1. Introduction
Rhubarb is a perennial plant from the genus Rheum. It has short and thick rhizome, and is characterized by a large inverted triangular leaf on a long petiole called the stalk (Clementi and Misiti, 2010). It is native to the mountainous and desert regions of the Tibetan Plateau in China, and also grows in Asia and North America, with approximately 60 species worldwide (Li et al., 2003; Wang et al., 2005). The root of Rheum officinale (called da huang in China) and R. undulatum (called jong da huang in Korea) is commonly used in medicine (Khattak et al., 2020). Anthraquinone, rhein, emodin, aloe-emodin, chrysophanol, and physcion have been identified as the main medicinal components of rhubarb root (Aichner and Ganzera, 2015; Zheng et al., 2013). These compounds have strong anti-bacterial (Lu et al., 2011), anti-cancer (Huang et al., 2007), anti-inflammatory (Tang et al., 2007), and antioxidant (Cai et al., 2004) properties. Several studies have focused on the pharmacological functions of rhubarb root, such as its role in nerve cell protection in the brain (Li et al., 2019; Lu et al., 2015; Wang et al., 2016).
In the West, rhubarb (R. rhabarbarum) stalk, which is rich in dietary fiber, anthocyanins, and the flavonol quercetin and its derivatives, is mainly consumed raw, as a vegetable (Pussa et al., 2009; Takeoka et al., 2012), and in wine, jam, and juice. After adding sugar and heating, the sour stalk (pH 3.0-3.6) tastes sweet. Therefore, it is a popular ingredient of pies and tarts. Hence, rhubarb is commonly called a pie vegetable (Clementi and Misiti, 2010). While rhubarb leaf contains a large amount of oxalic acid and is not edible, it is commonly used as a natural pesticide (Pucher et al., 1938). Unlike medicinal rhubarb (da huang), which has been studied extensively, studies on the physiological and chemical activities of edible rhubarb are lacking.
Free radicals generated during normal metabolic processes in the body are highly reactive and easily oxidize the surrounding substances. Oxygen compounds derived from this process (for example, H2O2, OḢ ·, OH-, and O2-) are called reactive oxygen species (ROS) (Liou and Storz, 2010). A moderate amount of ROS prevents pathogen infection and facilitates intracellular signaling (Valko et al., 2006). However, when ROS overwhelm the cellular antioxidant defenses, oxidative stress ensues (Charde et al., 2012), which results in oxidative and tissue damage, with cell destruction. This potentially harmful effect of ROS limits cellular antioxidant activity, enhancing cell aging and inflammation, and increasing the incidence of various cancers (Aruoma, 1998).
Inflammation is a local defense mechanism that protects the body from physicochemical assault, such as bacteria and foreign substances, and involves various biochemical phenomena (Lordan et al., 2019). Macrophages are important immune control cells that are involved in the detection, phagocytosis, and destruction of bacteria and other harmful organisms, and also in repair processes, such as muscle regeneration, wound healing, and others (Caputa et al., 2019; St Pierre and Tidball, 1994). Inflammation is initiated by the release of ROS such as NO, NO2, and NO3– (Brune et al., 2003; Qian and Pollard, 2010). NO, an indicator of macrophage activation, is a free radical formed from l-arginine by NO synthase (NOS) (Rosenfeld et al., 2012). Upon excessive accumulation, reactive forms of nitrogen, such as NO, act as an oxidative trigger in the body, causing inflammatory reactions and cell damage (Sharma et al., 2007). Inflammatory cytokines and lipopolysaccharides promote the activation of nuclear factor-kB, which enhances the expression of inflammatory reaction-induced genes, such as inducible NOS (iNOS), and cyclooxygenase 2 (COX2) (Posadas et al., 2000). When NO reacts with ROS, it becomes a powerful oxidizer, causing various diseases, such as diabetes (Assmann et al., 2016), atherosclerosis (Forstermann, 2010), stomach cancer (Cheng and Fan, 2013), and uterine cancer (Elsehemy et al., 2016). There is substantial interest in the different effects of ROS in the human body, and physiologically active substances that affect ROS generation and detoxification.
In the current study, we evaluated the antioxidant properties of the leaf, stalk, and root extracts of edible rhubarb (R. rhabarbarum). To investigate its potential as a medicinal material, we compared the antioxidant and anti-inflammatory properties of these extracts with those of da huang root extracts as a control. We show that edible rhubarb root has strong free-radical scavenging activity, equivalent to that of da huang root extract. It also reduces NO production by activated RAW 264.7 macrophages, indicating a promising anti-inflammatory activity. Hence, the potential use of edible rhubarb as a functional material should be investigated further.
2. Materials and methods
Edible rhubarb (R. rhabarbarum) was grown in 30-cm pots in commercial soil with pearlite in a greenhouse at the College of Applied Biotechnology Science, Yeungnam University (Gyeongsan, Korea). Three-year-old rhubarb was harvested, cleaned, and separated into roots, stalks, and leaves. Each part was individually squeezed to produce juice using a juice extractor (ZN35, Tefal, Rumilly, France) at room temperature (RT). The juice was purified using filter paper (No. 2, Advantec, Tokyo, Japan) and frozen at −20°C. Frozen juice was then individually freeze–dried to obtain a powder, for 48 h, using a freeze-dryer (FDS8508, Ilshinbiobase, Yangju, Korea). Dried Chinese da huang root was imported from China by Herb pharm (www.herb-pharm.co.kr) and dried Korean da huang (Dong Il, Yeong Cheon, Korea) was obtained from Hwa sang dang store. Then, 3-5 g of dried roots were ground using a grinder (HR3752, Philips, Amsterdam, Netherlands) and then made into an even finer powder using liquid nitrogen. Next, 200 mg of individual samples were extracted with 70% EtOH in a shaking incubator (VS-203D, Vison Scientific Co., Daejeon, Korea) for 24 h at RT. The extracted samples were filtered with Whatman® filter papers (No. 2) and condensed using a rotary evaporator (EYEL4, Rikokai, Tokyo, Japan). The concentration of extract stocks was 50 mg/mL in 70% EtOH.
Free-radical scavenging activity assay was performed using the method developed by Lee and Moon (2019). 2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) was from Wako Pure Chemical Ind. Ltd. (Osaka, Japan); potassium persulfate and dimethyl sulfoxide (DMSO) were from Sigma-Aldrich (St. Louis, MO, USA); and EtOH was from Merck (Darmstadt, Germany). For the assay, 7 mM of ABTS was dissolved in sterilized H2O and potassium persulfate was added for a 2.45 mM solution. The solution was stored at RT for 16 h in the dark to generate ABTS free radicals. The ABTS+ stock was wrapped with aluminum foil and stored at −20°C. Immediately prior to the actual experiment, the ABTS+ stock was diluted using sterilized H2O for an optical density (OD) value of 0.6 at 734 nm. When ABTS reacts with potassium persulfate, it is oxidized to turquoise-colored free radicals (ABTS+), which can be converted to ABTS by antioxidants. The prepared rhubarb extracts amples (50 mL) were placed in wells of a 96-well plate (3680, Corning Inc., Corning, NY, USA). Then, 50 mL of ABTS+ stock was added and allowed to react for 5 min at RT. Next, sample OD was measured at 734 nm using a microplatereader (UVM340, Biochrom, Cambridge, UK). ABTS+ scavenging ability was determined using the following equation:
S: sample (sample / ABTS+)
SC: control (H2O / ABTS+)
BS: blank of sample (sample / H2O)
BC: blank of control (H2O / H2O)
RAW 264.7 macrophages were obtained from the National Institute for Korean Medicine Development (NIKOM; Gyeongsan, Korea). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Cytiva, Marlborough, MA, USA) containing 10% fetal bovine serum (FBS; HyClone, Cytiva) and 1% penicillin–streptomycin (Welgene, Gyeongsan, Korea), in a T25 flask (CytoOne T25 TC flask, USA Scientific, Ocala, FL, USA). The cells were incubated at 37°C in a 5% CO2 incubator (MCO-17AIC, Sanyo, Osaka, Japan). When they reached 70-80% confluency, they were collected using a cell scraper (Celltreat, Pepperell, MA, USA). The collected cells were mixed with 10 mL of trypan blue (Sigma-Aldrich) at a 1:1 ratio and stained for 5 min. The cells were counted using a hemocytometer. Different concentrations of macrophages were prepared in DMEM for further experiments, as described.
NO production by RAW 264.7 macrophages was measured using a modified Griess assay (Kim et al., 2013). The cells were placed in a 96-well cell plate (cell culture plate, Thermo Fisher Scientific, Waltham, MA, USA) and diluted in DMEM to 5×104 cells well. Each well containing 160 mL of cells was incubated in a 5% CO2 incubator at 37°C for 24 h, and then subcultured in fresh DMEM. The cells were treated with 20 mL of 1 mg/mL LPS (Sigma-Aldrich) for 6 h to activate them. Induced cells were treated with 20 mL of each rhubarb extract and incubated in a 5% CO2 incubator at 37°C for 12 h. Next, 100 mL of the cell solution were transferred to a new 96-well plate for the Griess assay (Promega, Madison, WI, USA). Cells were treated with 50 mL of 1% sulfanilamide solution (in 5% phosphoric acid) for 5 min in the dark, followed by the addition of 50 mL of 0.1% N-1-naphthyl-ethylene-diamine dihydrochloride (NED) solution for an additional 15 min. Sample absorbance was read at 540 nm using a microplate reader (Epoch, BioTek, Winooski, VT, USA). The NO production in each sample was normalized by the absorbance value of LPS-treated cells as a control. The readings with the Korean rhubarb root sample were used as a reference.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay is routinely used to test cell viability, cell proliferation, or cytotoxicity. However, it has several shortcomings, such as cell loss during washing and harvesting, and the utilization of DMSO, which is toxic to eukaryotic cells. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay compensates for these shortcomings and can be used to measure cell viability more accurately than MTT assay, using a colored formazan dye that is soluble in cell culture media. Accordingly, MTS assay was conducted using the CellTiter 96® AQueous One kit (G3580, Promega). RAW 264.7 macrophages (180 mL) were dispensed in a 96-well microplate (1×104 cells well). The macrophages were stabilized at 37°C for 24 h in a 5% CO2 incubator. Then, 20 mL of rhubarb extract were added per well, and the cells incubated for additional 16 h. Next, 10 mL of MTS were added per well and the incubation continued for 3 h to allow red formazan formation. Sample absorbance was measured using a microplate reader (Epoch, BioTek) at 490 nm. To investigate cell toxicity of the rhubarb extracts, cell viability was also determined without extract addition. The readings with the Korean rhubarb root sample were used as a reference.
All experiments were conducted in triplicate. Statistical analysis was performed using IBM SPSS Statistics version 21.0 (IBM, New York, NY, USA). One-way analysis of variance (ANOVA) was used, followed by Duncan’s multiple range test for multiple comparisons. Differences were considered significant at p⟨0.05.
3. Results and discussion
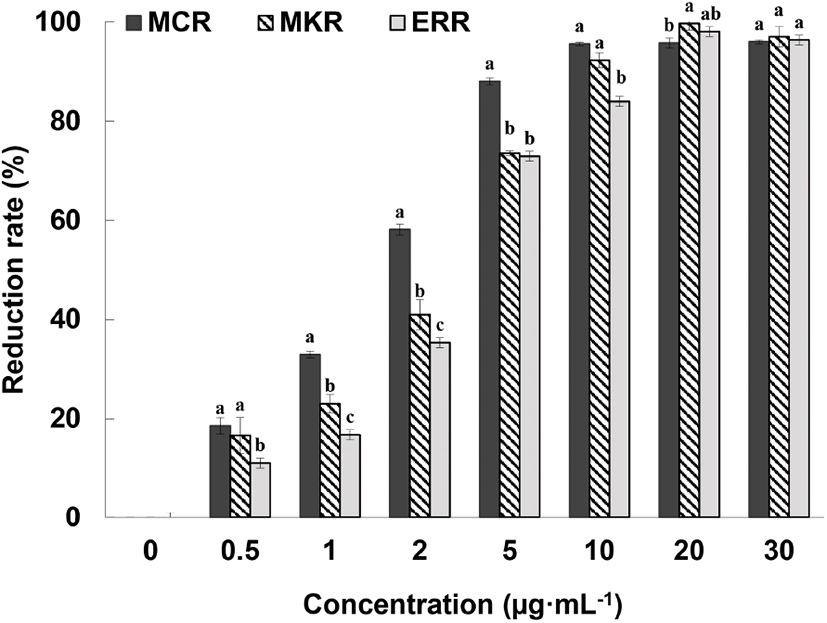
The ABTS+-scavenging ability of different concentrations of medicinal Chinese da huang root (MCR), medicinal Korean da huang root (MKR), and edible rhubarb root (ERR) in 70% ethanol extract is shown in Fig. 1. At relatively low concentrations (0.5-5 mg/mL), the free radical reduction rate of MCR was significantly higher than that of MKR and ERR (p⟨0.05). However, no statistical difference in the free-radical scavenging capacities of MCR and MKR was noted at a concentration of 10 mg/mL. We were unable to unambiguously conclude that the free-radical scavenging ability of MCR is higher than that of MKR as the age of plants used for the preparation of MCR and MKR was unspecified by the manufacturer. At concentrations of 20 mg/mL and above, MCR and MKR both demonstrated 100% reduction of free radicals, thus confirming the lack of statistically significant difference between the samples. In addition, the free-radical reduction rate of ERR was the same as that of MCR and MKR, indicating high antioxidant capacity of ERR.
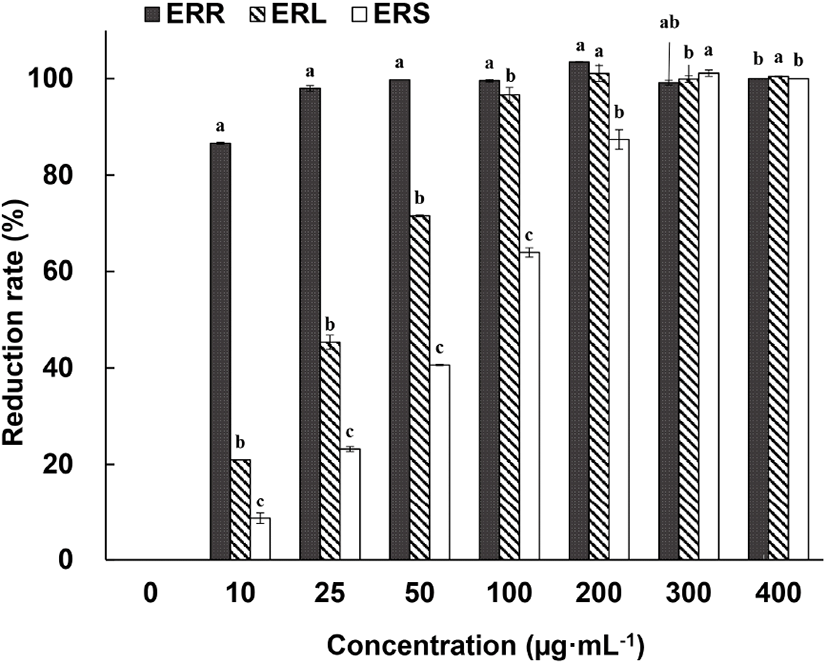
The free-radical scavenging ability of 70% EtOH extracts of ERR, edible rhubarb leaf (ERL), and edible rhubarb stalk (ERS) is shown in Fig. 2. ERR showed 100% ABTS+ reduction ability at concentrations of 25 mg/mL and above, whereas ERL and ERS showed 100% ABTS+ reduction rate at concentrations above 200 mg/mL and 300 mg/mL, respectively. Using DPPH assay, Raudsepp et al. (2013) reported that the antioxidant activity depends on the extraction solvent used, with the highest activity for blue honeysuckle berries extracted with buffered water and Siberian rhubarb (R. rhaponticum) stalk in 30% EtOH and rhubarb roots showed the highest antibacterial effect. Ozturk et al. (2007) used the DPPH assay to test edible rhubarb (R. ribes) consumed in Turkey. In that study, methanol extracts of the root and stalk were tested, at a concentration of 100 mg/mL. In the cited study, the free-radical scavenging activity of the stalk extract (87.1%) was higher than that of the root extract (60.1%) (p⟨0.05), which differs from the observations described in the current study. This difference can be explained by the findings of Komatsu et al. (2006), who noted several differences in the active ingredients of rhubarb associated with the differences in cultivation area and genetic differences. We propose that the differences noted herein could be explained by the different subspecies tested (R. rhabarbarum vs. R. ribes), or extraction solvents used (ethanol vs. methanol).
As shown in Fig. 3(A), MKR suppressed NO production by activated macrophages to 35% that of the positive control at a concentration of 30 mg/mL, and ERR suppressed NO production to 38% at a concentration of 40 mg/mL. These results are encouraging, as both MKR and ERR suppressed NO production to the negative control level. When activated RAW 264.7 macrophages were exposed to 70% EtOH extracts of ERL and ERS, we observed suppression of NO production at a concentration of 100 mg/mL and above, with the suppression efficiency depending on the extract concentration (Fig. 3(B)). Collectively, these experiments revealed similar suppression level of NO production in activated RAW 264.7 macrophages by extracts of all edible rhubarb parts, and also demonstrated high free-radical scavenging ability of these extracts. Choi et al. (2014) reported that stilbene derivatives in 70% EtOH extract of MKR (R. undulatum) down regulate the expression of iNOS in RAW 264.7 cells with an enhanced anti-inflammatory effect. Accordingly, we propose that the ERR extract contains phenolic compounds or derivatives, and that these compounds inhibit NO production and, consequently, support the anti-inflammatory effect of the extract.
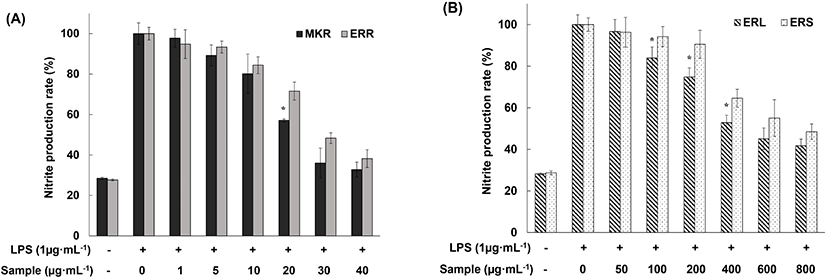
As described above, at the phytochemical level, 70% EtOH extracts of ERR, ERL, and ERS showed high antioxidant activity. Next, to confirm their anti-inflammatory effect at the cellular level, we investigated their ability to inhibit NO production by LPS-activated RAW 264.7 macrophages. LPS is a known toxin produced by gram-negative bacteria that induces inflammatory responses in cells. In the current study, 100% NO production in positive control (LPS added) was detected indicating an inflammatory induction, while 30% NO production was detected in negative control (no LPS added).
In this study, we verified that edible rhubarb root (ERR) has the same, high, antioxidant properties and the ability to inhibit NO production by activated macrophages as the roots of medicinal rhubarb (da huang) used in Korea (MKR) and China (MCR). We also confirmed that the extracts of each part of edible rhubarb exert anti-inflammatory effect on activated macrophages most likely by reducing the production of inflammatory cytokines and various inflammatory mediators that initiate the inflammatory response.
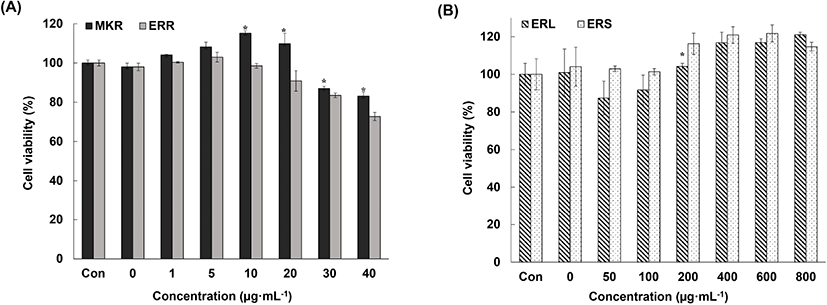
To check whether the decrease in NO production by activated macrophages observed upon exposure to rhubarb extracts was associated with cell death caused by toxins present in the extracts or the antioxidant activity of the extracts, we analyzed cell viability using the MTS assay.
We observed 85% cell viability upon exposure to 70% EtOH extracts of MKR and ERR at concentrations of 30 mg/mL and lower (Fig. 4(A)). This demonstrated that these extracts are not cytotoxic at certain concentrations, and that the inhibition of NO production by activated macrophages was associated with the anti-inflammatory effects of these extracts but not with cytotoxicity. In addition, we observed over 90% cell viability upon exposure to 70% EtOH extracts of ERL and ERS at concentrations of 100 μg/mL and lower (Fig. 4(B)). Cell proliferation was assessed as the concentration of the extracts increased.
4. Conclusions
Even though many studies related to rhubarb have been published, most of them focus on da huang (R. officinale), used as an oriental medicinal herb, and not on R. rhabarbarum. Since edible parts of these plants are different, i.e., roots for da huang and stalks for edible rhubarb, we compared specific activities of the extracts of these different parts to examine the potential of their usage. We showed that the free-radical scavenging activity of the root of edible rhubarb is pronounced and as high as that of da huang root. In addition, edible rhubarb extracts dramatically reduced NO production by activated macrophages, indicating a good anti-inflammatory effect. Hence, the edible rhubarb could potentially become a new vegetable crop in Korea. In addition, leftover (leaves and stalks) from da huang root harvest could be used as potential functional materials.
