Introduction
Taro (Colocasia esculenta L. Schott), a starchy tuber crop belonging to the Araceae family, is one of the most widely grown crops in the tropical and sub-tropical areas of the world (1,2). There are several different taro species including Alocasia macrorrhizos (giant taro), C. esculenta (true or ordinary taro), Cyrtosperma chamissonis (giant swamp taro), and Xanthosoma sagittifolium (tannia). Among them, the true taro (C. esculenta) is the most important species for agriculture and human consumption (3). The large underground corms of taro are especially one of the most popularly consumed staple foods in Asia, Africa, the Caribbean, Middle and South America and the Pacific Islands (2,4). In Fiji, taro is an important staple food and cash crop for Fijian communities (5).
The taro corm is a rich source of carbohydrates and fibers as well as small starch granules that play an essential part in its digestibility and the enhanced bioavailability of nutrients (6). In addition, taro corms are good sources of phosphorus, zinc, potassium, iron, magnesium, calcium, vitamins, flavonoids, carotenoids, and phenolic compounds, which play important roles in reducing the risk of cancer, cataracts, cardiovascular diseases, and gall bladder and inflammatory bowel diseases (6-8). In addition, the corms contain functional compounds including phenolic and flavonoid compounds such as cyanidin, delphinidin, isorhamnetin, kaempferol, myricetin, quercetin, chlorogenic acid, and p-coumaric acid (9). Baiao et al. reported (9) that quercetin is the major phenolic and flavonoid compound found in taro corm. These compounds provide several benefits such as antioxidant, antiallergic, anti-inflammatory, antibacterial activities, and reducing risk of type-2 diabetes (9).
Previous research using taro corms has focused on the identification of its nutritional compositions and health benefits (7,10). In addition, Kaushal et al. (7) reported the effects of extraction conditions (solvent types and processing methods) on the production of taro-based products. Melese and Negussie (6) found that the heat treatment of taro corms, such as steaming and boiling, reduces the anti-nutrients in taro corms such as oxalic acid, mucilage, tannins, cyanide, and protease inhibitors. In addition, Chumyam et al. (11) reported the effects of steaming on the antioxidant activities of vegetables. Although steaming provides beneficiary aspects in food processing and consumption to prevent browning and reduce anti-nutrients, there is still room for controversy on whether the steaming of taro corms enhance the functional compounds and antioxidant activity. In addition, no studies have been conducted to identify the effect of steaming on functional compounds (total phenolic content (TPC) and total flavonoid content (TFC) and antioxidant activity of Fiji taro corm. The goal of our research is to develop healthy taro snacks with Fijian taro corms. Thus, as a first step, the functional compounds and antioxidant activity of steamed Fijian taro corms were compared with those of un-steamed Fijian taro corms.
Materials and methods
Fijian taro (C. esculenta L. Schott) corms were provided by the Sigatoka Research Station (Nacocolevu, Fiji), and stored in a well-ventilated room at 4℃ with 85% RH before use. After the Fijian taro corms had been washed and peeled, the peeled corms were cut into 0.5-cm-thick slices using a stainless-steel knife, placed into a pot with a sieve, and steamed for 10 min. Alternatively Fijian taro corms were cut into small pieces without any exposure to heat. After the Fijian taro corms had been freeze-dried, 10 g of each freeze-dried steamed or un-steamed sample was mixed with 200 mL of filtered distilled water (FDW), 80% methanol, or 80% ethanol, The corms and solvent were then agitated at 180 rpm agitation for 48 h at room temperature. For the extract yields, the mixture was filtered with filter paper (Whatman Inc., Maidstone, Kent, UK) and evaporated by rotary evaporation (IKA Works Inc., Staufen, Germany). The extract yields for each solvent were calculated by subtracting the dried weight of the Fijian taro corm residues after extraction from the weight of the freeze-dried Fijian taro corm. The Fijian taro corm extracts were stored at 4℃ until further use.
TPC was determined by the Folin-Ciocalteu’ s colorimetric method, and gallic acid (0, 2.5, 5, 10, and 20 mg gallic acid equivalents/g) was used as a standard solution (12). An aliquot of 0.2 mL of each Fijian taro corm extract was mixed with 1.8 mL of FDW and 0.2 mL of Folin-Ciocalteu reagent and vortexed. After 6 min, 2 mL of 7% sodium carbonate solution (Daejung Chemicals & Metals Co., Siheung, Korea) was added to each mixture, which was then incubated for 90 min. The absorbance of the mixture was measured at 750 nm using a spectrophotometer (Mecasys Co., Daejeon, Korea). The average absorbance values obtained at different concentrations of gallic acid were used to plot the calibration curve. The TPC is expressed as milligrams gallic acid equivalents (mg GAE/g- freeze-dried Fijian taro corm sample).
TFC was determined using the aluminium chloride colorimetric method (13) with minor modifications. An aliquot of 0.5 mL Fijian taro extract was mixed with 2 mL of FDW and 150 μL of 5% sodium nitrite (NaNO2, Daejung Chemicals & Metals Co.). After 5 min, 150 μL of 10% aluminium chloride (AlCl3, Daejung Chemicals & Metals Co., Siheung-si, Korea) was added and the reaction mixture was incubated for 1 min at room temperature. After adding 1 mL of 1 N sodium hydroxide, the absorbance of the mixture was measured at 510 nm. Catechin at concentrations of 0, 2.5, 5, 10, and 20 mg catechin equivalents (CE)/g was used as a standard solution. The average absorbance values obtained at different concentrations of catechin were used to plot the calibration curve. The results are expressed as milligram catechin equivalents (mg CE/g freeze-dried Fijian taro corm sample).
The 2,2-diphenyl-1-picrylhydrazyl (DPPH)-free radical scavenging activities of the Fijian taro corm extracts were determined using Blois’s method (14) with minor modifications. An aliquot of 2 μL of the Fijian taro corm extract was added to a 198 μL methanolic solution of DPPH radicals (50 μM DPPH) and left to stand in the dark for 10 min at room temperature. Then, the absorbance was measured at 517 nm. The DPPH content is expressed as the percentage inhibition.
The ferric reducing antioxidant power (FRAP) activity of the sample extract was determined following the method of Benzie and Strain (15) with minor modifications. A FRAP solution was prepared by mixing 50 mL of 0.3 M acetate buffer (3.1 g sodium acetate (C2H3NaO2) and 16 mL acetic acid (C2H4O2), pH 3.6), 5 mL of 10 mM 2,2,6-tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl, and 5 mL of 20 mM FeCl3·6H2O solution, and the mixture was warmed to 37℃ before use. An aliquot of 25 μL of each Fijian taro corm extract was mixed with 175 μL of FRAP reagent and left in the dark at room temperature for 30 min. Trolox (Sigma-Aldrich Co., St. Louis, MO, USA) was used as a standard solution, and the average absorbance values obtained at different concentrations of Trolox (0, 12.5, 25, 50, and 100 mM) were used to plot the calibration curve. The absorbance of the mixture was measured at 590 nm and the results are expressed as mM Trolox equivalent (mM TE).
The 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay was carried out according to the method described by Thaipong et al. (16). The ABTS radical cation (ABTS.+) solution was prepared by mixing the two stock solutions (7.4 mM ABTS.+ solution and 2.6 mM potassium persulfate solution) in equal quantities and left to react for 12 h at room temperature in the dark. The solution was then diluted by mixing ABTS.+ solution with ethanol to obtain an absorbance of 0.70±0.02 units at 595 nm using the spectrophotometer. An aliquot of 25 μL of each Fijian taro corm extract was allowed to react with 195 μL of the ABTS.+ solution for 20 min in the dark condition and the absorbance was measured at 517 nm. The ABTS content is expressed as the percentage inhibition.
All experiments were performed in triplicate and the data were expressed as mean±standard deviation (SD). The data analysis was performed using the GraphPad Instat 3 (GraphPad Software Inc., San Diego, California, USA). The comparison between the groups was performed using a Student’s t-test and one-way analysis of variance (ANOVA) and differences were considered statistically significant at p<0.05.
Results and discussion
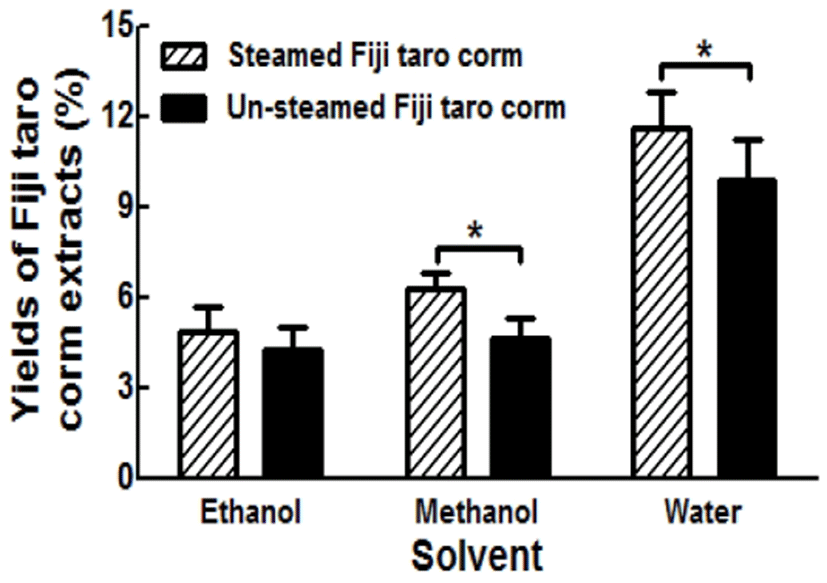
Because the polarity of the solvent could affect the taro corm extraction yields, three different solvents were used, and the percentage yield of each taro corm extract is presented in Fig. 1. As reported by Goli et al. (17), the extraction yield is dependent on the polarity of the solvent, as well as the extraction method, and the yields of steamed taro corms were greater than those of the un-steamed taro corms for all extractions. Further, as the polarity of the solvent increased (water > methanol > ethanol), the yields also increased. The steamed taro corm treated by aqueous extraction showed the greatest yield of 11.58±1.20%. In contrast, the un-steamed taro corm treated by ethanol extraction had the lowest yield of 4.24±0.77%. Statistically, the yields of steamed Fijian taro corm were significantly higher than those of un-steamed Fijian taro corm after methanol and ethanol extraction (p<0.05). The better yields of steamed taro corm over those of un-steamed taro corm in all extractions may be attributed to the hydrolysis of starch and softening effect of the steaming process, which result in the greater extraction of functional compounds and other nutrient compounds (7,18).
The TPCs and TFCs of steamed and un-steamed Fijian taro corm extracts are expressed in terms of gallic acid equivalents (linear regression: y = 0.0512x+0.0634, r2 = 0.99) and catechin equivalents (linear regression: y = 0.0379x+0.0366, r2 = 0.99), respectively. As shown in Table 1, the steamed Fijian taro corms exhibited significantly greater TPCs than the un-steamed Fijian taro corms after both ethanol and methanol extraction (p<0.05). However, there were no significant differences in the TPCs of the ethanol and methanol extracts for both the steamed and un-steamed taro corms, although the methanol extract of steamed Fijian taro corm showed the greatest TPC (42.77±3.39 mg GAE/g dry weight). In contrast, there were no significant differences observed in the TFCs after the extraction of the steamed and un-steamed taro corms with ethanol and methanol, although the methanol extract of steamed Fijian taro corm showed the greatest TPC (42.77±3.39 mg GAE/g dry weight). In addition, the TPC and TFC of the aqeous extract were significantly lower than those of the ethanol and methanol extracts, and no significant effects of steaming were observed (p<0.05). Overall, the steaming process increased the TPC after extraction with methanol and ethanol, whereas there was no significant change in the TFC when using water extraction. In addition, the TPC:TFC ratio is consistent with that reported by Smisek and El (2), which indicates that one-quarter of the taro TPC was TFC.
The TPC and TFC vary with extraction method and conditions, as well as the region of taro cultivation (climatic variations and soil conditions) (2). For example, the TPCs of Turkish (2), Japanese (19), Singaporean (20), and Fijian taro corms (21) were determined to be 187±53, 46, 180, and 12-39 mg GAE/100 g dry weight, respectively. With respect to the work of Jeon et al. (22), our TPC values (31.92-42.77 mg GAE/g dry weight) were greater than their TPC values obtained using aged black taro corms (20.61-28.20 mg GAE/g dry weight), even when using un-steamed taro corms. Overall, the TPCs of the Fijian taro corms were greater than those reported in previous studies, and steaming increased the TPCs in both the ethanol and methanol extracts.
The effect of steaming vegetables on the TPC and TFC is dependent on the type of vegetable. For example, the TPCs of eggplant, spinach, sweet corn, and pepper increase after three heating processes (steaming, boiling, and micro-waving) (11), but the TPCs of tomatoes, garlic, pungent peppers, and fresh-cut broccoli decrease, presumably because of the reduction of antioxidant capacity (11). The final goal of our project is to develop a healthy taro-based snack using steamed taro; thus, the positive effect of steaming on the TPC and corresponding increase in antioxidant activity (because TPC rather than TFC is associated with antioxidant activity) (11,18) is very promising.
The antioxidant activity of Fijian taro corm extracts were determined on the basis of the DPPH radical scavenging activity, FRAP activity, and ABTS radical scavenging activity (Table 2). In regards to the DPPH radical scavenging activity, the steamed Fijian taro corm extracted with methanol and ethanol exhibited excellent DPPH radical scavenging activities of 34.82±2.91% and 27.23±2.58%, respectively, which are significantly greater than those of the other treatments (p<0.05). Meanwhile, the DPPH radical scavenging activities of un-steamed Fijian taro corms were lower than those of steamed Fijian taro corms in all extracts. Our results are consistent with those of Saikia and Mahanta (23). In their study, the DPPH radical scavenging activities of banana blossom, cauliflower, beet root, green pea, tomato, and carrot were increased after steaming and ranged from 10.30±0.16% for green peas to 92.89±0.21% for banana blossom.
The FRAP results of steamed and un-steamed Fijian taro corm extracts are expressed in terms of Trolox equivalents (linear regression: y = 0.002x+0.1249, r2 = 0.98). The trend in FRAP activity is similar to that of DPPH activity; that is, the methanol-extracted steamed Fijian taro corm (339.08±20.50 mM TE) exhibited the greatest FRAP activity of all samples (p<0.05); in contrast, there was no significant difference in the FRAP activities observed between the water extracts of the steamed and un-steamed Fijian taro corm. In addition, the FRAP activity of the ethanol and methanol extracts of steamed and un-steamed Fijian taro corms were significantly greater than those of the aqueous extracts (p<0.05).
In regards to the ABTS activity, steamed Fijian taro corm extracted with methanol exhibited the greatest ABTS activity (56.34±3.54%) (p<0.05), followed by the ethanol-extracted steamed taro corm (46.86±2.38%). Furthermore, steamed Fijian taro corm showed better ABTS activity than the un-steamed Fijian taro corm. The resulting pattern of ABTS activity was similar to the DPPH radical scavenging activity results, which is consistent with the results of other studies (22,24,25) in that a greater ABTS activity led to an increase in DPPH activity.
Our results agree with those of previous studies (2,11,18) that have shown that antioxidant activity is mainly correlated with the TPC containing TFC. Moreover, most studies have found that DPPH and ABTS activities correlate with the TPC. We found that the steaming of Fijian taro corms resulted in softening, so the TPC and TFC extraction was increased, which led to an increase in the extraction of functional components and enhanced the total antioxidant activity. Thus, the positive effect of steaming on the TPC and antioxidant activity of Fijian taro corms will provide benefits for developing a healthy snack based on Fijian taro.
Conclusion
In this study, the effects of steaming Fijian taro corms on both their functional compound contents and antioxidant activities were investigated by comparison with un-steamed corms. The yield of the steamed Fijian taro corm extracted with water was the highest at 11.58%, while that of the un-steamed Fijian taro corm extracted with ethanol was the lowest at 4.24%. The TPC (42.77 mg GAE/g dry weight) and TFC (12.68 mg CE/g dry weight) of the steamed Fijian corms extracted with methanol were significantly greater than those of other extracts (p<0.05). In the same way, the methanol-extracted steamed Fijian taro corms exhibited significantly greatest activities in DPPH, FRAP, and ABTS (p<0.05). Hence, this study will contribute to the development of healthy functional snacks derived from steamed Fijian taro corms.
