Introduction
In recent years, broccoli has increasingly been consumed as a ready-to-eat product (1). This involves cutting or slicing it to provide fresh cut product, which results in tissue damage and the release of intracellular contents (2). The leakage of cellular substrates can also promote microbial contamination (3).
Food can become contaminated by a number of pathogens, such as the harmful microbe Listeria monocytogenes. The growth conditions for L. monocytogenes are known to range from 1-45℃, pH 4.0-9.2, and water activities below 0.90 (4). Due to its viability at low temperatures, this pathogen is often encountered in refrigerated ready-to-eat foods (5). There have been reported cases where L. monocytogenes was isolated from broccoli in modified atmosphere packages (6). Furthermore, L. monocytogenes can generate biofilms, which protect L. monocytogenes from environmental stresses and sanitizing agents in food processing (7,8). In light of these characteristics of L. monocytogenes, an appropriate sterilization process is needed to ensure food safety.
In this study, the potential use of chlorine dioxide and ultrasound in the disinfection process was assessed. Chlorine dioxide is used as a disinfectant as it has a broad and potent sterilizing effect (9). Chlorine dioxide is known to be GRAS (Generally Recognized As Safe) and it does not generate cancer-causing agents when it reacts with nitrogen-containing compounds (10). Aqueous chlorine dioxide was recently approved for sanitization of vegetables and fruits and it is hence likely that there will be an increase in the use of chlorine dioxide in the future (11).
As ultrasound is safe, non-toxic, and eco-friendly, it has also been applied in food processing (12). A substantial number of studies have indicated that ultrasonic irradiation can inactivate microorganisms (13,14). Alternating compression and expansion cycles are generated when longitudinal ultrasound waves propagate in a liquid substance. Small bubbles are created in the medium during these cycles and they continuously undergo growth and collapse. This phenomenon is called cavitation (15), and it causes thinning of cell membranes, localized heating, and the formation of free radicals (16,17), thereby inactivating microorganisms.
The main objective of this research was to assess the inactivating effect of aqueous chlorine dioxide and ultrasonication on L. monocytogenes. The ability of the disinfection treatments to reduce in the number of L. monocytogenes was measured. The results for the sterilizing effect were applied to a Weibull model to analyze the microbial inactivation. Furthermore, color values and electrolyte leakage were measured to assess whether the wash solutions and ultrasonication adversely affected the broccoli.
Materials and methods
Broccoli (Brassica oleracea) was purchased from a local market (Daegu, Korea). Broccoli florets with stems were cut into small pieces (1 cm of the stems) using a sharp knife. Each sample weighed between 15 and 20 g.
Chlorine dioxide solution was obtained as Vibrex (AGRANCO Co., Ltd., Miami, FL, USA). The solution was diluted with distilled water to obtain working stocks with concentrations of 20, 60, and 100 ppm.
L. monocytogenes (NCCP 10943) was incubated in tryptic soy agar (Difco-Becton Dickinson, Sparks, MD, USA) at 37℃ for 24 h. A single colony was suspended in 10 mL of Listeria enrichment broth (Difco-Becton Dickinson). The culture was grown by shaking in an incubator (SI-600R, Jeio-tech, Daejeon, Korea) at 37℃ for 24 h at 130 rpm.
After incubation (as described above), the culture was centrifuged at 2,688 ×g for 10 min (at 4℃). The cell pellet was washed twice with sterilized 0.1% peptone water. The cell solution was diluted with 0.1% peptone water to a cell concentration of approximately 8 log CFU/mL. Broccoli florets were contaminated by adding 0.1 mL of the L. monocytogenes cell suspension at 10 or more sites. The samples were dried in a biological safety cabinet at 22℃ for 2.5 h.
The inoculated broccoli samples were immersed in glass beakers filled with various wash solutions. Plastic sieves were placed on top of the glass beakers to fully submerge the samples.
For the combined treatment with ultrasound, the glass beakers were placed in an ultrasonication apparatus (JAC-3010, Kodo Technical Research Co., Ltd., Hwasung, Korea). The ultrasonication apparatus was set at a frequency of 40 kHz and a power level of 300 W. The temperature in the ultrasonication bath never exceeded 28℃. The samples and the wash solutions were treated at a ratio of 1:20 (w/v). The treatment time was either 1, 5, 10, or 20 min.
For simultaneous treatment (US+ClO2), ultrasonication and chlorine dioxide were applied concurrently for 20 min. For pre-treatment and post-treatment (US/ClO2, ClO2/US), the samples were irradiated for 10 min at the beginning or at the end of the treatment, respectively. For the remainder of the ultrasonication time, the samples were kept in a beaker for 10 min.
Following the disinfection treatment, the samples (15 g) were placed in 135 mL of 0.1% peptone water in a sterile filter bag (sample bag 1930F, 190×300 mm, 3M, Seoul, Korea). The samples were homogenized using a Stomacher (SH-001, SHIMSKYU, Tokyo, Japan) for 3 min. The filtrate was diluted in 0.1% peptone water, and 0.1 mL of aliquots were then plated on PALCAM agar (KisanBio Co., Ltd., Seoul, Korea). The plates were incubated at 37℃ for 48 h. The results were expressed as the log of the colony-forming units (CFU/g).
The Weibull model (Equation A) was applied to determine the inactivating effect. Where N is the population of surviving cells according to the treatment time (log CFU/g), N0 is the initial population of cells (log CFU/g), and t is the treatment time (min). Where ⍺ and β are the scale and shape parameter, respectively. The value of tR was calculated by the non-linear least squares regression method using Microsoft Excel 2010. The shape of the survival curve was assigned a value. If =1, the curve exhibits linearity, if >1 or <1, the curve indicates a downward or an upward concavity, respectively. Two parameters including the shape and the scale were used to compute tR value (Equation B), where tR, which is similar with the traditional D-value, means the time required for a 90% reduction in the cell population (18).
The color of the broccoli was measured using a chroma meter (CR-300, Minolta Co., Osaka, Japan). Prior to measurement, the machine was calibrated using a calibration plate (L=97.55, a=0.03, b=1.63). Three points of floret surface were randomly selected. Each measurement was repeated five times. The results were expressed as L (lightness), a (redness-greenness), b (yellowness-blueness), and yellowing index (YI). The yellowing index was calculated as described (19).
Electrolyte leakage was analyzed according to the method described by Das and Kim (20) with minor modifications. The electrolyte leakage of fresh-cut broccoli was measured after the various disinfection treatments. Samples weighing 20 g were incubated at 23℃ in 200 mL of deionized water. The samples were agitated at 150 rpm for 30 min. The conductivity (s/cm) of the samples was determined with a conductivity meter (MC126, Mettler Toledo, Schwerzenbach, Switzerland). Each measurement was repeated five times.
Results and discussion
The results for the inactivation of L. monocytogenes in broccoli by the combined chlorine dioxide solution and ultrasound treatment are shown in Table 1. Three levels of chlorine dioxide concentration (20, 60, and 100 ppm) were used to treat the broccoli for 1, 5, 10, and 20 min. Overall, there was more inactivation of L. monocytogenes as the chlorine dioxide concentration increased.
Previous studies have investigated the mechanism of bacterial disinfection by chlorine dioxide. It was reported that chlorine dioxide could potentially disrupt protein synthesis by inhibition of amino acid activation, the destruction of ribosomes, or inactivation of mRNA (21). Another researcher has suggested that chlorine dioxide reduces transmembrane ion gradients by reacting with proteins and fatty acids of the cell membrane (22). One would hence expect that the chlorine dioxide concentration would correlate with inhibition or destruction of microorganisms. However, bacterial inactivation by 20 ppm chlorine dioxide and combined treatment for 1 min was not significantly different from the inactivation at 60 and 100 ppm (p<0.05). The reason for this may be that the complex structure of broccoli can limit contact between the wash solution and the microbes (23).
Reductions of L. monocytogenes in the range of 0.82-1.59 log CFU/g and 1.10-1.87 log CFU/g were achieved by chlorine dioxide treatment (ClO2) on its own and by combined treatment (US+ClO2), respectively. Overall, it was observed that the inactivation effect was greater with longer treatment times. The combined treatment (US+ClO2) resulted in more pronounced disinfection than the single treatment (ClO2). Similar results have been reported by other researchers (24,25), who showed that chlorine dioxide with ultrasound enhanced the inhibitory effect on microbes. Out of all of the tested treatments, 100 ppm chlorine dioxide with ultrasound for 20 min resulted in the greatest reduction of the microbial population.
Wash solutions including tap water and 100 ppm chlorine dioxide were treated with ultrasound. Furthermore, different process sequences were tested to compare the disinfection effect on L. monocytogenes (Fig. 1).
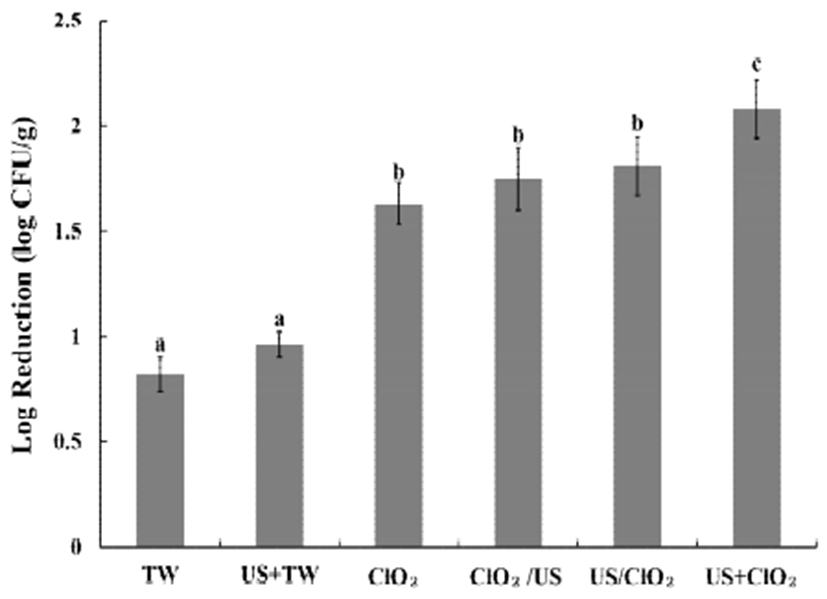
The sterilizing effect of 100 ppm chlorine dioxide was significantly greater than tap water, both as a single treatment and when combined with ultrasound (p<0.05). Simultaneous treatment (US+ClO2) was more effective at inactivating L. monocytogenes than chlorine dioxide treatment on its own (ClO2).
Zhou et al. (26) reported that ultrasound pretreatment resulted in a higher disinfection efficiency than simultaneous treatment. However, the inactivating effect of a pre-treatment and a post-treatment (US/ClO2, ClO2/US) was not significantly different from chlorine dioxide treatment (ClO2) only (p<0.05). Huang et al. (25) reported that structural differences are a critical factor in the decontamination process. The complicated internal structure of broccoli may limit the effect of ultrasonication.
On the other hand, the reduction of L. monocytogenes by tap water (TW) was not significantly different from tap water combined with ultrasound (US+TW). A similar result was obtained by Piyasena et al. (27). They reported that a single process employing ultrasound did not effectively inactivate microbes in food. A likely reason for this is that the process of cell death is different in case of tap water. Physical or chemical treatments can generate small-sized pores, which induce the flow of water into the cells, thereby resulting in cell turgor (28). Ultrasound treatment has also been shown to destroy L. monocytogenes cells (29). Therefore, ultrasound allowed the wash solutions to more readily penetrate the microbial cells (30). When the wash solutions can permeate into the cells, tap water merely increases the turgor pressure, while chlorine dioxide can inactivate entities on the cell membrane. This is probably why chlorine dioxide combined with ultrasound is more effective at inactivating microbes than tap water.
Kinetic models of microbial inactivation are useful means for assessing food preservation processes (31). Among these, the Weibull model is a suitable representative model in light of its simplicity and flexibility, and because it can be adapted to a wide range of trends including upward, downward, and linear (32,33).
The kinetic parameters are presented in Table 2. The regression coefficient (R2) was used to evaluate the suitability of the curves. In this experiment, the regression coefficients (R2) for all of the treatments were above 0.92, indicating that the Weibull model is appropriate for estimation of the inactivation curve.
The tR values were 11.55, 1.93, and 1.09 min for treatments with 20, 60, and 100 ppm chlorine dioxide, respectively. The values for chlorine dioxide with ultrasound treatment were 0.32-1.64 min. These results show that the tR values decreased as the chlorine dioxide concentration increased.
The kinetic curve can be categorized as an upward (<1) or downward (>1) concavity by the shape factor. In this study, the survival curve of L. monocytogenes in broccoli exhibited an upward concavity. An upward concavity means that sensitive cells are inactivated rapidly and that the remaining cells have the capability to resist the applied stress (34). Fig. 2 indicates that the remaining populations, which exhibit a degree of resistance, were decreased by the ultrasound process.
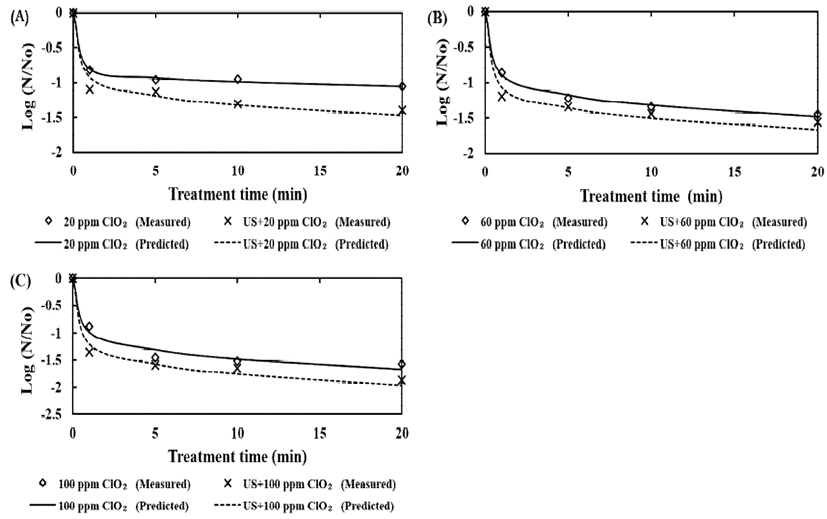
Regarding the tR value and shape of the survival curve, it was considered that a wash solution with ultrasonication was effective at removing resistant populations of L. monocytogenes.
Color is the most important factor of quality that influences consumer acceptability and the shelf life of broccoli (35,36). As the oxidative effect of chlorine dioxide can cause discoloration, the color of the broccoli was measured after the various disinfection treatments (11). In order to assess the effect of the treatments, a color value for the broccoli was measured after treatment with tap water and chlorine dioxide (100 ppm) with ultrasound for 20 min (Table 3). The L, a, and b value of samples did not exhibit any significant differences between the treatments (p<0.05).
The yellowing index (YI) is a parameter to evaluate the color of broccoli. An increase in the yellowing index is known to reduce consumer preference for broccoli (19). The results show that the yellowing index did not differ according to the type of wash solution (p<0.05). Ultrasonication did not increase the yellowing index either. Thus, chlorine dioxide and ultrasound did not result in discernible discoloration of the broccoli.
Electrolyte leakage is an indirect measure of tissue damage (37). There is a connection between electrolyte leakage and the quality of the fresh cut product, since the cutting process increases solute release from the cytosol (38). The electrolyte leakage from broccoli after the disinfection treatments was evaluated (Fig. 3).
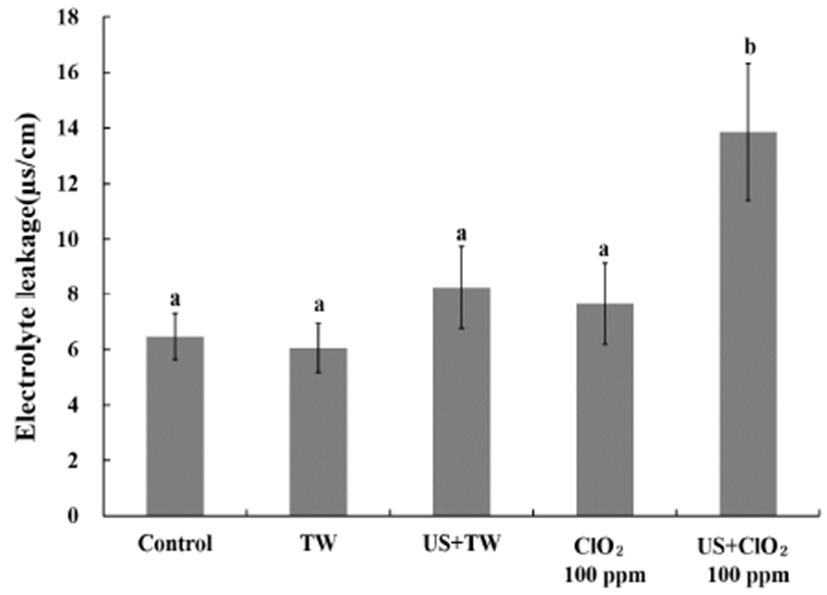
Chlorine dioxide treatment and tap water did not exhibit significant differences in terms of electrolyte leakage. Das et al. (39) reported that the type of wash solution influenced electrolyte leakage from fresh cut iceberg lettuce. However, Kim et al. (40) reported that because broccoli has a firm tissue, no differences in electrolyte leakage were observed according to the type of wash solution. It was, therefore, assumed that the cell structure of broccoli would be damaged less by chlorine dioxide treatment. However, it was observed that electrolyte leakage increased after the combined process with ultrasound. Approximately, 13.86 s/cm of electrolyte leakage was observed for the combined process with ultrasound (US+ClO2), while this value was 7.66 s/cm for the chlorine dioxide treatment. Leadley and Williams (41) reported that cavitation effects cause a collapse of the cell wall and cell leakage. Therefore, it was predicted that cavitation by ultrasound could increase electrolyte leakage from broccoli.
A previous study demonstrated that cutting cantaloupe melon, which has a high respiration and ethylene production rate, resulted in a high level of electrolyte leakage (42). In addition, it has been reported that plant tissue damage enhances the respiratory activity of broccoli (38). Based on these studies, it would appear that electrolyte leakage correlates with respiration and ethylene production.
Ethylene is one of the respiratory gases released from broccoli. Although broccoli produces a small amount of ethylene, it is very sensitive to ethylene exposure (43). Indeed, ethylene has been shown to accelerate yellowing and to reduce the shelf life of broccoli (44). Therefore, it is possible that tissue damage by ultrasound can affect the quality of fresh cut broccoli during storage. There is a need, therefore, to investigate whether tissue damage by exposure to ultrasound can significantly influence the shelf life of broccoli.
