1. Introduction
With the recent changes in dietary culture and interest in health, the demand for fresh products such as convenience foods of vegetables and fruits has increased. Therefore, simply processed fruits and vegetables are gaining popularity. These simple processed products are frequently used by busy modern people because they can be consumed immediately after purchase without preprocessing (Kim, 2017). However, as these products undergo processing such as peeling, cutting, coring, and slicing, quality deterioration such as moisture loss, tissue softening, and microbial contamination are unavoidable, resulting in respiration, ethylene generation, and browning. Therefore, preservability and stability are significantly lower compared to raw agricultural products. The main cause of the deterioration of preservability is tissue damage that occurs during the peeling and cutting processes, which are essential manufacturing processes. Generally, browning occurs first under modified atmospheric packaging (MAP), followed by odor, softening, and decay (Park et al., 2003). In particular, browning is a major factor in discarding fresh convenience foods during distribution, with a high distribution loss rate of about 25% (Jeong, 2012), as it is a criterion for consumers’ freshness judgment and purchasing behavior. In most cases, when the appearance and flavor of these products are damaged, consumer preference is lowered, thereby directly or indirectly affecting the commercial value. Therefore, food storage and processing are critical (Kim and Uyama, 2005; Park et al., 2013). In particular, the browning of peeled root vegetables is the main quality deterioration phenomenon, which significantly affects consumer purchasing decisions and is a major cause of waste during distribution (Choi et al., 2013; Oh et al., 2019). Therefore, synthetic compounds are used to inhibit browning and disinfect during the manufacturing processes of washing, peeling, and cutting. However, as consumers’ interest in health has increased recently, their use has been discouraged (Lee and Kim, 2020). Typical additives widely used in peeled root vegetable products are sulfites and chlorine dioxide (Kim et al., 2000). These sulfites generate sulfurous acid with strong reducing power. When sulfurous acid is oxidized to sulfuric acid, a strong bleaching action reduces colored substances. Therefore, it is widely used to prevent browning by strongly inhibiting the action of polyphenol oxidase, which is involved in the browning of foods (Kim et al., 2000). The sulfur dioxide (SO2) management is subject to ‘Standards and Specifications for Food Additives’ (Ministry of Food and Drug Safety Notice No. 2021-19, 2021. 3. 9.) II. Food Additives and Mixed Formulations 5. Standards for Use by Item a. Food Additives’ (MFDS, 2021). In the United States, the EU, and Canada, sulfites are considered allergens. Therefore, if more than 10 mg/kg of SO2 is used in food, it must be labeled on the product. Like sulfur dioxide, chlorine dioxide used in food is a chlorine-based compound. Hypochlorous acid series (for example, sodium hypochlorite) and aqueous chlorine dioxide are mainly used. They exist as chlorite ions and chlorate ions, which are major decomposition products (KFDA, 2008). The effects of chlorine-based disinfectants on health were mainly reported in drinking water. Chlorine dioxide, which is used to disinfect drinking water, decomposes rapidly in water into chlorite (ClO2−), chlorate (ClO3−), and chloride (Cl−). In particular, chlorite is the main chemical species (WHO, 2016). As the use of chlorine-based disinfectants in food increases, there are concerns about health risks. However, a systematic and standardized analysis method for chlorite and chlorate, which are decomposition products, has not yet been proposed worldwide. The Ministry of Food and Drug Safety has included “chlorine dioxide (ClO2)” in the list of the ‘Food Additive Analysis Method in Food’ (MFDS, 2021). In addition, a quantitative analysis method by ion chromatography (IC) of chlorite (ClO2−) and chlorate (ClO3−), which are by-products of chlorine dioxide in seasoned dried fish, has been established. However, because this method is for seasoned dried fish, it is necessary to develop a wide range of analysis methods for each medium. According to “Standards and Specifications for Food Additives” (Ministry of Food and Drug Safety Notice No. 2021-19, 2021. 3. 9.) II. Food Additives and Mixed Formulations 5. Standards for Use by Item a. Food Additives, chlorine dioxide should be used only for sterilization of food according to the standards for the use of additives containing chlorine in food. In addition, it is specified that chlorine dioxide should be removed before completion of the final food (MFDS, 2021). However, although there are standards for using chlorine-based compounds in food, the standards for residues of decomposed products are not established (KFDA, 2008).
Therefore, this study checked the residual concentrations of chlorite ions and chlorate ions, which are decomposition products of sulfur dioxide and chlorine-based compounds used as antioxidants, preservatives, and bleaching agents for peeled root vegetable products distributed online and offline. In addition, this study investigated the actual use of synthetic compounds. Based on the results, this study sought to secure the safety of peeled root vegetables by developing a naturally-derived anti-browning agent to replace synthetic compounds.
2. Materials and methods
The materials used in this experiment were peeled root vegetables (balloon flower, deodeok, lotus root, and greater burdock) sold in online marts, supermarkets, and traditional markets from February to October 2021. All the materials were fresh. The residual amounts of sulfur dioxide and chlorine ions were investigated for 67 cases of 4 types of vegetables—38 cases of balloon flower, 6 cases of deodeok, 13 cases of lotus root, and 10 cases of greater burdock. A list of tested products is shown in Table 1.
| Group | Balloon flower | Deodeok | Lotus root | Burdock |
|---|---|---|---|---|
| Domestic products | 17 | 4 | 4 | 9 |
| Imported products | 19 | 2 | 9 | 1 |
| Not registered | 2 | 0 | 0 | 0 |
| Total | 38 | 8 | 13 | 10 |
For sulfur dioxide analysis, sodium bisulfite, dimedon (5,5-dimethyl-1,3-cyclo hexanedione), sodium azide, and pararosaniline were purchased from Sigma Chemical Co. (St. Louis, Mo, USA). HPLC-grade ethanol was purchased from Fisher. 171.0 mg of sodium hydrogen sulfite (100 mg as sulfur dioxide), a standard material, was precisely weighed, and 0.1 N sodium hydroxide was added to make 100 mL, which was used as the standard stock solution. For the analysis of chlorite ion (ClO2−) and chlorate ion (ClO3−), each standard solution (100 μ/mL) was purchased from a high-purity standard company. Sodium carbonate concentrate (Sigma Chemical Co.) and sulfuric acid (95-98%, Sigma Chemical Co., St. Louis, MO, USA) were used. Distilled water purified by a water purification system (Milli-Q Direct 16, Merk Millipore, Germany) was used as purified water. A standard solution for a calibration curve was prepared by diluting a standard substance, 100 μ/mL of each chlorite and chlorate ions, with ultrapure water.
In a flask, 20 mL of water, 1 mL of 5% dimethone/ethanol solution, 1 mL of 1% sodium azide solution, 2 mL of ethanol, 2 drops of an antifoaming agent, and 10 mL of 25% phosphoric acid were added, and the flask was installed in the heating distillation apparatus (Jeong et al., 2003; Kim et al., 2000). Next, 20 mL of 0.1 N sodium hydroxide solution was added to the sulfur dioxide collection flask, and the flask was purged with nitrogen for 5 min. A certain amount of the sample was placed in the flask and heated for 20 minutes. When measuring absorbance, a solution obtained by adding 0.1 mL of water to 5 mL of the collected sulfur dioxide (SO2) solution was designated as (A) solution, and a solution obtained by adding 0.1 mL of 0.1% hydrogen peroxide solution to 5 mL of a newly collected solution was designated as (B) solution. To solutions (A) and (B) each, 1 mL of para-rosaniline/formaldehyde solution was added, shaken, and left at temperature for 20 min. Absorbance was measured at a wavelength of 580 nm with a UV spectrophotometer (Agilent, USA). The concentration of SO2 in the test solutions (μ/mL) was obtained from the calibration curve using the absorbance of the test solutions (absorbance of A-absorbance of B). The samples’ SO2 content (g/kg) was calculated using the following equation (MFDS, 2022).
C = SO2 concentration in the test solution (μg/mL)
W = Sample weight (g)
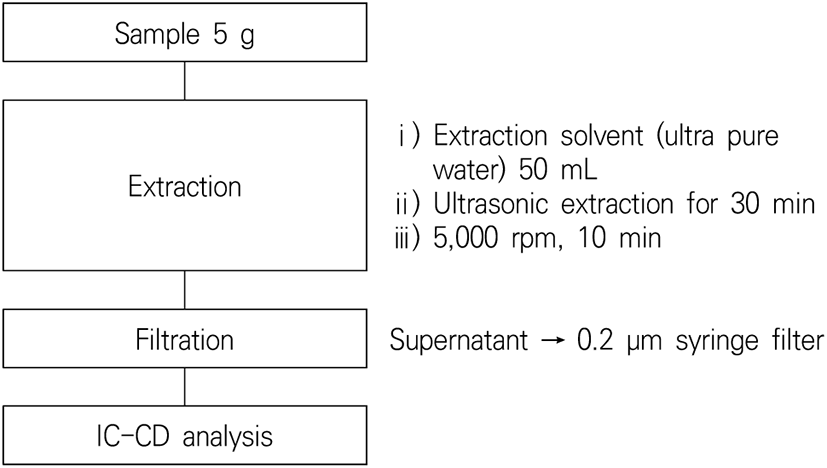
Sample pretreatment was performed by referring to the ‘Test method for by-products of chlorine-based disinfectant in seasoned dried fish (NIFDS Additives Packing, 2014)’ (Fig. 1). The selected four types of peeled root vegetables were cut into small pieces, with 5 g of each vegetable precisely weighed. Then, 50 mL of ultrapure water was added, followed by ultrasonic extraction for 30 min. After centrifuging the extract at 5,000 rpm for 10 min, the supernatant was filtered with a 0.2 μm syringe filter (Advantec, Otowa, Tokyo, Japan) to use as test solutions. A Professional IC Vario model was used as an ion chromatography-conductivity detector (Metrohm, USA). The conditions of the instrument analysis used for quantitative analysis of chlorite ions and chlorate ions are shown in Table 2.
C = Measured ion concentration in the sample solution (μg/mL)
V = Volume (mL) of ultrapure water added to the sample
m = Weight of sample used for analysis (g)
3. Results and discussion
As the test method for sulfur dioxide is specified in the food code, the test method was not validated (MFDS, 2022). However, the specificity, linearity, accuracy, precision, detection limit, and quantitation limit of the test methods for chlorite ion and chlorate ion in peeled root vegetables are investigated to verify the test method because there is currently no standardized test method for chlorine dioxide. And its validity was confirmed (MFDS, 2016).
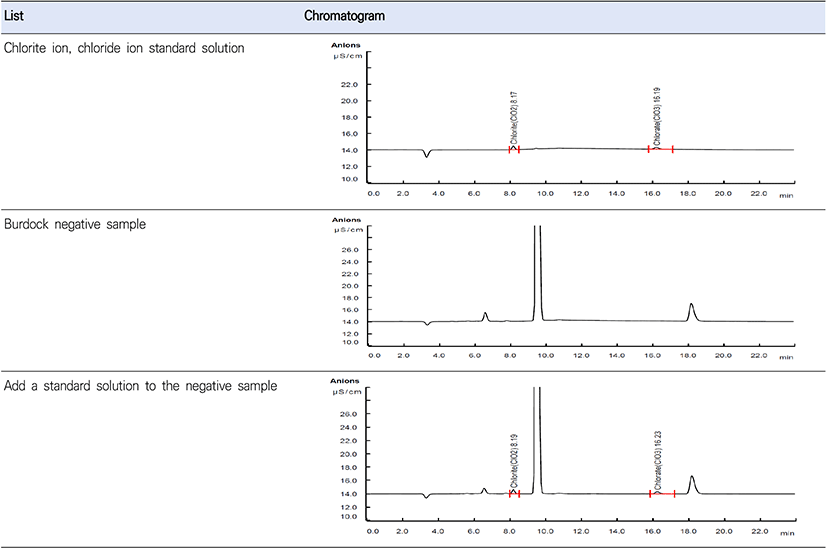
To investigate whether the analysis method performed was specific to chlorite ion and chlorate ion, each ion was added at a concentration of 1.5 μg/mL to greater burdock, a peeled root vegetable in which the corresponding ions were not detected, and then the greater burdock was analyzed. In the greater burdock negative sample and greater burdock negative sample spiked with standard material, chlorite ion in standard material was found at 8.2 minutes, chlorate ion was detected at around 16.2 minutes, and the peak was detected at the same retention time in the greater burdock sample to which standard material was added. In addition, the sample was selectively analyzed because the peak was separated from the baseline, and there were no other interfering substances (Fig. 2).
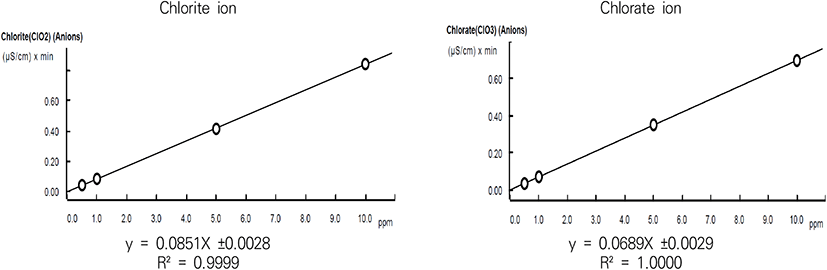
Standard solutions of chlorite ion and chlorate ion were prepared at 0.5, 1, 5, and 10 μg/mL, respectively, and a calibration curve was obtained through three repeated tests. As a result of regression analysis of the integrated area of the peak, the slope and standard deviation were 0.0851±0.0028 for chlorite ion and 0.0689±0.0029 for chlorate ion in the corresponding concentration range. In addition, the correlation coefficient (R2) was 0.999 or more, showing excellent linearity (Fig. 3).
To investigate the accuracy, the standard material of each ion (1.5, 2, and 4 μg/mL when measured by the instrument) was added to greater burdock, a peeled root vegetable in which the corresponding ions were not detected, and the recovery rate was calculated. As a result of examining the recovery rate in 3 repeated tests for 3 different concentrations, it was confirmed that the recovery rate of chlorite ions from burdock was 83-89.1%, and that of chlorate ions was 96.6-102.0%. The measured recovery value was within the 80-110% recovery range, consistent with the criteria suggested in EC Regulation and AOAC International Appendix F. Therefore, the established test method was confirmed to have good accuracy (Table 3).
| Element | Spiked concentration (μg/mL) | Recovery (%)±SD |
|---|---|---|
| Chlorite ion (each n=3) | 1.5 | 89.1±4.7 |
| 2 | 88.8±5.8 | |
| 4 | 83.0±3.4 | |
| Chlorate ion (each n=3) | 1.5 | 102.0±5.2 |
| 2 | 96.6±0.8 | |
| 4 | 97.2±1.0 |
The precision was evaluated with the relative standard deviation (RSD) of the recovery rate obtained from the accuracy test. The precision RSD of chlorite ions was 4.1-6.5%, and the precision RSD of chlorate ions was 0.9-5.1%. These values were within the range of precision standards presented in EC Regulation, AOAC International Appendix F. Therefore, the analysis method was confirmed to have good precision (Table 4).
The limits of detection and quantitation were calculated by the following formula using a method based on the reaction’s standard deviation and the calibration curve’s slope.
σ: Standard deviation of the y-intercept in the regression line
S: The slope of the calibration curve
As a result, the detection limit was 0.1 mg/kg, and the quantitation limit was 0.3 mg/kg.
Recently, the market for fresh food consumption, such as livestock and aquatic products, as well as agricultural products such as vegetables and fruits, has been steadily expanding due to an increase in single or two-person households and increased interest in health care. This study validated the established analytical method by analyzing sulfur dioxide and chlorine dioxide contained in peeled root vegetable products and identified residual concentrations that may exist, reflecting the situation. Among the target peeled root vegetables, lotus loot products had the highest number of labels containing sulfur dioxide with 10 cases (83.3%) out of 12 cases, followed by greater burdock with 1 in 10 cases (10%) and balloon flower with 1 in 23 cases (4.3%). No deodeok products had the label (Table 5). Suppose food additives are added to primary products such as agricultural products for simple washing, sterilization, and film coating. In that case, they are judged as agricultural products (simple processed agricultural products). When sulfur dioxide is added to prevent browning, it should be classified as processed food (processed fruits and vegetables, pickled foods, and other processed agricultural products). However, products labeled as containing sulfur dioxide in peeled root vegetables investigated in the study were limited to processed fruits and vegetables and pickled foods. In addition, there was no indication that simple processed agricultural products contained sulfur dioxide. In particular, there were no cases labeled as containing sulfur dioxide in other agriculturally processed products that do not have standards for using sulfur dioxide. Moreover, as a result of analyzing sulfur dioxide in peeled root vegetable products that are not labeled as containing sulfur dioxide, there were no such cases violating the labeling regulation regarding sulfur dioxide in processed fruits and vegetables, other agriculturally processed products, and pickled foods. However, a small amount (2.8 mg/kg) of sulfur dioxide was detected in one simple processed agricultural product of greater burdock. According to the ⌜Study on the Establishment of the Sulfur Dioxide Instrumental Analysis Method for Food⌟ of the Korea Food and Drug Administration (2011), the detection limit of the modified Rankine method used in this experiment is 0.4 mg/kg, while the limit of quantitation is 1.0 mg/kg (NIFDS, 2011). In the current food code, however, 10 mg/kg or less is considered non-detection. Therefore, it was concluded that the greater burdock, in which a small amount of sulfur dioxide was detected, did not violate the “Standards and Specifications of Food”. In addition, as a result of examining the concentrations of chlorite ions and chlorate ions in dermabrasion root vegetables for 67 cases of 4 items of target samples, chlorite ions were found to be 5.01 mg/kg, 5.22 mg/kg, and 10.94 mg/kg in 3 cases of bellflower (Table 6). In one case of lotus root, 21.74 mg/kg of chlorate ion was detected. However, in Korea, there are only standards for using additives containing chlorine in food, but there is no standard for residual chlorate ions in food. Therefore, it is not possible to determine suitability. In 2008, as a result of measuring the free residual chlorine concentration twice for a total of 32 fresh convenience foods (salads) from major discount stores and department stores in Seoul, no chlorine was detected in all samples (MFDS, 2008). Therefore, when compared with the previous results, attention should be paid to the results of this study. Therefore, based on these results, as chlorine-based disinfectants are still used in the distribution of products such as fresh convenience foods, it is necessary to review the test methods and standards for providing safe food. In addition, as there are concerns about the safety of residues in food, efforts should be made to supply safe food to consumers by developing a browning inhibitor using natural substances as an alternative to chlorine-based disinfectants.
4. Conclusions
This study investigated the synthetic compounds (sulfur dioxide, chlorite ion, and chlorate ion) contained in 67 peeled root vegetables (bellflower, deodeok, lotus root, greater burdock) from February to October 2021. Out of 67 cases of 4 types of peeled root vegetables, 12 cases contained sulfur dioxide. Chlorite ion was detected in three cases of bellflower and chlorate ion in one case of lotus root. Although the detection rate and amount were insignificant, it was possible to confirm the use of the synthetic compound, which was the purpose of this investigation. By confirming specificity, linearity, accuracy, precision, detection limit, and quantification limit, this study verified the reliability of the chlorine dioxide analysis method for peeled root vegetables, for which an analytical method has not been established. As chlorine-based disinfectants are still used in food to increase storage and processing efficiency, health risks remain a concern. Nevertheless, the systematic and standardized management of chlorite and chlorate, which are decomposition products, has not yet been proposed. Accordingly, it is urgent to prepare countermeasures. More studies are necessary to develop a browning inhibitor using natural materials as an alternative to synthetic compounds to supply safe food to consumers.