJ was significantly lower than that of the RPJ (p<0.05).
4. Conclusions
The findings unequivocally demonstrated that bentonite and silica sol were significantly efficacious in enhancing the quality of the raw juices. It can be generally asserted that the minimal protein content in the RDG and RPG juice samples was documented for treatment P5, which incorporates activated carbon and bentonite. Conversely, the maximal protein content was identified in treatment P4, which pertains to the amalgamation of gelatin and silica sol. The activated carbon exhibited a considerable capacity to adsorb substantial quantities of colloids and proteins. Collectively, the findings of the investigation elucidated that all employed treatments manifested substantial effects on impurities, including ash, color, protein, and colloids, in sugar beet juice. Noteworthy synergistic effects were discerned for the concurrent utilization of clarifying agents, particularly in the case of bentonite combined with activated carbon or gelatin. The aforementioned results were documented in both juice variants obtained via pressing and diffusion, corroborating the enhanced efficacy of clarifying agents when utilized in conjunction as opposed to their isolated application. In general, the comparative analysis of the two extraction methodologies (raw press juice and raw diffusion juice) indicated that in most of the evaluated samples (control sample and treatments P1 to P6), the diffusion method (raw diffusion juice) exhibited a markedly superior efficiency compared to the pressing method (raw press juice). The concentration of impurities such as ash, colloids, proteins, and turbidity were observed to be greater in the raw press juice sample. The rationale for the disparity in the concentrations of these impurities between raw press juice and raw diffusion juice is likely attributable to the divergent mechanisms of cellular disruption and the release of constituents into the juice. The findings of this study may hold potential in demonstrating that beetroot is not solely relegated to sugar production, and that beetroot juice can be processed directly akin to other fruit juices (apple and grape), through a straightforward clarification procedure. At present, the sugar processing sector expends considerable energy on clarifying beet juice utilizing lime juice and carbon dioxide gas, as well as on concentration and crystallization processes to convert it into sugar. The methodology proposed in this study has the potential to conserve substantial energy, time, and costs, thereby facilitating the direct utilization of beet juice in food products such as beverages. Constraints to industrial application may encompass the more intricate transportation of beet juice in comparison to sugar and the seasonal availability of fresh beets, in which case the juice may be preserved as a concentrate until it is processed similarly to other fruit juices.