Introduction
The immune system is essential for health and is a complex system of tissues, cells, and molecules that mediate resistance to infections (1). Immunostimulation is regarded as a potential strategy to enhance the body's defense mechanism, especially in elderly people and cancer patients. Therefore, the search for biomaterials that can activate and increase immune function has increased in the immunopharmacological and therapeutic research communities. In particular, the role of activated macrophages in defense against tumor cells has been investigated extensively. Activated macrophages has been shown to recognize and lyse tumor cells, including those that are resistant to chemotherapeutic drugs (2). Moreover, macrophages play an important role in regulating the innate and adaptive immune responses via the production of cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) (3,4).
Polysaccharides are macromolecules produced by most living organisms, including plants, animals, marine algae, mushrooms, microorganisms; therefore, they are also known as biomacromolecules and are widely distributed in nature (5). These polysaccharides are composed of monosaccharides linked together through glycosidic bonds. As well as serving as energy reserve and structural components, polysaccharides participate in signal recognition and cell-cell communication (6). In addition, polysaccharides have been shown to possess immunomodulatory activity through their ability to modulate macrophage function (7). As a result, polysaccharides can influence specific, non-specific, humoral, and cellular immunities (8). The fact that polysaccharides can profoundly affect the immune system, makes them immunomodulators with potentially broad clinical applications (9). Recently, because of relatively limited resources, many researchers have focused on searching for novel compounds with biological activity amongst the natural marine resources. Ascidians (commonly known as sea squirts) are known to contain many bioactive compounds with significant pharmaceutical and biomedical potential (10). Previous studies have revealed the abundance of bioactive substances present in ascidians, as well as their antioxidant, anticancer, and immunomodulatory activities (11-13). Styela plicata is a solitary, hermaphroditic ascidian species that is commonly found in ports, harbors and marina, usually in high density. S. plicata is considered an introduced species in many regions of the world (14,15). S. plicata extract has antigenotoxic and anticarcinogenic effects, minimizing colon carcinogenesis (16). Further, compounds isolated from S. plicata can activate phagocytosis and increase IL-2 secretion by mammalian peripheral blood mononuclear cells (17).
In the present study, we extracted polysaccharides from S. plicata and isolated three fractions (designated SF-1, SF-2, and SF-3). The analysis of the immunomodulatory effects on murine-derived macrophages demonstrated that the polysaccharides of S. plicata activate the production of nitric oxide and cytokines.
Materials and methods
S. plicata was purchased from a local supermarket (Daegu, Korea). The S. plicata was thoroughly washed with water and dried in hot air (50℃). The dried samples were milled using a Wiley-type cutting mill (J-NCM; Jisico Co., Seoul, South Korea), sieved (<0.5 mm), and stored at -20℃ before the extraction of the polysaccharides. The enzyme neutrase (0.8 AU/g) was donated by Novo Co. (Novozyme Nordisk, Bagsvaerd, Denmark). Dextran, pullulans, glucose, D-glucuronic acid, potassium sulfate, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals were of the highest grade available commercially.
The S. plicata powder (100 g) was suspended in 2 L of distilled water. Then, 2% of neutrase (enzyme to substrate ratio) was added. The hydrolytic reaction was performed for 15 h at 60℃. After centrifugation at 3,000 rpm for 30 min (Supra 30K; Hanil Science Industrial Co., Seoul, South Korea) to remove debris, the filtrate was concentrated in a rotary evaporator, and then precipitated with five volumes of 95% ethanol for 24 h at 4℃. The precipitate was acquired after centrifugation and dissolved in distilled water.
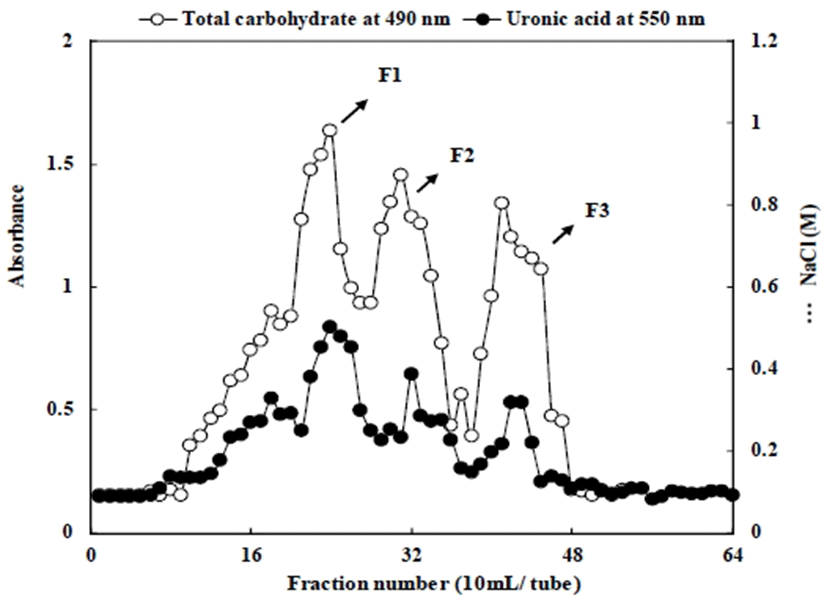
The crude polysaccharide (200 mg), denoted SP, was dissolved in distilled water (10 mL), and the solution was injected into a DEAE-Sepharose CL-6B column (GE Healthcare Bio-Science AB, Uppsala, Sweden). The polysaccharide was eluted with distilled water to obtain a non-adsorbed fraction and subsequently developed by stepwise NaCl gradient (0-1.0 M) to elute the polyanionic polysaccharides. The three fractions obtained by the chromatographic separation, denoted SF-1, SF-2, and SF-3, were dialyzed (molecular weight cut-off (MWCO) 14,000) against distilled water and lyophilized.
The total carbohydrate content of the polysaccharides was determined by the phenol-sulfuric acid method (18) using glucose as a standard. The uronic acid content was determined by the carbazole reaction (19) using D-glucuronic acid as a standard. The sulfate content was determined by the BaCl2-gelatinmethod (20) using potassium sulfate as a standard. Protein was determined by Lowry method (21) using BSA as a standard.
The average relative molecular mass of fractionated polysaccharides was estimated by size-exclusion high-performance liquid chromatography (HPLC, Dionex, Sunnyvale, CA, USA) using a Shodex OHpak column (SB-806HQ, 8.0×300 mm, Showa Denko Co., Tokyo, Japan). In this process, 10 μL of each sample (1%, w/v, in water) was injected, eluted with water at a flow rate of 0.8 mL/min at 60℃, and detected using an evaporative light scattering detector (ELSD, Alltech, Deerfield, IL, USA). The standards used were blue dextran (2,000 kDa) and pullulans (800, 400, 200, 100, and 20 kDa).
The monosaccharide composition of the crude polysaccharide and fractionated polysaccharides was determined using a HPLC system, which consisted of a pump (Waters 2695, Waters Co., Milford, MA, USA), a column (SUGAR SH1011, 8.0 mm×300 mm, Shodex, Showa Denko, Tokyo, Japan) and a refractive index detector (Waters 2414. Waters Co., Milford, MA, USA). After the hydrolysis of the polysaccharides (60 mg) in 6 N HCl at 100℃ for 4 h, HCl was removed by the evaporation with a dried stream of nitrogen. The hydrolysates (10 μL) were injected into the HPLC system, and 20 mM H2SO4 was used as amobile phase at a flow rate of 0.6 mL/min. The column temperature was maintained at 60℃. The following monosaccharides were used as references: mannose, galactose, glucose, glucosamine and galactosamine (Sigma-Aldrich Co.).
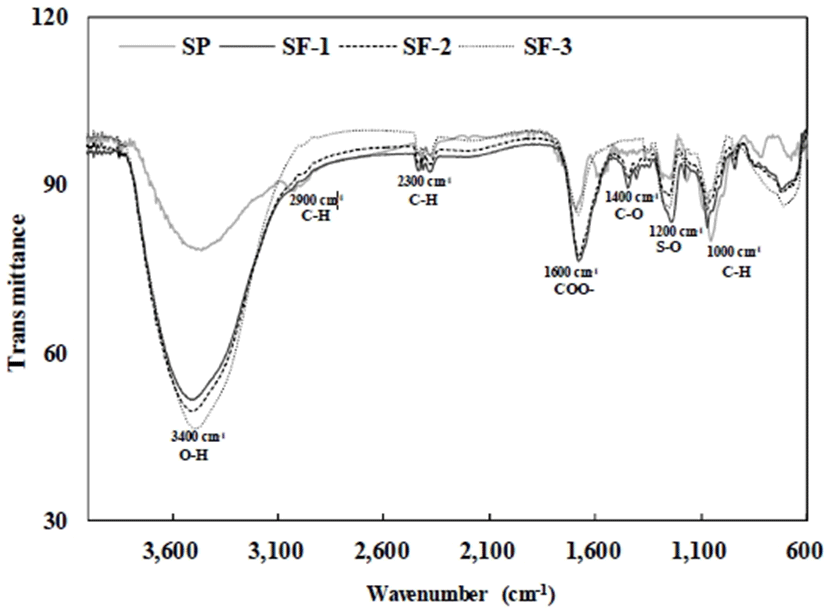
The crude polysaccharide and fractionated polysaccharides were pressed into KBr pellets. The FT-IR spectrum of polysaccharides was then obtained using a JASCO FT-IR spectrometer (300E; Jasco, Tokyo, Japan). The spectra were recorded at an absorbance mode from 4,000 cm-1 to 600 cm-1. The background was determined by using a blank KBr disk.
Murine macrophage cells (RAW 264.7 cells, Korean Cell Line Bank, Seoul, Korea) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, WELGENE Inc., Daegu, Korea) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; GIBCO, Rockville, MD, USA) at 37℃ in a humidified atmosphere with 5% CO2.
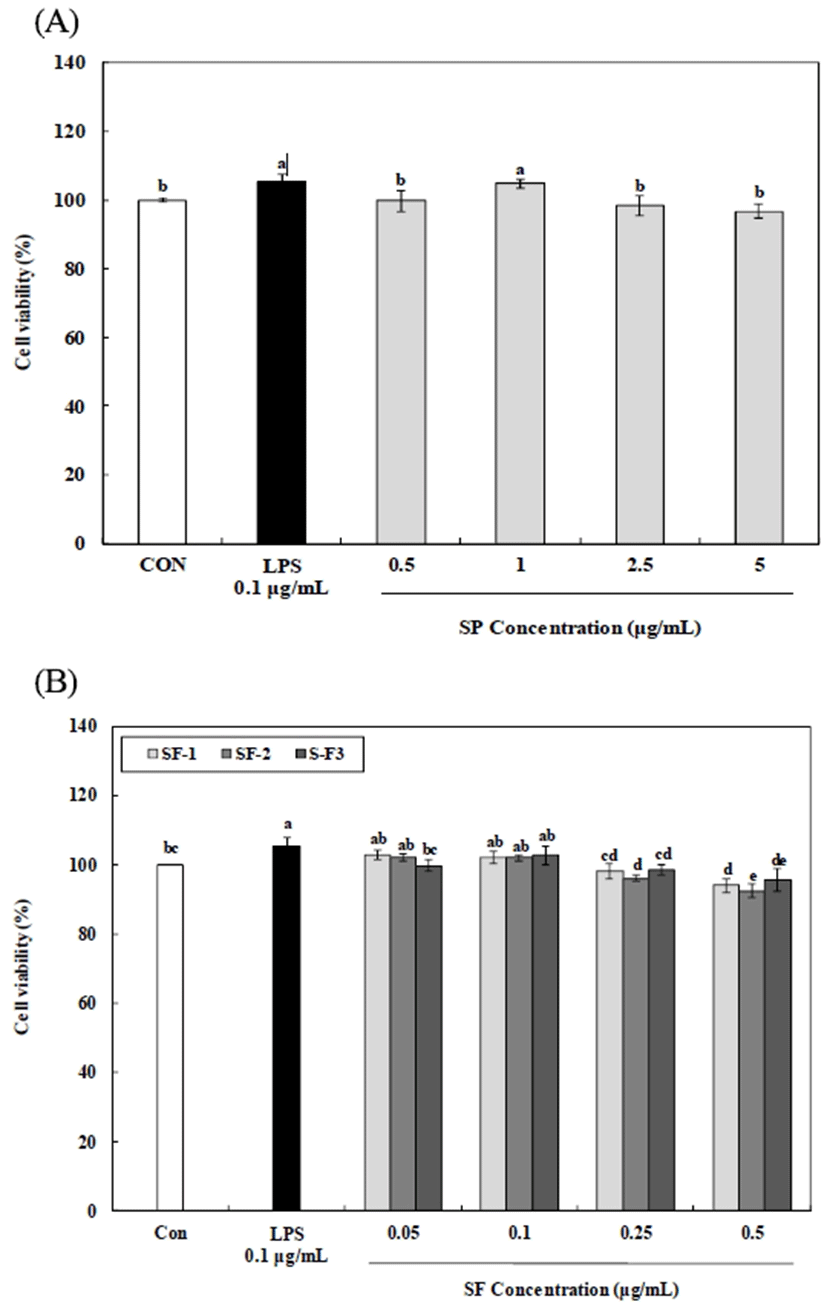
The MTT assay was used to evaluate the toxic effect of crude polysaccharide and fractionated polysaccharides on RAW 264.7. The RAW 264.7 cells in DMEM medium containing 10% FBS were plated in a 96-well microplate (5×104 cells/well in a volume of 100 μL) for 24 h and then cultured with different concentrations of crude polysaccharide (0.5, 1, 2.5, and 5 μg/mL), fractionated polysaccharides (0.05, 0.1, 0.25, and 0.5 μg/mL) or lipopolysaccharide (LPS, 0.1 μg/mL) for 24 h. After incubation, MTT was added to each well to a final concentration of 0.5 mg/mL. The plates were further incubated for 4 h, and the produced formazan crystals were dissolved in dimethyl sulfoxide (Junsei Chemical Co., Osaka, Japan). The absorbance (A) at 540 nm was measured using an enzyme linked immunosorbent assay (ELISA) reader (UVM-340, Biochrom Ltd., Cambridge, England). Proliferation was calculated as follows: activity (%) = (Asample/Acontrol)×100, where Asample and Acontrol are the absorbance of the sample and control, respectively.
Nitric oxide (NO) secretion from macrophages and the nitrite concentration was determined using the Griess test. RAW 264.7 cells were placed in a 96-well plate (5×104 cells/well in a volume of 100 μL) and incubated for 24 h at 37℃. The cultured cells were treated with 100 μL crude polysaccharide (5 μg/mL), fractionated polysaccharides (0.05, 0.1 and 0.25 μg/mL) or LPS (final concentration 0.1 μg/mL), which was used as a positive control. After incubation for 24 h at 37 ℃, 100 μL of the cultured cell supernatant was mixed with an equal volume of griess reagent (1% sulfanilamide, and 0.1%N-[1-naphthyl]ethylenediamine hydrochloride in distilled water) and left at room temperature for 10 min. The absorbance at 540 nm was measured using a micro plate reader. The NO secretion from RAW264.7 cells was calculated by reference to a standard curve obtained with NaNO2.
For cytokine determination, RAW 264.7 cells were cultured in the presence of crude polysaccharide (5 μg/mL), fractionated polysaccharides (0.05, 0.1, and 0.25 μg/mL) or LPS (final concentration 0.1 μg/mL). The cells were incubated for 24 h, and the supernatant was collected and used to determine cytokine production following stimulation by crude and fraction polysaccharides. The production of TNF-α and IL-6 was measured using commercial ELISA kits (Pepro tech Inc., Rocky Hill, NJ, USA).
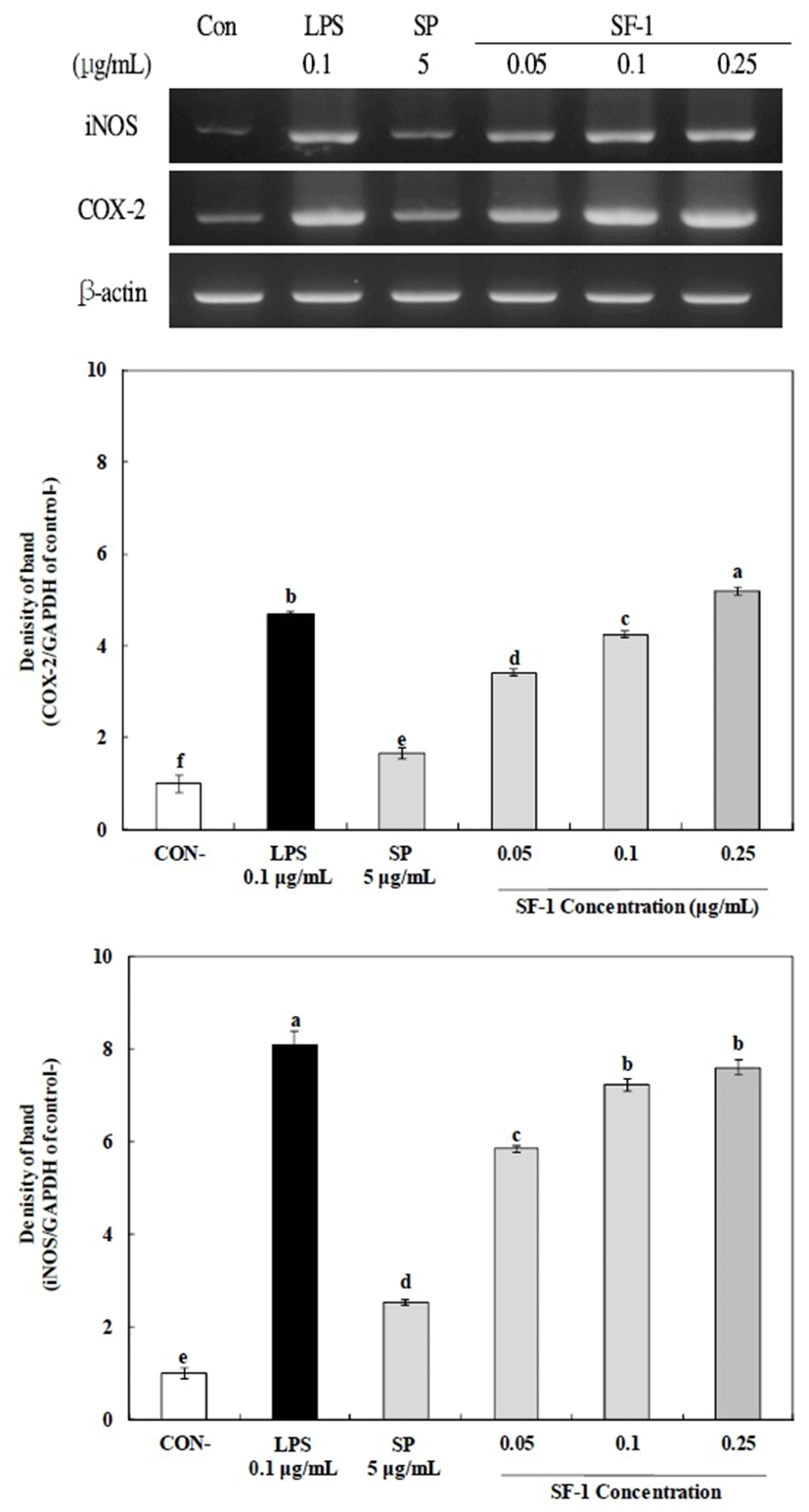
To evaluate the mRNA expression levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), total RNA from the crude polysaccharide and SF-1 (0.05, 0.1, and 0.25 μg/mL) or LPS (final concentration 0.1 μg/mL)-treated RAW 264.7 cells were prepared using a total RNA (1 μg) extraction kit (Qiagen., Hilden, Germany). The reverse transcription polymerase chain reaction (RT-PCR, Mastercycler® nexus, Eppendorf AG, Hamburg, Germany) was performed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Reverse transcriptase amplification was conducted with an initial denaturation (94℃ for 30 s), annealing (55℃ for 30 s), and extension (72℃ for 1 min), followed by a final extension step at 72 ℃ for 10 min. The products obtained by RT-PCR were separated by electrophoresis (Mupid-ex, Advance, Tokyo, Japan) using a 1.2% agarose gel and stained with Eco view (Dae myung Science Co., Seoul, Korea). The gels were then viewed under UV transillumination. The sequences of primers used in this experiment were: 5ʹCCCTTCCGAAGTTTCTGGCAGCAGC-3ʹ (forward), and 5ʹGGCTGTCAGAGCCTCGTGGCTTTGG-3ʹ (reverse) for iNOS,; 5ʹCACTACATCCTGACCCACTT-3ʹ (forward), and 5ʹATGCTCCTGCTTGAGTATGT-3ʹ (reverse) for COX-2,; 5ʹGTGGGCCGCCCTAGGCACCAG-3 (forward), and 5ʹGGAGGAAGAGGATGCGGCAGT-3ʹ (reverse) for β-Actin.
All experiments were carried out in triplicate, and means±standard deviation (SD) or average values are reported. Duncan’s multiple-range test was used to adjust for multiple comparisons, and null hypotheses were rejected at the 0.05 level. All data were analyzed using SPSS/Windows software (Version 19.0, SPSS Inc., Chicago, IL, USA).
Results and discussion
The immunomodulatory effects of marine polysaccharides have been found to be related to their structural features, such as chemical composition (uronic acid and sulfate content), average molecular weights (Mw), conformation, and functional groups (22,23). The polysaccharides from S. plicata were fractionated using a preparative DEAE-Sepharose-CL-6B column to obtain three main fractions, which were selected based on the total carbohydrate and uronic acid elution profile (designated as Styela plicata polysaccharides SF-1, SF-2, and SF-3) (Fig. 1). The yield, chemical properties, and monosaccharide compositions of the crude polysaccharide and fractionated polysaccharides from S. plicata was shown in Table 1 and Table 2. The yields of fractionated polysaccharides were SF-1 (7.02%), SF-2 (24.18%), and SF-3 (10.80%). The constituents of the fractionated polysaccharides were mainly total carbohydrate (28.03-42.65%) with various contents of uronic acid (17.01-23.76%) and protein (1.48-9.23%). The total carbohydrate slightly decreased from 42.65% (SF-1) to 28.03% (SF-3) when the NaCl concentration of the eluent at the ion-exchange chromatography increased to 1.0 M. The average molecular weights of SF-1, SF2 and SF-3 were estimated to be approximately 1,187, 136, and 28 kDa, respectively. The biological activity of polysaccharides depends on their structural features such as molecular weight, degree of sulfation, type of sugar, and glycosidic branching. Therefore, when polysaccharides of different molecular weights are studied, different biological activities can be expected. Moreover, the molecular interactions may be affected by the molecular weights of polysaccharides, there by resulting in different biological properties (24,25).
1)SP, crude polysaccharide from Styela plicata; SF-1, polysaccharide fraction-Ⅰ; SF-2, polysaccharide fraction-Ⅱ; SF-3, polysaccharide fraction-Ⅲ.
The major monosaccharide compositions of the crude polysaccharide were galactose (44.08%), glucose (37.63%) and glucosamine (10.74%), as well as a minor amount of mannose (4.06%) and galactosamine (3.49%). In general, the monosaccharide composition of ascidian polysaccharides differ significantly with species (26). Galactose (27.91-62.21%), glucose (14.79-34.97%), and glucosamine (13.35-28.31%) are the three main sugars of the three fractionated polysaccharides, although they also contained considerable amounts of galactosamine (2.51-8.81%). Furthermore, in the tunic of S. plicata, these polymers are the three main fractions, and they are markedly distinct in their molecular mass and chemical composition. The high-molecular-mass fraction contains a high proportion of galactose, whereas the other two fractions of low molecular mass contain a higher proportion of amino sugars, and glucose (27,28). These results suggest that it is possible to obtain polysaccharides with various ionic strengths and chemical compositions by fractionating the crude polysaccharide using ion-exchange chromatography.
FT-IR spectroscopy was used to investigate the structural features of the polysaccharides such as the monosaccharide type, glycosidic bonds, and functional groups (29). The FT-IR spectra of SP, SF-1, SF-2 and SF-3 are shown in Fig. 2. Two characteristic absorptions of polysaccharides, a strong and wide absorption band of about 3,400 cm-1 for O-H stretching vibrations and a strong absorption peak of about 2,900cm-1 for C-H stretching vibrations, were observed. Extensive absorption bands at 1,000-1, 400cm-1 corresponding to coupled C-H and S-O stretching and C-O bending vibrations were observed in the FT-IR spectra of SP, SF-1, and SF-2, and these are the characteristic absorption bands of polysaccharides (11). Furthermore, the band at 1,600 cm-1 is characteristic of the deprotonated carboxylic acid group (COO-); this, considered with the analytical results (30), is evidence that S, SF-1, SF-2, and SF-3 contain uronic acid (12). In addition, the absorption peak at 1,200 cm-1 was assigned to the asymmetric stretching vibrations of SO, an indication of sulfate esters, confirming directly that S, SF-1, SF-2, and SF-3 were sulfated polysaccharides (31).
Macrophage cells are phylogenetically conserved and plays a key role in host defense and innate immune response. Macrophage activation is one self-defense mechanisms used to protect the host against pathogen infection (32). The crude polysaccharide and fractionated polysaccharides at concentrations between 0.5-5 μg/mL, and 0.05-0.25 μg/mL, respectively, has no toxicity on the RAW 264.7 (Fig. 3). Thus, test concentrations within these ranges were used to investigate the immunomodulatory effects.
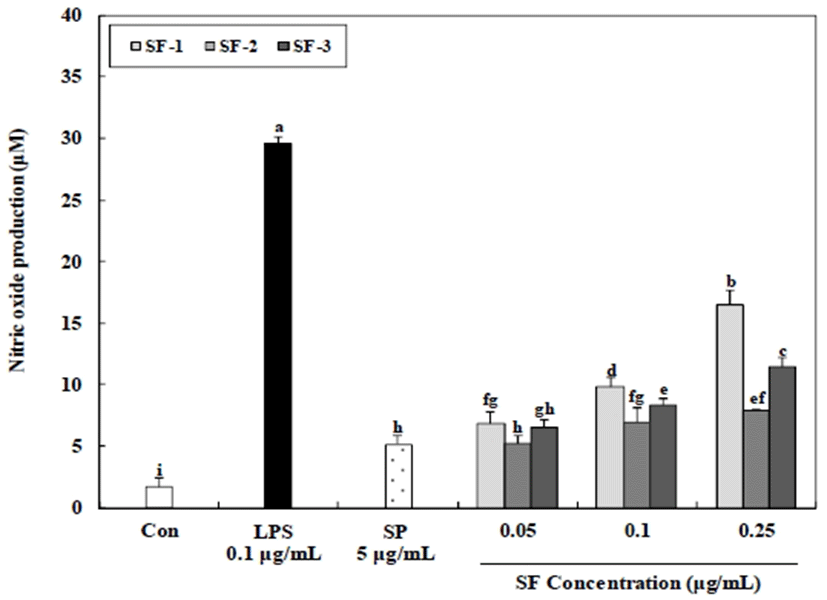
Nitric oxide (NO) has been identified as a major effector molecule produced by macrophages and is involved in the regulation of apoptosis and in host defenses against microorganisms and tumor cells (33). The synthesis of NO by activated macrophages is an important cytotoxic/cytostatic mechanism of non-specific immunity (34). The NO production from RAW 264.7 cells treated with SF-1 was dose dependent, and 16.53 μM NO was produced at the highest polysaccharide concentration (0.25 μg/mL). Significantly lower production of NO (maximum 7.89-11.43 μM) was released from RAW 264.7 cells treated with the SF-2 and SF-3 (0.25 μg/mL) fractions. Minimal NO-releasing capacity, less than 5.17 μM, was observed for SP (5 μg/mL). Therefore, these results indicate that the SP fractions could have beneficial pharmacological effects via their ability to modulate macrophage function (Fig. 4), especially because these compounds have been shown to increase macrophage cytotoxicity against tumor cells, increase reactive oxygen species (ROS) and NO production, and enhance secretion of cytokines (35). According to a previous study, the level of NO release was proportionally related to the sulfate content of the polysaccharide (36). In addition, observed a relationship between the Mw and NO production from macrophages, reporting that higher Mw polysaccharides induced a greater production of NO from Raw 264.7 cells (37).
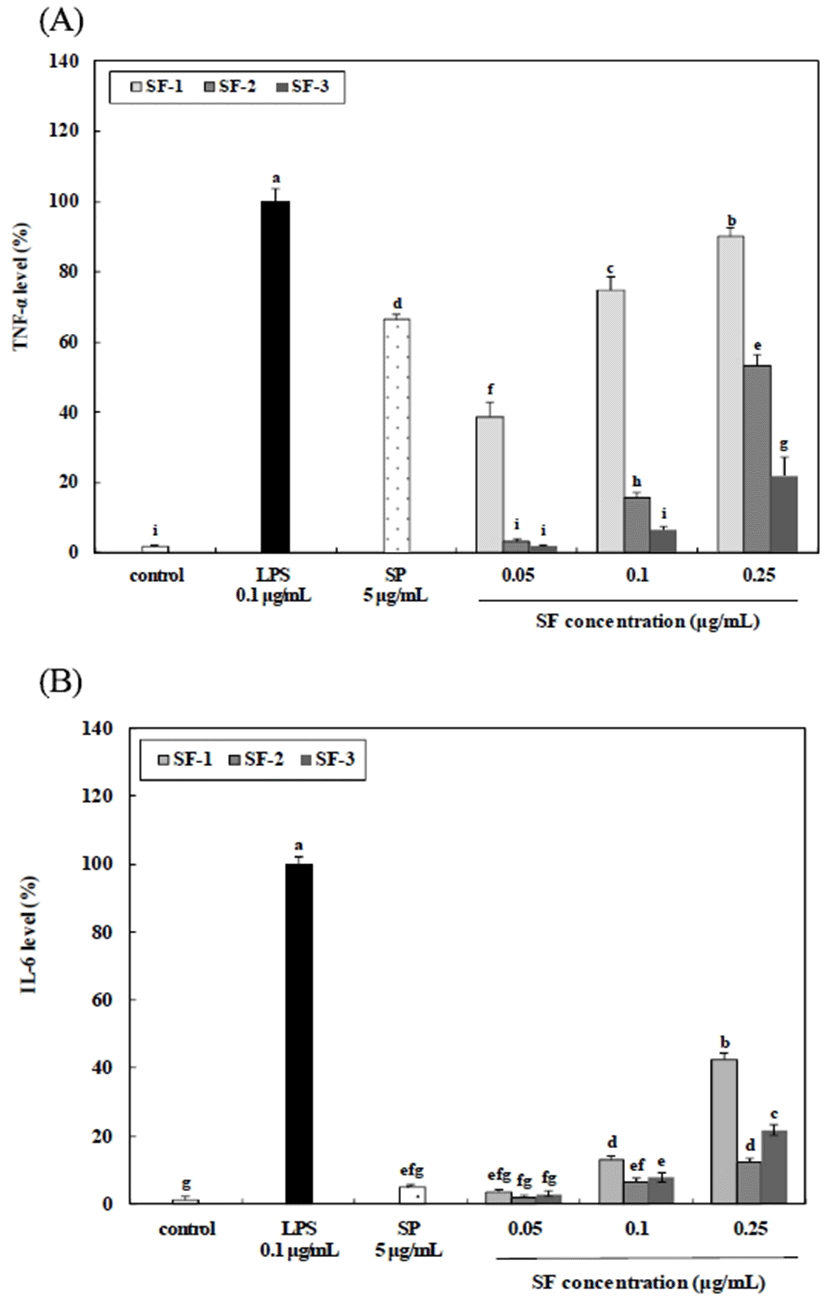
Cytokines are intercellular signaling proteins released by both immune and non-immune cells. Cytokines play important roles in controlling the homeostasis of the whole organism by the induction of cell differentiation, proliferation, and apoptosis, as well as defense functions such as immune responses and inflammatory reactions (38). To evaluate the effects of the crude polysaccharide and fractionated polysaccharides on cytokine secretion in RAW 264.7 macrophages, ELISA assays were performed to measure TNF-α and IL-6 levels in the culture supernatants of RAW 264.7 cells treated with the polysaccharides. As shown in Fig. 5, SF-1 increased the secretion of TNF-α, and IL-6 in a dose-dependent manner, when the concentration of SF-1 was 0.25 μg/mL, the production of TNF-α, and IL-6 was 89.96% and 42.45%, respectively. These results show that SF-1 has significant immunomodulatory activity. In general, polysaccharide are highly diverse in their monosaccharide composition, glycosidic linkage patterns, molecular mass, conformation, degree of branching, and other physicochemical properties. These factors influence their immunomodulatory activity, as well as other bioactivity (13). The ability of SF-1 to stimulate RAW 264.7 macrophages to produce these cytokines strongly suggested that the SF-1 may have beneficial effects on early immunomodulation.
The expression of iNOS, and COX-2 in macrophages is largely regulated by transcriptional activation, It has been suggested that activated macrophages also release some immune factors including iNOS, COX-2, and others (39). The mRNA expression of iNOS and COX-2 in RAW 264.7 cells by the crude and fractionated polysaccharides was determined by agarose gel analysis of the RT-PCR products using primers for iNOS and COX-2 mRNA (Fig. 6). The levels of mRNA expression of iNOS and COX-2 increased in a dose-dependent manner (0.05-0.25 μg/mL) after the application of SF-1, and this is similar to the levels generated by SP (5 μg/mL) and LPS (0.1 μg/ mL) treatment. In addition, the expression of iNOS, and COX-2 was found to be statistically significant above 0.05 µg/ml. Therefore, these results suggested that the increased NO production might be due to the enhanced mRNA expression of iNOS and COX-2 that was up-regulated in RAW 264.7 cells via activation by the polysaccharides (40). In conclusion, SF-1 has potent immunomodulatory activities, and our results suggest that these polysaccharides can be used as a functional food material.