Introduction
The demand of natural food constituents derived from animal or plan origin has escalated on global scale owing to increased awareness level in health-conscious people (Earnest et al., 2015). Among historically used medicines of animal-origin, velvet antler (VA) usage could be traced back to ancient times of more than 2,000 years ago and exhibit several pharmacological benefits for immune, cardiovascular, nervous and reproductive systems and are used frequently to develop functional foods, nutraceutical supplements and other medicinal products (Jang et al., 2020; Zhao et al., 2016). Bioactive compounds from animal sources include essential oils, vitamins, minerals, dietary fiber, peptides, protaglandinds, glycoaminoglycans, proteins and fatty acids (Cheng et al., 2017). All these bioactive compounds are widely employed in pharmaceutical sector to prepare medicines and supplements to mitigate lifestyle related disorders (Sui et al., 2014; Zhao et al., 2016).
Animals of Cervidae family like elk, deer, moose and caribou usually have VA in the form of whole cartilaginous antler at pre-calcified growth stage. Global supply of VA is usually met by antlers originated from Red deer, Sika deer, Alk and Wapiti (Elaphurus davidianus) (Huo et al., 2014). In historical recorded, Chinese traditional medicine has a detailed account of medicinal uses of VA with name of Pinyin Lu Rong for treating deficiency syndromes and most notable deer species in this regard include Red deer and Sika deer (Zhao et al., 2016). In recent years, VA’s sourced from Red and Sika deer have become increasingly popular in China, Korea and Japan in terms of supplements usage for disease prevention (Jeon et al., 2009). VA usually consumed orally and according to recently published reports, the total production of VA on global scale was 1,300/year and demand is increasing gradually every year. Hence, the researchers are actively exploring new bioactive compounds from VA and VA extraction methods and its properties (Huo et al., 2014; Zhao et al., 2016).
VA product is usually sold as nutraceutical in encapsulated form through health food stores and pharmacies. Mostly, the pharmacies and health food stores set their own quality standards for VA products. In this regard, product safety concerns include bacterial contamination (E. coli, Salmonella spp.) or physical contamination like dander (Huo et al., 2014). Consistent VA product safety and quality parameters increase processor reputation and allows to get market penetration. Moreover, VA product safety is also an important aspect of good manufacturing practices in processing plants (Cooney, 2001). Siliac acid is categorized as an acidic sugar and is member of family of derivative compounds and most commonly also known as N-acetylneuraminic acid. Sialic acids are widely found in distributive manner in animal tissues, mostly in glycoproteins and gangliosides (Dhar et al., 2019). In terms of glycobiology, the sialic acid forms glycan chains and plays a vital role on cell surfaces and soluble proteins and is involved in tissues structural configuration and modulation of variety of normal physiological processes (Varki, 2008). Similalry, uronic acid is also included in class of sugar acids. Uronic acid is widely distributed in liver and adipose tissues. In humans, uronic acid pathway is involved in glucose metabolism as an alternative oxidative pathway and catalyzes conversion of glucose to glucuronic acid (Garron et al., 2010). Major function of uronic acid pathway is to synthesize D-glucuronic acid which has role in detoxification of foreign chemicals and synthesis of mucopolysaccharides (Zhao and Brasier, 2019).
Therefore, this study was aimed to analyze the proximate composition (moisture, crude protein, crude fat, crude ash, carbohydrate) of hot water VA extract and total yield and bioactive compounds (uronic and sialic acids) were determined at two different extraction temperatures (90 and 100°C) and extraction times (12, 24 and 36 h). Moreover, the amino acid profile of VA extract extracted at 24 h were evaluated at 90 and 100°C followed by identification of the microbiological profile of pathogenic bacteria in VA.
Materials and methods
VA of deer (Cervus canadensis Erxleben), raised in Korean local farm, was supplied by the Deer Cluster (Cheongju, Korea). Dried VA samples subjected to sectioning at thickness of 1-3 mm followed by grinding using a grinder into fine powder (160-180 mesh). Equal mixture of powder obtained from top, middle, and bottom sections of meshed VA was obtained, and was further used for extraction. Analytical grade chemicals were employed in this study.
VA and distilled water were added to a round-bottom flask and extracted with a reflux extractor was used for VA extraction as per described method (Ameer et al., 2017). No stirring bar was used. For extraction, an aliquot of VA mixture (5.625 g) was mixed with 1 L distilled water (DW) at specified extraction conditions of 90 and 100°C temperatures at extraction times of 12, 24 and 36 h at each temperature. After extraction, the extracts were filtered through a Whatman filter paper No. 4 and centrifuged at 4,005 ×g for 30 min. The VA extract was concentrated using a rotary evaporator (N-2110, Sunileyela, Sungnam, Korea), followed by freeze-drying (Ilsinbiobase, Dongduchen, Korea) and storage at −20°C prior to use.
The general proximate analysis procedures of moisture, crude protein, crude fat, crude ash, and carbohydrate were performed following the AOAC standard methods (AOAC, 1996). The moisture content was measured by drying at 105°C in a drying oven. The crude protein content was determined by semi-micro Kjeldahl method (AOAC, 1996).
The extraction yield was calculated by weighing the dried samples obtained after freeze-drying according to the Eq. 1 given below.
Sialic acid content was determined using the thiobarbituric acid (TBA) assay and N-acetylneuraminic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as a standard (Warren, 1959). An aliquot of 0.2 mL of VA extract was mixed with 0.1 mL periodate solution for 20 min at room temperature. After that, 1 mL of arsenite and 3.0 mL of TBA solutions were added and reacted at boiling temperature for 15 min followed by standing time of 5 min to cool down. An aliquot of 1 mL of the reaction mixture was added into 1 mL of cyclohexanone and centrifuged at 11,124 ×g for 3 min. The optical density of the supernatant at 549 nm was measured using a spectrophotometer (UVmini 1240, Shimadzu, Kyoto, Japan). Sialic acid of VA extract was also determined using a HPLC equipped with Phenomenex Luna C18 column (4.5 mm×250 mm, 5 μm pore size, Phenomenex Co., Torrance, CA, USA) and multi fluorescence detector (Waters 2475, Minford, MA, USA). The detailed HPLC conditions for sialic acid determination are presented in Table 1.
Uronic acid content was determined by carbazole reaction using a 96-well plate according to the described method of Cesaretti et al. (2003). The sample was added into a 96-well plate followed by addition of sulfuric acid into the well. The plate was placed in an oven at 100°C for 10 min and cooled down at room temperature for 5 min. Afterwards carbazole solution (0.125%) was added and the plate was placed in an oven at 100°C for 10 min, and cooled down at room temperature for 15 min. The optical density at 550 nm was measured using a spectrophotomer (UVmini 1240, Shimadzu).
Approximately 0.1 g of 5% trichloroacetic acid was added to freeze-dried VA extracts and diluted 10 times using distilled water (Jeon et al., 2009). Then, the supernatant was collected by centrifugation at 11,124 ×g for 10 min, and filtered through a membrane filter (0.45 μm). The amino acid analysis of the filtrate was performed using an amino acid analyzer (L-8900, Hitachi, Tokyo, Japan).
VA extract sample (25 g) was aseptically weighed into filtered stomacher bags and homogenized with 225 mL (0.1% w/v) peptone water for 3 min. Samples were then serially diluted and 1 mL of dilution was incorporated into blood agar plate (BAP) followed by incubation at 37°C for 10-26 h (Payment et al., 1994). All isolates were separated based on their colony formation, time and color. Separated colony was further purified by streak plating. Identification was performed by analyzing the 16S rRNA sequence and detecting the homology of the nucleotide sequences in BLAST of National Center for Biotechnology Information (NCBI) (Mignard and Flandrois, 2006).
Results and discussion
Mostly, the pharmacies and health food stores set their own quality standards for VA products and chemical composition analysis allows to elucidate dynamic compositional and quantitative changes in VA extract for standardization (Cooney, 2001). Proximate composition of VA extract was analyzed and results are given in Table 2. Overall, the extracts recovered at 100°C temperature showed higher proximate parameters as compared to extracts recovered at 90°C temperature and these parameters were as: moisture (4.66%), crude protein (86.63%), crude fat (3.23%), crude ash (4.1%) and carbohydrate content (5.43%). Actually, the crude fat content exhibited increasing tendency with corresponding rises in extraction temperature and time. This can be possibly attributed to the fact of increased mass transfer of solutes from matrix of VA mixture due to rises in extraction temperature (Ameer et al., 2017). Elevated temperatures have been reported to accelerate mass transfer of solutes from samples matrices irrespective of their origin. Moreover, reflux extraction at elevated temperature of 100°C resulted in higher amounts of crude fat due to water absorption resulting in dilution which consequently accelerated mass transfer rate (Onyeike and Oguike, 2003). These findings are in agreement with the previous findings of Jeon et al. (2009) who inferred that bone composition of antler may vary depending on specific antler farming region and weight specifications of the antler from that region.
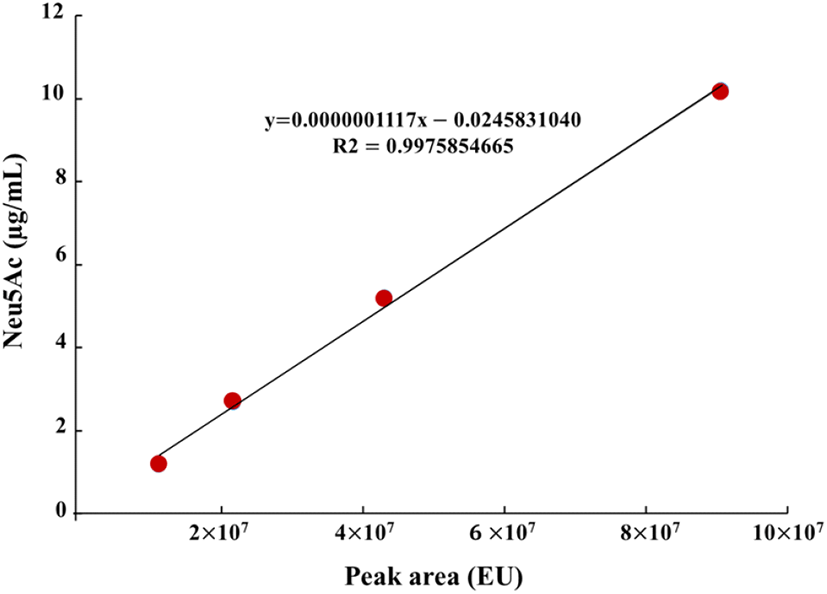
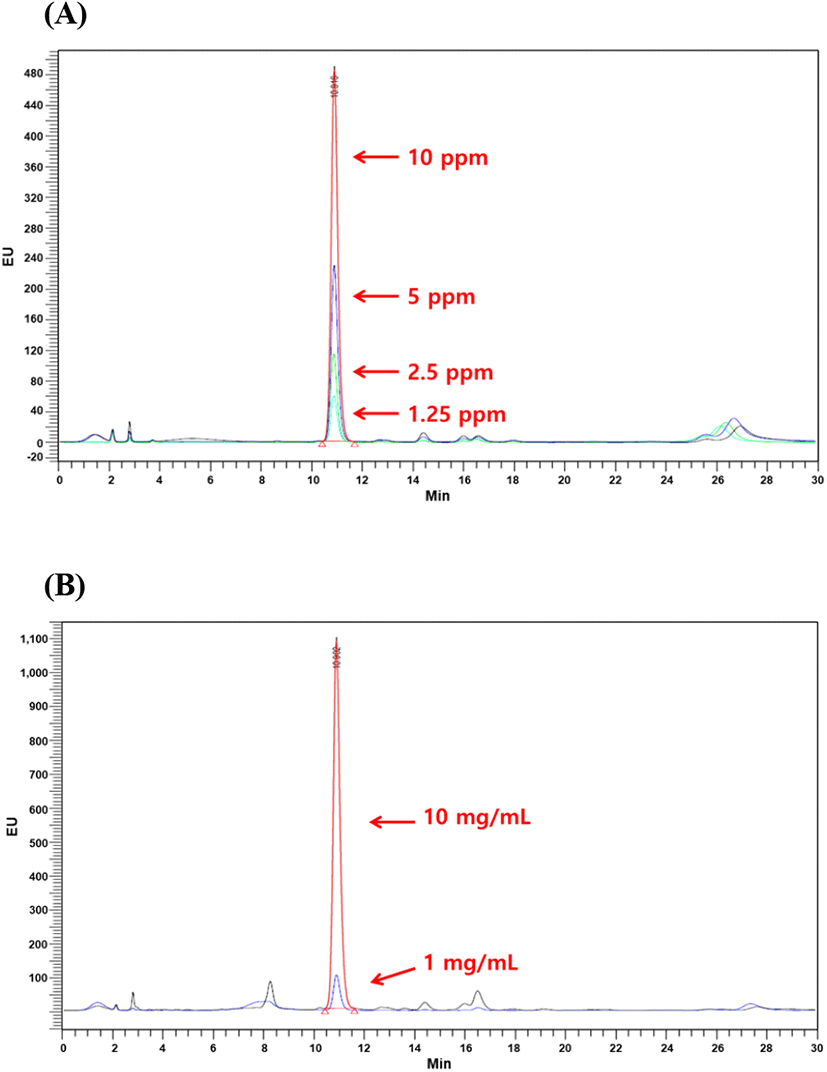
Sialic and uronic acids are found as glycosaminoglycans in VA extract (Huo et al., 2014). Therefore, the determination of sialic and uronic acids as biomarker may provide useful basic information for the association between structural properties and chemical composition during antler growth period, which should facilitate efficient production of high-quality antlers for food consumption and as pharmaceutical agents (Cooney, 2001). In this study, single factor experiments were carried out by keeping one variable constant and varying the second at different levels to determine the individual effects of variables on target responses (yield, uronic and sialic acid contents). The results tabulated in Table 3 showed that yield exhibited linear increases with corresponding rises in extraction time. At 90°C and 100°C extraction temperatures, the highest VA extract yield was 37.07% and 39.97%, respectively at 36 h of extraction time. In case of uronic acid content, VA extract obtained at 90°C temperature showed linear rises and maximum uronic acid content at 36 h was 28.06 mg/g, while uronic acid content decreased at 36 h of extraction time and 100°C temperature. At 100°C, maximum uronic acid content was 22.68 mg/g at 24 h of extraction time while increase in extraction time resulted in decline of uronic acid content. Standard curve for sialic acid (N-acetylneuraminic acid) is shown in Fig. 1 which exhibited coefficient of regression of 99.75%. HPLC chromatograms of selected concentrations of sialic acid standards and selected concentrations of velvet antler extract are shown in Fig. 2. Likewise, similar to uronic acid content, sialic acid content showed the same tendency and maximum sialic acid contents were 0.56 mg/g and 0.73 mg/g at 90 and 100°C temperatures, respectively. Conclusively in comparison with 90°C extraction temperature at 24 h, the best extraction conditions were extraction temperature of 100°C at 24 h of extraction time. These extraction conditions led to recovery of higher yield (39.29%), uronic acid content (22.68 mg/g), and sialic acid content (0.73 mg/g). Similar results have been reported by Jin et al. (2015) regarding hot water extraction of bioactive compounds from middle parts of deer antlers originated from New Zealand. Hot water extraction showed comparable extract yield (6.72%) and sialic and uronic acids contents were 0.11 mg/g and 0.75 mg/g of solid Optimal conditions for HE were 90°C for 20 h with a solvent ratio of 1:29.34. Temperature played the pivotal role in case of extraction yield followed by solvent ratio which generally exerted significant effect on recovery of sialic and uronic acids. Similarly, in another report by Sunwoo et al. (1997), sialic and uronic acids yields were 0.16% (w/w) and 0.35% (w/w), respectively obtained from growing antlers of wapiti (Cervus elaphus).
Amino acid content from two different VA extracts are shown in Table 4. Arginine, aspartic acid and serine were not detected in VA extract obtained from both extracts at 90°C and 100°C temperatures at extraction time of 24 h. Total amino acid content was found to be higher in the extract recovered at 90°C (22.84 mg/g) as compared to 100°C extract (8.48 mg/g). Alanine, glycine, leucine, phenylalanine, hydroxyproline and valine were the predominant amino acids among all VA extracts and these accounted for the 33-37% of the total amino acid content. This can be attributed to the presence of collagen in large amounts in VA structural matrix. It was evident that 90°C temperature led to the increased recovery of the hydroxyproline in comparison with extraction temperature of 100°C. In case of proline, 90°C temperature resulted in higher content in VA extract (0.28 mg/g) in comparison with VA extract obtained at 100°C temperature (0.04). Kim et al. (2002) has reported that glutamic acid, proline, aspartic acid and glycine constituted the major proportion of amino acid profile of VA from both Elk and Sika deer. It was also implied that composition of the amino acid may be an indicative of quality of the VA and amino acid profile is influenced by the elongation, mineralization, growth period and section specificity of the VA.
Pathogenesis of human diseases may rise from the microbial colonization of various human organs and especially bacteria may proliferate from the natural products such as, extracts of plant or animal origins and infusions. Therefore, the bacterial flora need to be identified in the extracts obtained from both plants and animals to have overview of the pathogenic and non-pathogenic bacterial types (Barbour et al., 1997). As the VA extracts are usually employed for development of processed and functional foods/health promoting supplements, therefore, the bacteria may get introduced into the processing lines or through other food ingredients by coming into contact with extracts or infusions (Barbour et al., 1997). Keeping this in view, the VA extract was analyzed for pathogenic bacteria present in VA extract (Table 5). Among all bacteria, Chryseobacterium indologenes, Shigella flexneri ATCC 29903, Staphylococcus equorum, Staphylococcus xylosus, Vagococcus fluvialis and Staphylococcus succinus demonstrated 100% identities in the VA extract. The morphological status of C. indologene, S. flexneri ATCC 29903 and V. fluvialis was Bacilli, while Cocci was the morphology of S. equorum,S. succinus and S. xylosus. While other identified bacteria were in the order as given; Bacillus amyloliquefaciens, Escherichia fergusonii ATCC 35469, Klebsiella oxytoca ATCC 13182, Macrococcus caseolyticus ATCC 13548 and Staphylococcus warneri showed 99% identities from the VA extract followed by Mannheimia granulomatis ATCC 49244 (97% identity). The bacterial flora identified from the VA extract may serve as the reference database regarding microbiological assessment studies of the VA extract from deer. Furthermore, the identified bacterial types were characterized with respect to gram staining (either positive or negative) and morphology. As shown in Table 5, five bacteria including Chryseobacterium indologenes, Escherichia fergusonii ATCC 35469, Klebsiella oxytoca ATCC 13182, Mannheimia granulomatis ATCC 49244, and Shigella flexneri ATCC 29903 were idneified as gram (-) bacteria. Contrary, all other bacteria were identified as gram (+) bacteria. All gram (-) bacteria had rod shape whereas gram (+) bacteria consisted of cocci and rod shape. These analysis data will provide the microbiological profile of VA extract for using as a basic resource. Even though 6 species were identified as pathogenic bacteria in VA extract, no colony-forming ability from the entire range of identified bacterial species was observed in the VA extract. Kumar et al. (2014) have characterized the bacteria from extract of the Boerhaavia diffusa plant. Among all bacteria, Aeromonas hydrophila, Pseudomonas fluorescens and Flavobacterium branchiophilum were found to be pathogenic bacteria.
In conclusion, velvet antler (VA) has been known since ancient times for its pharmaceutical and health benefits in traditional and oriental medicine. Some of the prominent pharmaceutical functions include anti-inflammatory, anti-aging, anti-arthritis effects along with enhancement of the cognitive and brain activities. In this study, analysis of chemical components of VA extract (having pharmaceutical importance, such as amino acids, uronic and sialic acids) was carried out. Moreover, bacterial profile of VA extract was characterized. Results showed that after reflux extraction, the yield and contents of uronic acid and sialic acid of the VA extract were determined to be 39.29%, 22.68 mg/g and 0.73 mg/g, respectively at 100°C temperature and 24 h extraction time. The contents of moisture, crude protein, crude fat, and crude ash of VA extract were 4.26-4.66%, 85.32-86.63%, 1.55-3.23% and 3.19-4.17%, respectively. Alanine was the highest amino acid and 0.35 and 0.27 mg/g hydroxyproline was detected in 90°C and 100°C VA extracts, respectively. Bacterial characterization of VA extract was also performed to identify the existing bacteria. Twelve bacteria were isolated and identified in the VA extracts with 100% identities (Chryseobacterium indologenes, Shigella flexneri ATCC 29903, Staphylococcus equorum, Staphylococcus xylosus, Vagococcus fluvialis and Staphylococcus succinus), 99% identities (Bacillus amyloliquefaciens, Escherichia fergusonii ATCC 35469, Klebsiella oxytoca ATCC 13182, Macrococcus caseolyticus ATCC 13548 and Staphylococcus warneri), and 97% identify (Mannheimia granulomatis ATCC 49244). The morphological status of C. indologene, S. flexneri ATCC 29903 and V. fluvialis was Bacilli, while Cocci was the morphology of S. equorum,S. succinus and S. xylosus.
