Introduction
Spirulina (Oscillatoria spirulina) is one of the oldest algae on earth. Spirulina has been designated as a future food by the United Nations Food and Agriculture Organization because it contains a variety of physiologically active substances, such as carbohydrates, protein, vitamins, minerals, and gamma-linolenic acid (1). Spirulina is also classified as an alkaline super-food that provide health and prevention of chronic disease. It is a valuable food ingredient for illness, old age, and in infants, because of its high absorption rate of over 95% (2). The biological activity of spirulina was investigated. The nutritional content of spirulina, and the anti-oxidant, anti-aging, and anti-proliferating effects of spirulina extract were reported (3).
Spirulina has an excellent immune activity, and has been patented in Russia for use in radiotherapy. Furthermore, the National Aeronautics and Space Administration (NASA) has been developing spirulina-based space food (4). Spirulina has diverse physiological effects, including strong antioxidant effect, tumor cell suppression activities, inhibition of the production of lipid peroxides, improved immune function and circulation (5), suppressed aging and prevention of disease in adults. The increasing recognition of the physiological benefits of spirulina has led to its use as a high-value natural functional food ingredient.
Fermentation has been used as a classical method for preservation in Eastern and Western cultures. Yeast is an important organism in brewing industry because it provides valuable nutrient ingredient and various wine. Yeasts can obtain energy by breaking down hexose through anaerobic respiration (6). During anaerobic respiration, pyruvic acid is broken down and alcohol and carbon dioxide are released. Yeasts can grow between 10℃ and 37℃, showing limited growth at lower and higher temperatures. Yeast fermentation provided alcohol as well as condiments with pleasant flavor for food industry. β-Glucans as biologically active compound could be extracted from yeast cell wall. It could strengthen innate immunity in the elderly, and anti-cancer activity (7).
In spite of health promoting effect, the spirulina had some limitations as food ingredients because of indigenous odor. The removal of the distinctive smell and optimizing the production of physiologically active substances from spirulina by yeast fermentation has not been studied. Particularly, the strain K. servazzii KM 2017 has a low alcohol producing ability but a good gas generating ability, when comparing the strains S. cerevisiae, K. serazzii, and W. hamomyces (8,9). In addition, the K. servazzii strain has not only excellent gas generating ability but also fruit flavor during the growth period (10).
Therefore, it was judged that K. servazzii KM2017 is optimal for the strain which increases physiologically active substance while eliminating the odor of spirulina.
In this study, the yeast fermentation of spirulina was optimized using K. servazzii KM2017 to enhance the extraction of bioactive compounds. In particular, the effect of cold storage on the morphology and phytochemicals (phycocyanin, β-glucan, phenolics compounds) of yeast fermented spirulina were analyzed. The present study will provide the valuable information for utilizing as a novel food ingredient of spirulina.
Materials and methods
Spirulina powder was obtained from USA (Nutraceuticals International Group LLC, Paramus, NJ, USA). Yeast extract from Choheung Corporation (Ansan, Korea) was used as the nutrient in the medium for fermentation of spirulina. All reagents were of high grade.
The strain K. servazzii KM2017 (KCCM 11989P) isolated from fermented apple juice was confirmed by PCR and used for yeast fermentation. The genomic DNA was extracted using the boiling method based on Chelex beads and amplified by PCR (Veriti R TM 96-well Thermal Cycler (ABI).
DNA amplification involved initial denaturation at 95℃ for 15 min, 1 cycle; denaturation at 95℃ for 20 sec, 30 cycles; annealing at 50℃ for 40 sec, 30 cycles; extension and final extension at 72℃ for 1 min 30 sec and 5 min, respectively, 1 cycle. Lastly, sequencing was carried out using Big Dye® Terminator v3.1 Cycle sequencing Kits using an ABI prism 3730XL DNA analyzer (capillary 50 cm).
The nucleotide sequence was analyzed by Blast N. Sequence analysis of NCBI was performed to confirm the results. The reliability of NCBI sequence analysis is 99.9% similarity with K. servazzii. K. servazzii KM2017 was smeared onto medium containing DifcoTM Lactobacilli MRS broth agar (Becton, Dickinson and Company, Sparks, MD, USA) and cultured at 30℃ in an IS-971R incubator (Jeio Tech., Kimpo, Korea) for 24 h. Next, a single colony was collected for two-time subculture, inoculated onto MRS broth sterilized for 15 min at 121℃, and cultured for 24 h at 30℃ to generate the starter.
To prepare the spirulina paste, 20 g of spirulina powder was added to 45 mL of distilled water and homogenized (Shaking incubator HK-S125C) for 5-10 min. To remove other microorganisms, the solution was heated to 85℃ in a water bath for 30 min. Next, yeast fermentation was performed by adding 10 mL of 10% YE and 25 mL of 20% glucose as nutrients, inoculating with 1% K. servazzii KM2017, and culturing for 2 days at 30℃ with shaking. Yeast fermented spirulina paste was kept for 5 days at 1℃.
The pH was measured using a model 420+ pH meter (Thermo Scientific, Waltham, MA, USA). Titratable acidity was calculated, as lactic acid content (%, v/v), by adding 1 mL of reagent to 9 mL of distilled water, and titrating with 0.1 N NaOH to make the solution pH 8.3, as shown in the following equation:
Viable count was investigated by adding 1 g of the fermentation products to 9 mL of sterilized water followed by serial dilutions to 104, 105, and 106, with 20 μL of each dilution spread onto MRS agar plates. Following culture for 24 h at 30℃ in a thermostatic incubator, the colony forming units (CFU/mL) was determined.
Approximately 5 g of the sample is suspended in 100 mL of distilled water, and then approximately 120 mg of amylase (20 units) was added. pH was adjusted to 6.9 with 0.1 N sodium hydroxide solution, followed by shaking at 20℃ for 2 h. The solution was adjusted to pH 5.0 with 0.1 N hydrochloric acid solution. Cellulase (50 units) was added and the mixture was shaken at 37℃ for 2 h for enzymatic degradation. After then, 60 mg of aminoglucosidase and 0.1 N hydrochloric acid solution were added. and then mixture was hydrolyzed at 60℃ for 2 h. A 400 mL of ethanol (95%) is added to the above enzymatic degradation product, followed by precipitation at 4℃ for 12 h, followed by centrifugation (10,000 rpm, 10 min) to obtain a precipitate of the sample. After 500 mL of 80% ethanol was added. The precipitate was obtained by centrifugation (10,000 rpm, 10 min). The final precipitate was homogenized and resuspended in distilled water. Sample solution (1 mL) was mixed with 1 mL of 5% phenol solution and then quickly added 5 mL of sulfuric acid. Absorbance was measured at a wavelength of 470 nm (11).
Reducing sugar content was measured using the dinitrosalicylic acid (DNS) method (12). Spirulina paste was centrifuged at 13,200 rpm for 10 min. The supernatant was mixed with 3 mL of DNS and left to react for 5 min at 100℃. After cooling for 40 min at room temperature in a dark place, absorbance was measured at 550 nm. Reducing sugar content was calculated by comparison to a standard curve of reference glucose.
Fermented spirulina paste was centrifuged at 10,000 rpm for 10 min. The supernatant was diluted 4-fold and added with 5 mL of Bradford dye reagent (13). Thereafter, the reaction solution was reacted at 20-30℃ for 30 min and the absorbance of reaction solution was measured at 595 nm. Protein content was obtained by comparison to a standard curve of bovine serum albumin.
Polyphenol content was measured by the Folin-Denis method (14). A 60 μL of 2-fold diluted Folin reagent was added to cold storage spirulina (60 μL), and the mixture was homogenized and left for 3 min. To this, 60 μL of 10% Na2CO3 was added, and the mixture was left to react for 60 min in a thermostatic incubator at 30℃. After the reaction was completed, the absorbance of the sample was measured at 700 nm. Total polyphenol content was calculated by comparison to a standard curve for the reference substance garlic acid.
Flavonoid content was measured as previously described (15). Each sample (125 μL) 1,080 μL of 80% ethanol, 30 μL of 10% aluminum nitrate, and 30 μL of 1 M potassium acetate. After leaving the mixture for 40 min at room temperature, the absorbance was measured at 415 nm. Total flavonoid content was calculated by the comparison to a standard curve of quercetin.
The free radical scavenging activity of fermented spirulina and cold storage product was analyzed by measuring the ability to reduce DPPH. Each sample (160 μL) received 40 μL of 0.15 mM DPPH solution. The mixture was left for 30 min and the absorbance was measured at 517 nm. The free radical scavenging activity was analyzed as the concentration (IC50) that indicated the half inhibition of full activity. Trolex was used as the positive control.
For measurement of green phycocyanin pigments, 10 mL of fermented spirulina product was left for 10 h in a dark room at 5℃. Thereafter, the spirulina paste was centrifuged at 13,200 rpm, and then the supernatant was collected. The absorbance was measured at 562 nm, 620 nm, and 652 nm. Phycocyanin content was obtained using the following equations (16).
The chromaticity of fermented spirulina products was measured using a colorimeter (Color Reader, CR-10, Minolta, Osaka). Color values for lightness (L), redness (a), yellowness (b) were measured. The values of the standard white plate used were L: 96.12, a: -4.41, and b: 7.20.
Results and discussion
After yeast fermentation for 2 days at 30℃, 20% spirulina paste (w/v) was subjected to cold storage for 5 days in a refrigerator. The initial pH of spirulina paste was 6.92±0.01. Thereafter, pH decreased slightly and acidity increased from 0.26 to 0.51% for 2 days due to the organic acids produced by the yeast. This may reflect the production of oxalic acid, malic acid, acetic acid, citric acid, succinic acid, and lactic acid by yeast (17).
During the cold storage at 1℃, pH increased slightly and the acidity decreased slightly. After cold storage for 5 days, the final product showed pH 6.16 and acidity of 0.41%. The pH increase during cold storage may be the release of neutral amino acids and proteins from spirulina degradation. This result is similar to a study in which β-glucan and protein increased, resulting in decreased acidity during cold storage of yeast cell at low temperatures (18). In another study, cold storage of yeast led to the production of neutral amino acids, proteins, and organic compounds (19).
On yeast fermentation day 1, the viable count of yeast in the spirulina paste increased from 3.24×106 CFU/mL to 1.21×108 CFU/mL, Although the production of bioactive compounds increased with yeast fermentation, the spirulina paste fermented for 2 days maintained a viable count of 107 CFU/mL indicating yeast viable cell of 5.33×107 CFU/mL. This is similar to the result that when yeast cultivation was carried out for about 3 days, the number of yeast cells was decreased (20). It was confirmed that the number of viable cells of yeast decreased as cold storage progressed as shown in Table 1. During cold storage of fermented spirulina the viable cell of K. servazzii decreased from 5.33×107 CFU/mL. Although the spirulina paste provided a good growth environment for yeast, the cold storage of fermented spirulina resulted in the cold storage, indicating the viable cell counts of 4.39×107 CFU/mL for 5 days.
Reducing sugars have an aldehyde group or form an aldehyde group in solution (21). Glucose was used as the reference substance to measure reducing sugars. The initial reducing sugar concentration was 4.97%. As yeast fermentation progressed, the reducing sugar concentration decreased, reaching 0.68% on fermentation day 2. This is similar to reports that yeasts decrease level of reducing sugars by breaking down sugars during fermentation. After fermentation was complete, cold storage at 1℃ showed the increasing trend of reducing sugar content, reaching 1.53% for 5 days. The increase in reducing sugar content during cold storage is due to the cell lysis of yeast as well as cell disruption of spirulina. This is similar to another study, in which yeast extract was prepared by cold storage and enzymatic hydrolysis, and there was a slight increase in reducing sugar concentration during cold storage (22,23).
During the yeast fermentation of spirulina, β-glucan content increased from was 0.04 mg/g to 4.75 mg/g for 2 days as shown in Table 1. The results of the physicochemical analysis after yeast growth showed a somewhat similar result to that of 0.09 mg/g of β-glucan. As the yeast fermentation proceeded, it was confirmed that the amount of β-glucan was increased. It was concluded that β-glucan of yeast was eluted with decreasing viable cell count by yeast fermentation for 2 days. Yeast cell lysis is depending on the fermentation date during yeast fermentation (24). The content of β-glucan increased by cold storage of yeast fermented spirulina. The content of β-glucan was 6.21 mg/g after 3 days. It was judged that various physiologically active substances were also eluted due to the cold storage of yeast cells while the sample was stored at a refrigeration temperature of 1℃ The β-glucan content was 8.53 mg/g after the cold storage for 7 days.
It has been reported that the lower temperature or higher temperature of 50℃ resulted in the collapse of the cell wall of the yeast, resulting in the elution of physiologically active substances (25). However, during the storage at high temperature, physiologically active substance of yeast was increased. But spirulina could change its unique color (26).
The initial protein content in 20% spirulina paste was 242.98 μg/mL as shown in Table 1. This result is similar to the values of 1,102.88 μg/mL and 1,200.04 μg/mL in raw spirulina in another study (27), considering 5-fold dilution of fermented spirulia paste. The protein content increased during yeast fermentation, reaching 423.98 μg/mL on fermentation for 2 days. The increase may be because of the release of protein as the increased viable cell count on fermentation day 1 decreased on day 2. Also, it is due to the lysis of spirulina cells. Another study reported the similar results increasing in protein, where the initial protein content was 0.03 g/g and increased to 0.25 g/g after yogurt fermentation for 3 days (28).
It was concluded that the yeast fermentation of spirulina and its cold storage higher content of bioactive compounds due to the cold storage through cell death and cell licking,
Antioxidant activity of fermented spirulina paste was determined by DPPH radical scavenging activity, and analysis of polyphenol content and flavonoid content analyses. The results for polyphenol and flavonoid content are shown in Fig. 1. Polyphenolic compounds are widely distributed in nature, and mostly exist in vacuolar. Anthocyanins, flavonoids, pro (pancho) cyanidin, remunerators, ligands, and tannin are included (29).
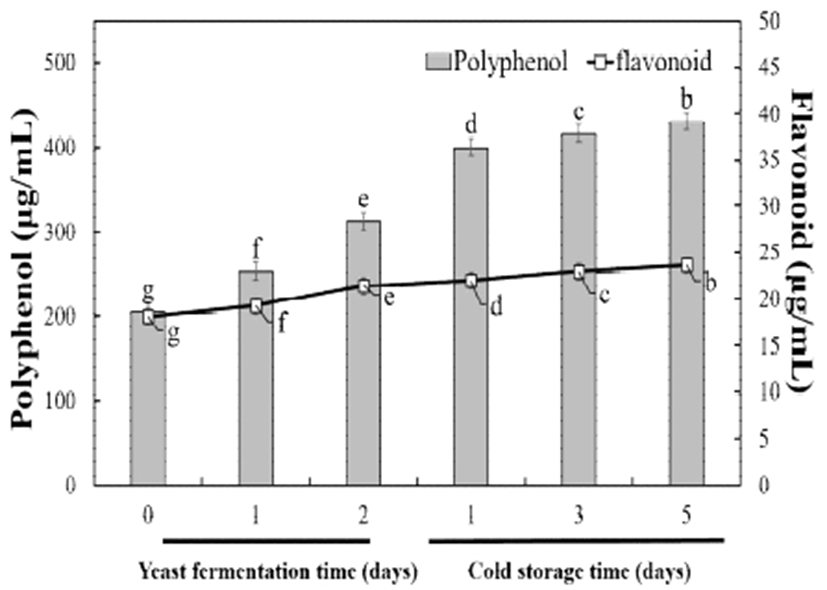
Polyphenol have various physiological and pharmaceutical effects, including anticancer, antihypertensive, anti-inflammatory, antidiabetic, and anti-aging activities. The polyphenol and flavonoid contents before yeast fermentation were value 213.79 μg/mL, 23.26 μg/mL, respectively. In particular, the flavonoid content was similar to the value flavonoid content of 120.24 μg/mL previously measured in pure spirulina extract, considering the 5 fold dilution of fermented spirulina paste.
During yeast fermentation, the polyphenol and flavonoid contents increased gradually. On fermentation for 2 days, their content were 350.84 μg/mL and 28.41 μg/mL, respectively, with a large increase in the polyphenol content. This may be because the alcohol produced during yeast fermentation causes a large amount of the total polyphenol to be released (30). On fermentation day 2, antioxidant substances are thought to be released due to the cell disruption of spirulina.
During low-temperature storage, the polyphenol and flavonoid contents increased gradually, reaching 420.55 μg/mL and 34.01 μg/mL, respectively, for 5 days. After the copious release of protein, organic compounds, and phenolics compounds during yeast fermentation, storage at low-temperature resulted in the death of yeast cell and accompanied by the release of bioactive compounds from both yeast and spirulina cells. In effect of temperature on growth and cold storage of Spirulina pliantness the low temperature increased the autolysis (31). This is similar to the description of increased polyphenol and flavonoid content, as indicator of antioxidant ability, during yeast fermentation of wine (32).
Table 2 shows the DPPH radical scavenging activity, which was an indirect measure of the antioxidant activity of the fermented spirulina. In the similar analysis, an alcohol-free wine is reacted with the stable free radical DPPH+, with the decrease in DPPH radical measured by spectrophotometric (33). The spirulina without yeast fermentation showed the IC50 value of 2.48 mg/mL and decreased to 1.21 mg/mL after yeast fermentation for 2 days at 30℃. After the end of fermentation and cold storage for 5 days at 1℃, the IC50 value decreased to 0.88 mg/mL, which was even lower than the value after 2 days of yeast fermentation. Conclusively, the cold storage of fermented spirulina may decreased IC50 value because the degradation of both yeast cells and spirulina during cold storage led to the release of bioactive compounds.
| Time (days) | IC50 | |
|---|---|---|
| Yeast fermentation | 0 | 2.48±0.411) mg/mL |
| 2 | 1.21±0.23 mg/mL***2) | |
| Cold storage | 5 | 0.88±0.11 mg/mL*** |
| BHA (standard) | 4.62±0.18 μg/mL | |
The phycocyanin content was also found to increase during yeast fermentation and cold storage. As shown in Figure 2, Table 3, the C-PC, APC and PE contents before yeast fermentation were value 0.078±0.003 mg/g, 0.055±0.003 mg/g, 0.150±0.001 mg/g. Especially, picocyanin, which is one of the specific coloring components of spirulina, is contained in a great number of cyanobacteria (34). These pigments are responsible for photosynthesis and have a great effect on nutrient supply and antioxidant effect (35). The C-PC value of the yeast fermentation day 2 was 0.121 mg/g and the APC value was 0.096 mg/g. Finally the second day PE value of yeast fermentation was 0.169 mg/g. It was confirmed that this value was increased by yeast fermentation. This is similar to the results of studies that various pigment components elute with partial lysis of spirulina (36). After cold storage, the C-PC, APC, and PE values of spirulina increased. The cold storage 5 day, C-PC value was 0.144 mg/g, APC value was 0.126 mg/g and the PE value was 0.192 mg/g. Especially, it was confirmed that the color became darker after cold storage. This is similar to the study that the color changes as the spirulina pigment elutes (37).
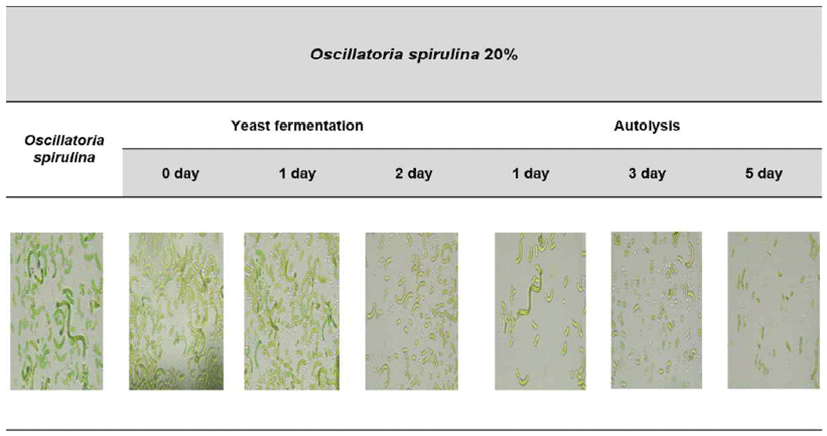
Table 4 shows the changes in the chromaticity of spirulina. The color value of the initial spirulina fermented product was L: 63.12, a: 0.23, b: 23.14. This is similar result to the low a value of red color in a study of the quality characteristics of tofu using spirulina powder (38). As the yeast fermentation, the L value decreased while the a and b values increased. Afterwards, the brightness dimmed as cold storage progressed. This is similar to the results of the lysis of spirulina, showing the dark color due to the physiologically active substance and chlorophyll-a, phycocyanin eluted (24). In particular, the value of a, which represents the red value, was also increased. It was reported that the spirulina analysis showed that the anthocyanin in spirulina was eluted and reddish (39).
Fig. 2 shows spirulina morphology during yeast fermentation and cold storage process of spirulina paste. As the yeast fermentation proceeded spirulina could not maintain the spiral shape, showing fragmented shape. It is inferred that spirulina was destroyed by shaking culture of yeast fermentation (24). It was also confirmed that spirulina was destroyed more by cold storage at refrigeration temperature. This is similar to the results of a study that cold storage of algae was performed at refrigeration temperature (40). Conclusively, it was judged that the morphological change of spirulina by yeast fermentation and cold storage could enhanced the production of physiologically active substances.
