Introduction
Cucumber (Cucumis sativus L.) is an important fruit that is consumed globally for its fresh crispy taste, high nutritional value and great health benefits. However, it is perishable owing to its high moisture content and is not amenable for long-term storage even at low temperatures. The peculiar deteriorative changes in cucumbers during their storage and distribution are mostly due to their high respiration, yellowing, and loss of moisture leading to shriveling and consequently microbial decay (1). Processing of fruits into more convenient product is considered to be an appropriate strategy to reduce their postharvest losses.
Minimal processing of fruits into ready-to-eat products is a recent trend which has attracted the attention of consumers (2,3). Currently, fresh-cut fruits are either packaged individually or as mixed fruits with others. Various fruits such as pineapple, watermelon, and apple are commonly sold in the market, but fresh-cut cucumber is yet to gain popularity globally. As the demand for fresh-cut fruits and vegetables is increasing, the shelf life of fresh-cut products is of concern, because their quality deteriorates during their storage. There have been various studies on extending the shelf life of certain fresh-cut fruits (4,5), but very few on preserving fresh-cut cucumber. Techniques such as mild heat treatment, and cold temperature storage are used to preserve fresh-cut produces for long durations (6,7). However, these treatments inevitably lead to the deterioration of some physiological and sensory characteristics of fresh-cut produces (8-10).
Recently, in order to maintain the quality and extend the shelf-life of fresh-cut products, edible coatings are applied or the produces are stored in modified atmospheric conditions (11-16). The applied coating essentially modifies the atmosphere surrounding the fruit/vegetable and ac as a semipermeable barrier that controls the gas exchange and water loss, and maintains the firmness of tissues, in addition to inhibiting microbial degradation of the produce (17).
Chitosan, a natural polysaccharide-based coating, regulates the internal tissue atmosphere, acts as a barrier to the movement of moisture, oxygen, carbon dioxide, and volatiles, and thus reduces the metabolism and delays the fruit senescence (18). The effect of the chitosan coating on the quality and safety of fruits has been studied extensively (19-22). After the coating of fresh-cut fruits, a suitable package may be required to protect them from further contamination and also to serve as a carrier during storage and distribution.
Packaging has a critical role in food storage and distribution. Apart from serving its primary function as a container, a packaging material must also meet the fundamental requirements of maintaining the food quality and safety during their storage. Packaging in polymer bags (such as polyethylene) is a commonly employed postharvest technique for maintaining the quality of fruits and vegetables during their storage and distribution (23). The field of food packaging has evolved over the years, as seen in the development of various beneficial polymer-based packaging materials with unique barrier properties against oxygen, moisture, light, volatiles, and both chemical and microbiological contamination (24,25). For low-temperature storage, a polymer film is suitable for a modified atmosphere packaging (MAP) and has the advantage of low cost and easy implementation at the commercial level (26-28). The successful use of polymer films for MAP is based on their specific permeation to oxygen and carbon dioxide to generate an atmosphere that is suitable for the retention of the food quality during storage (26-28). The choice of the packaging material for a fresh-cut product is important for its effective storage and distribution. Hence, many studies have investigated the effects of packaging materials on the quality and shelf-life of fruits (28-30). However, only few studies investigated the combined effects of coating and packaging material on the quality of fresh-cut cucumber. Therefore, the present study aims to evaluate the quality characteristics of fresh-cut cucumber samples coated with chitosan and packaged in various polymer films during its storage.
Materials and Methods
Freshly harvested cucumber fruits (Cucumis sativus) were purchased directly from a local farm (Gwangju, Korea) and stored in a refrigerator at 5℃ for 24 h until use. The fruits were selected based on their freshness, size, and greenness. Low-molecular chitosan powder (100%; 10.5 cps/20℃; Symbiosal Biotech Ltd., Mokpo, Korea) was used in this study because of its ease of solubility in the solvent. Analytical grade acetic acid was used (Daejung Chemical Ltd., Siheung, Korea). The fresh-cut fruits were packaged in 60 μm low-density polyethylene (LDPE) film (20 cm×30 cm; Thai griptech, Thailand), polypropylene tray (G-12157; GMPack tray, Paju, Korea) with 65 μm casted polypropylene (CPP) film (GMP CO., Ltd, Paju, Korea) used for heat-sealing, and 110 μm composite polyamide-polyethylene (PAPE) film (20 cm×30 cm; Solis, Switzerland). An airzero nozzle-type vacuum and gas flushing machine (AZ-450E) was used for heat sealing LDPE and PAPE, while a film adhesion machine (G320-Q12; GMP CO., Ltd, Paju, Korea) was used for heat sealing the CPP film to the tray.
Distilled water was used as control (0% chitosan), while chitosan solutions were prepared using the method described by Ali et al. (31). For preparing 1% and 2% solutions, 10 and 20 g of chitosan powder respectively were dissolved in 1 L of distilled water (60℃) containing 10 mL of glacial acetic acid and stirred using a magnetic stirrer for 5 h. The pH of the coating solution was adjusted to 5.6 using 1 N NaOH. The cucumber was washed with tap water and sliced uniformly into -10 mm thick pieces. The slices were then divided into three portions for different treatments. A single-layer chitosan coating was achieved by completely submerging 500 g of the fresh-cut cucumber in 1 L of the aqueous chitosan solution for 2 min and then the coated samples were allowed to dry at room temperature for 30 min inside a cabinet with laminar air flow. Fresh-cut cucumber of 100 g were packed into each packaging materials, heat-sealed and stored at 5℃, 60% RH in the refrigerator. The samples were prepared in triplicates for each treatment and their qualities were analyzed at 3 day intervals over 12 days.
The headspace gas compositions of the packaged samples were analyzed with a digital gas analyzer (Quantek Gas Analyzer, Model 902D, Grafton, USA). Gas concentration was measured by penetrating the package film containing the sample with the needle probe of the device. Values of CO2 concentration was recorded from the display screen on the instrument.
Visual quality was assessed using photograph figure of fresh-cut cucumber surface using an industrial camera (DFK 31AF03; Theimagingsource, Bremen, Germany) and visual quality score was assigned as previously described (32). The vsual quality was scored on a 1 to 9 scale, where 9 represents an excellent and fresh appearance, 7 represents good, 5 represents fair (limit of marketability), 3 represents fair (useable but not saleable), and 1 indicates that the product is unusable. Intermediate numbers were assigned where appropriate.
The samples were weighted using laboratory-scale digital balance. Weight losses were determined by comparing the weights of the sample (initial weight, 100±3 g) before and after the storage period. The values are expressed as weight loss percentages with regard to the initial weight.
where, Win is the weight of the produce on the first day and Wfin is its weight on the final day.
Firmness was measured as the maximum force (g) required to puncture the surface of fresh-cut cucumber. A penetration test was conducted on the surface of fresh-cut cucumber using a texture analyzer (Compac-100, Scientific Co., Tokyo, Japan) with a 5 mm diameter cylindrical probe. Samples were penetrated to a depth of 12 mm. The speed of the probe per penetration was 3 mm/s. Four measurements were carried out for each packaged sample.
Fresh-cut cucumber was homogenized, squeezed with cheesecloth, and the TSS and pH of the juice was analyzed. TSS (brix) was determined at 25℃ using a digital refractometer (PAL-1; Atago Co. Ltd., Tokyo, Japan) and pH was measured with a pH meter (Mettler-Toledo AG8603, Schwerzenbach, Switzerland).
The colors of the peel and fresh-cut surface of the cucumber samples were measured with a chroma meter (CR-300; Minolta Co., Osaka, Japan). L*, a* and b* represents the lightness, redness, and yellowness respectively. Here, b* and color change (ΔE) are used to express the color quality of the peel and fresh-cut surface of cucumber respectively. ΔE was calculated as follows;
All fresh-cut samples were analyzed for total bacteria count (TBC). For this, 10 g of fresh-cut cucumber was aseptically packed in a sample bag (190×300 mm, 3M Co. Yeoju, Korea) and diluted with 90 mL of 0.1% peptone water. The samples were homogenized using a stomacher (SH-001, Shimskyu, Tokyo, Japan) at a high speed for 3 min. The bacterial count was determined on plate count agar (PCA; Becton Dickinson, NJ, USA) after the incubation of the sample at 37℃ for 48 h and is represented as log CFU/g of the sample.
All experiments were carried out in triplicates and the data were analyzed using IBM SPSS (V.20). The results are expressed as the mean±standard deviation. Significant differences in means were calculated by analysis of variance (ANOVA) and Duncan multiple comparison test at 95% significance level.
Results and discussion
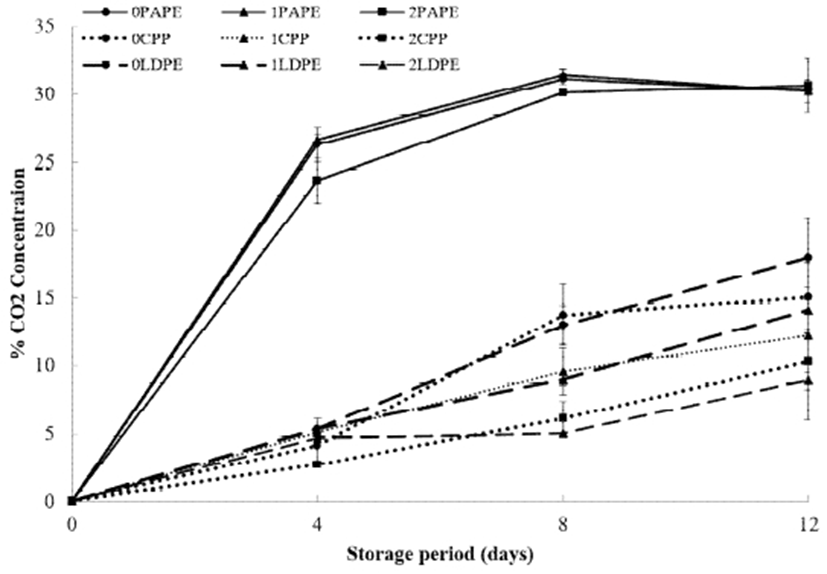
The headspace CO2 concentration for all the samples at 5℃ is shown in Fig. 1. The CO2 concentration increased throughout the storage period. Fresh-cut products generally have a higher respiration rate than whole products, probably due to the increased surface area exposed to the atmosphere and the increased metabolic activity of injured tissues (33). Higher accumulation of CO2 was observed in PAPE-packaged samples than in CPP- and LDPE-packaged samples. However, no obvious physiological defects and no off-flavor were observed in samples packaged in PAPE. The gas permeability of packaging materials has been reported to affect the O2 concentration and consequent accumulation of CO2 in packaged fresh-cut papaya (34). Changes in the CO2 concentration in the packages were also affected by chitosan coating, as respiration was more pronounced in uncoated samples than in chitosan-coated samples. A significant difference (p<0.05) was observed between samples coated by different concentrations of chitosan solution (1 and 2%) and uncoated (0%) samples, with 2% chitosan-coated CPP-packaged and LDPE-packaged samples showing the lowest CO2 concentrations of 10.3 and 9%, respectively. This result suggests that chitosan coating is useful for slowing down the respiration rate of fresh-cut cucumber. The tolerance of most fruits and vegetables to high CO2 concentrations has been established (35). Hence, it is important to identify safe concentrations that will result in maximum commodity storage life without injury (35,36). In this study, a high CO2 concentration of -30% was observed after 12 days, indicating that the storage life of fresh-cut cucumber was extended without a remarkable decline in the fruit quality. A similar result was also reported for chemically treated fresh-cut papaya, for which a high CO2 concentration (up to 41%) was recorded. The papaya fruit was found to still maintain better quality parameters compared to those of untreated samples after storage (34).
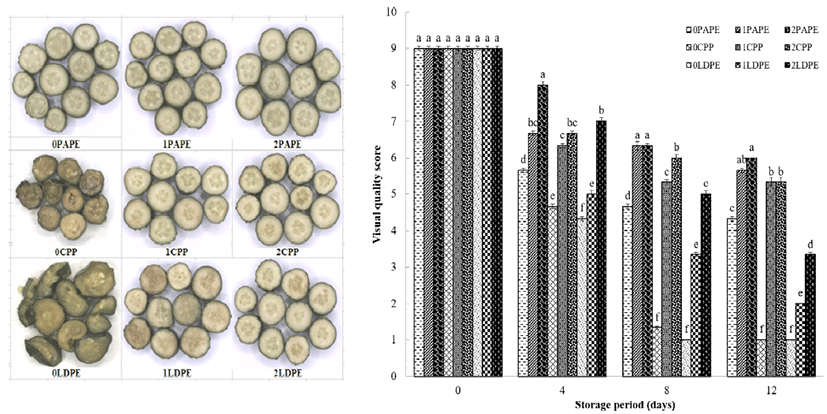
The visual quality score revealed a gradual decrease in the perceived quality of fresh-cut cucumber during its storage. Both chitosan coating and packaging material significantly affected the visual quality of fresh-cut cucumber, as shown in Fig. 2. Uncoated samples packaged in CPP and LDPE had already decayed at the end of the 12-day storage, while the PAPE-packaged sample showed better properties owing to the prevention decay. The coated samples packaged with PAPE and CPP had better visual ratings than samples packaged in LDPE. Overall, 2% chitosan-coated, PAPE-packaged fresh-cut samples had the best appearance after the 12 day storage period. Edible coating and packaging materials can slow down the deteriorative changes in products by reducing desiccation. According to a previous report, the visual quality of broccoli was also maintained using different packaging materials to create a modified atmosphere during its storage (32).
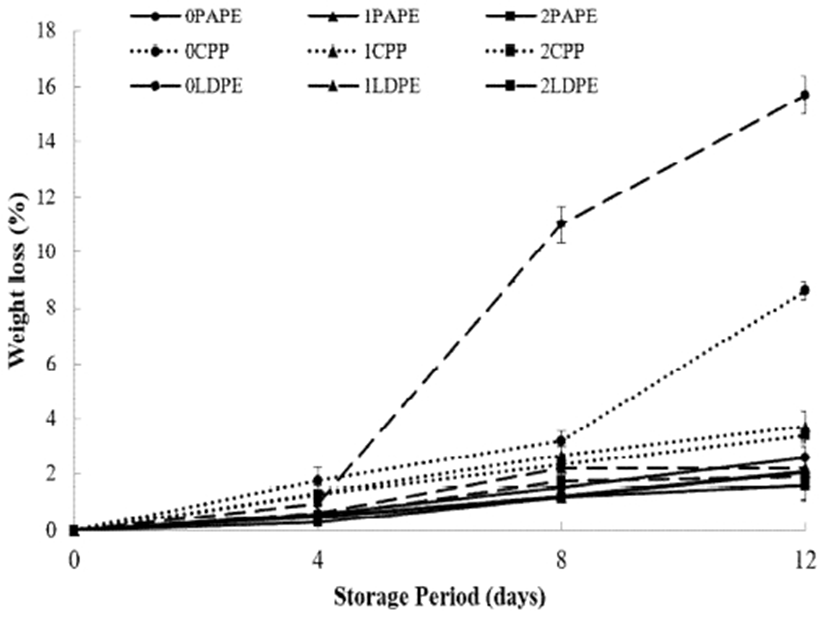
Moisture content is an important quality parameter of fruits, which is assessed by the loss in their weight. Fresh-cut fruits are highly susceptible to weight loss (36). Therefore, it is important to evaluate the weight loss during their storage (37). As shown in Fig. 3, all samples lost weight during the storage period. By reducing the water transfer, chitosan coating combined with packaging materials minimized the weight loss during the storage period, leading to delayed dehydration (38). Tissue disintegration caused by microbial decay was observed in uncoated fresh-cut samples packaged in LDPE and CPP, which caused a surge in the weight difference, resulting in high calculated weight loss (over 2%) after 4 days and 8 days of storage. These results indicate that the coating may be combined with LDPE and CPP packaging to improve their moisture barrier properties and reduce weight loss in fresh-cut fruits. Ali et al. (31) also reported the lowest weight loss in chitosan-coated bell pepper after storage for 20 days. Among the packaging materials, PAPE showed better moisture barrier properties for both chitosan-coated and uncoated samples. At the end of the storage period, 2% chitosan-coated fresh-cut cucumber packaged in PAPE showed the lowest percentage weight loss.
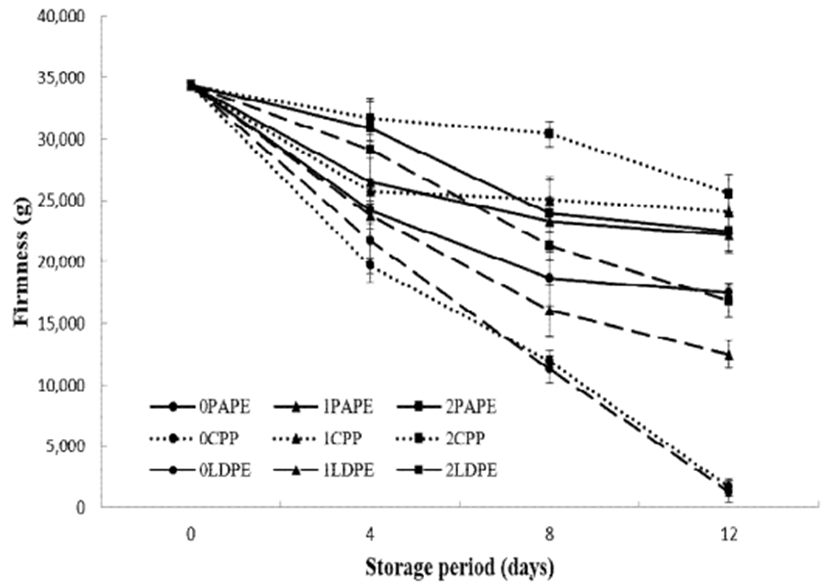
Firmness is also an important parameter that determines the quality and postharvest shelf-life of a fruit (39). Fresh-cut fruits and vegetables may lose their firmness owing to loss of moisture, microbial decay, ripening, tissue degradation, etc. As shown in Fig. 4, all samples suffered a loss in firmness (softened) during their storage. Fruit softening is caused by the action of tissue-degrading enzymes (40). Uncoated samples in LDPE and CPP suffered a drastic loss in firmness with -95% loss in firmness after the storage period. Apart from the loss of moisture, microbial decay casued a huge loss in the firmness of these samples. This speculation can be confirmed by the result of microbial analysis (Fig. 7) and monitoring of the visual quality (Fig. 2). Further, reduction in crispiness and tensile strength were also observed by the firmness test, which may be attributed to the dry surface and easy breakage caused by the loss of moisture. Chitosan-coated samples (1 and 2%) better retained their firmness than uncoated samples. This result is in agreement with those of other studies (31,41,42), wherein chitosan coating was found to be effective for suppressing factors that induce fruit softening and thus thence improve the firmness of coated fruits.
The TSS content determined as the brix value decreased in case of all treatments during the storage (Table 1). Chitosan coating and packaging materials had significant effects (p<0.05) on the TSS content. Moreover, the concentration of chitosan solution was observed to significantly affect the conservation of sugar in coated fresh-cut samples. The TSS content is closely related to the ripening of fruits (43). Overall, 2% chitosan-coated, PAPE-packaged samples have the highest brix value. Jiang and Li (44) observed a higher TSS value for longan fruits treated with a higher concentration of chitosan and stated that increasing the concentration of chitosan coating led to markedly enhanced beneficial effects. The pH of fresh-cut cucumber samples continuously increased during the storage period (Table 2). This increase may be attributed to their aging/ripening during storage. Chitosan coating significantly (p<0.05) lowered the pH of coated samples compared to those of uncoated fresh-cut cucumber samples. A similar result was obtained by Shao et al, (45) who also reported a lower pH value in chitosan-coated apple.
Color is one of the most important indicator of the quality of cucumber during its storage (46). Color change (ΔE) and b* were used to evaluate the discoloration of fresh-cut surface and peel of cucumber samples respectively. Discoloration is a common quality defect observed in fresh-cut cucumber during storage (46). As shown in Fig. 5, discoloration of the fresh-cut surface (as indicated by an increase in ΔE) was observed in all samples but it was more severe in uncoated LDPE-packaged samples. A ΔE value in the range of 0.5-1.5 indicates little color difference, that in the range of 1.5-3.0 represents a slight difference, whereas ΔE values in the range of 3.0-6.0 and 6.0-12 indicate remarkable and extremely significant differences, respectively (47). After the storage period, 2% chitosan-coated, PAPE-packaged samples had the lowest ΔE value (less than 3), indicating a slight color difference, compared to those of the other samples. Chitosan coating and packaging materials have been used by several authors to delay color changes in fruits (48-50).
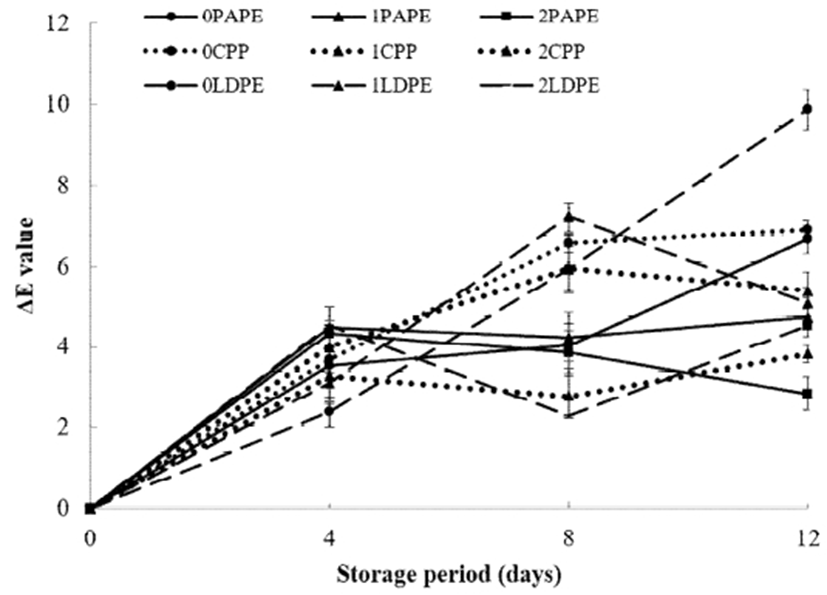
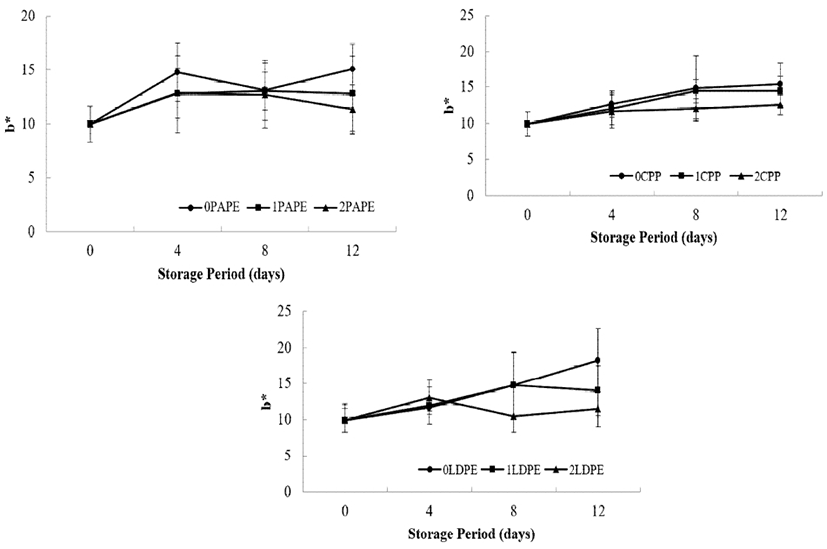
As shown in Fig 6, all samples showed an increase in b* during the storage period. The discoloration (as indicated by an increase in b*) was found to be more severe in uncoated samples than in samples coated with chitosan. There were also no significant differences (p>0.05) in b* values among the CPP- and LDPE-packaged samples until 8 days of storage. However, the difference in b* value of samples coated with different concentrations of chitosan (1 and 2%) was significant (p<0.05) in case of all packaging materials, with 2% chitosan-coated, PAPE-packaged sample having the lowest value and the uncoated LDPE sample having the highest b* value after the 12-day storage period. Overall, coating with chitosan significantly reduced the color change in the peel of fresh-cut samples. This might be becaused chitosan coating is capable of retarding the degradation of chlorophyll in the cucumber peel (51). The preservative properties of the packaging material in maintaining the natural green color of the cucumber peel, as sobserved in this present study, is in the order of PAPE > CPP > LDPE. Jia et al. (32) reported that packagings material inhibit yellowing and deterioration of the color broccoli florets.
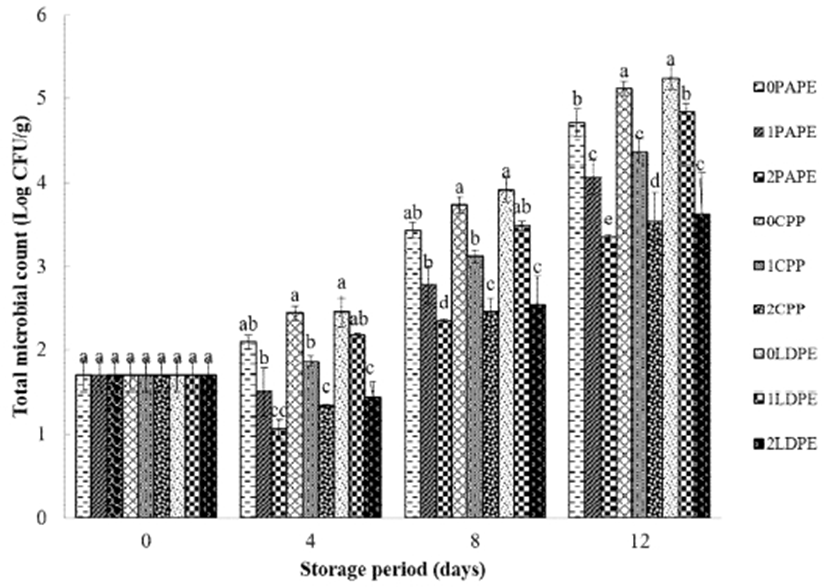
Achieving microbial safety in fresh-cut fruits is of crucial concern because they have exposed tissues, which can be easily contaminated (52). The total bacterial count (TBC, Fig. 7) reveals the effectiveness of chitosan coating in reducing the bacterial load. After 4 days of Storage, all chitosan-coated (1 and 2%) fresh-cut samples had a lower bacterial count. This result agrees with that reported by Li et al. (53) who also reported the effectiveness of chitosan coating as an antimicrobial agent. Park et al. (54) observed a reduction of 2.5 and 2 log CFU/g in the counts of Cladosporium sp. and Rhizopus sp., respectively, in strawberries coated with a chitosan-based edible film, immediately after the application of the coating. In this study, an increase in the TBC was observed during the storage period. No substantial difference in microbial reduction was observed between the different packaging materials. 2% chitosan-coated, PAPE-packaged fresh-cut cucumber samples had the lowest (log CFU/g) bacterial count after 12 days of storage.
Conclusion
The importance of chitosan coating in the preservation of fresh-cut fruits is further corroborated in this study. This study revealed that fresh-cut cucumber samples coated with chitosan using a 2% solution performed better in improving and preventing the deterioration of the quality of the samples during their storage. However, the effectiveness of this treatment goes alongside a suitable choice of a packaging material for storage. The commonly used LDPE film showed poor ability in maintaining the fruit quality, and hence may not be suitable in the absence of a preventive coating. PAPE performed better among the packaging materials evaluated in this study. Further studies are required to evaluate the effects of these treatments on the nutritional and sensory properties of fresh-cut cucumber. In general, chitosan coating enhanced the ability of packaging materials in the preservation of the quality of fresh-cut cucumber.
