Introduction
Wine aroma is one of the most important factors in determining wine character and quality. Wine aroma was determined by more than 1,300 volatile compounds, including alcohols, esters, acetates, acids, aldehydes, isoprenoids, lactones and ketones, with a wide concentration range (1). The aroma profiles are the results of complex interactions among several factors: grape variety (2), vineyard geographical origin (3), soil and climate characteristics (4) and winemaking techniques (5). Wine aroma contents may be used as a tool for characterization and differentiation of wines from varieties and for establishing criteria to improve the quality of the wines. In particularly, attention has been devoted recently to the analytical characterization and the quality improvement of the varietal aroma of wines. Previous studies have focused on the identification of volatile components and on the establishment of character impact odorants of different varieties (6-8).
Korean winemakers made wine using table grape cultivars, such as ‘Campbell Early’, ‘Muscat Bailey A’ and ‘Kyoho’ (9,10). Korea wines are produced using the most cultivated Campbell Early, however, these wines have the limitations of containing low levels of sugar, high malic acid concentration and negatively foxy aroma content (11). Therefore, development of wine cultivar with good wine aptitude and aroma is desperately required.
The present study was aimed to characterize aroma compounds of cultivated 11 wine grape cultivars to accumulate basic data for making red wine cultivars by aroma using headspace-solid phase microextraction (HS-SPME) combined with gas chromatography-mass spectrometry (GC-MS).
Materials and methods
Benzyl alcohol, 1-heptanol, Isoamyl alcohol, phenylethyl alcohol, Isobutyl alcohol, benzaldehyde, acetic acid hexyl ester, hexadecanoic acid ethyl ester, 1-hexanol, α-terpineol, 4-nonanol and sodium chloride (NaCl) were purchased form Sigma-Aldrich Korea Ltd. (Seoul, Korea). Fatty acid ethyl esters were purchased form Sigma-Aldrich Korea Ltd. (Supelco, Seoul, Korea). Calcium chloride (CaCl2) was purchased from Kanto Chemical Co., INC. (Tokyo, Japan). All used reagents were analytical grade.
Eleven grape cultivars, Cabernat Franc, Cabernet Sauvignon, Chanceller, Malbec, Marchel, MBA, Merlot, Narsha, Pinot Meunier, Sangiovetto and Sauvignon Vert were obtained from experimental vineyards of the National Institute of Horticultural and Herbal Science, Wanju, Korea. All grape varieties used for winemaking were harvested at August in 2016. Samples were frozen at -20℃ prior to analysis.
The winemaking process followed a modified Chang’s method (12) and micro-vinification method. Grapes were removed from grape bunches and potassium metabisulfite (K2S2O5) was added at a concentration of 200 mg kg-1. Before fermentation, grape must (800 g) was adjusted to 22 °Brix using table sugar. Five hours after the addition of potassium metabisulfite, active yeasts were inoculated into grape at a ratio of 0.02% (w/w). The yeast strain Saccharomyces cerevisiae (Fermivin, France) was used for fermentation in all winemaking processes. The grapes were first fermented for two weeks at a constant temperature of 25℃ in a 1,000 mL DURAN® laboratory bottle equipped with an airlock. After the initial fermentation, the fermented residual sugar and sediment were isolated from wine. The isolated wine was subjected to a second round of fermentation at a constant temperature of 15℃ for 1 week, after which the prepared wine samples were analysed.
The total soluble solids (TSS) of wine was determined using a digital refractometer (PAL-1; Atago, Tokyo, Japan). For measurement of the total acidity (TA), 20 mL of wine sample was titrated using 0.1 N NaOH to an end point of pH 8.2. The TA value was then converted to tartaric acid equivalents.
An SPME fibre coated with divinylbenzene/carboxen/ polydimethylsiloxane (50/30 μm, DVB/CAR/PDMS) (Supelco, Bellefonte, PA, USA) was used for the analysis owing to its high sensitivity for odorants and good reproducibility in grapes (13). Fermentation-finished wine (15 mL) was transferred into a capped 50 mL solid-phase microextraction vial, and 1.8 g of NaCl and 2 μL of internal standard (4-nonanol in EtOH, 1 mg/mL) were added. Samples were heated at 70℃ in an automated heating block (Wise Therm®, HB-48P). After 20 min of equilibration, the SPME fibre was manually inserted into the sample vial headspace. After 20 min, the fibre was withdrawn and introduced into the GC injection port for desorption at 250℃ and maintained for 10 min in splitless mode. All samples were examined in triplicate.
An Agilent gas chromatograph model 6890N coupled to an Agilent 5975 series mass selective detector was used to perform the analysis. Volatile compounds were separated on an HP-INNOWAX capillary column (30 m×0.32 mm×0.25 μm; Agilent technologies, Santa Clara, CA, USA), with purified helium as the carrier gas, at a constant flow rate of 2 mL/min. Desorption of the DVB/CAR/PDMS fibre was carried out for 10 min in the GC injection port at 250℃. The oven temperature was held at 40℃ for 5 min, increased to 220℃ at a rate of 3℃ min-1 and finally held at 220℃ for 5 min. The injector and source temperature were set at 250℃ and 230℃, respectively. The mass detector was operated in electron impact ionisation mode at a voltage of 70 eV, with the range set at 50-700 m/z. Selected GC-MS peaks were identified based on the comparison of mass spectra with the NIST11 (Agilent, Gaithersburg, MD, USA) mass spectral database. Content of all compounds was quantified relative to the known concentration of 4-nonanol internal standard.
A one-way analysis of variance (ANOVA) was performed to identify statistically significant differences between samples. The R Commander (version 2.13.0, A Basic-Statistics GUI for R statistical software package; McMaster University, Ontario, Canada) was employed for this analysis where statistical significance was defined as p<0.05.
Results and discussion
To compare the characteristic of eleven red wine grapes, the titratable acid (TA), the total soluble solids (TSS) and the total soluble solids/titratable acid (TSS/TA) were identified (Table 1). In all grapes, the TA range from 5.50 to 10.80 g/L, and TSS value were higher than 18.40 °Brix in all grapes. The TSS/TA ranged from roughly 1.98 to 4.15. An outstanding parameter to evaluate the maturity of grape is the total soluble solids, predominantly sugars, measured as °Brix (13). These results, all harvested grapes in Wanju region were reached their appropriate maturity times, with the exception of Cabernet Franc and Pinot Meunier grapes.
Seventy five compounds were identified as follows 16 alcohols, 11 volatile acids, 3 aldehydes, 28 esters and acetates, 4 ketones, 3 C13-norisoprenoids, 2 C6-compounds, 1 furan, 1 phenolic acid derivative, 1 terpene and 5 others in 11 wines by HS-SPME and GC/MS analysis (Table 2).
Higher alcohols are secondary products of yeast metabolism and have been related with pungent, sweet and fruity odors (14). Alcohols was the most abundant component in the aroma compounds of all wines like previous studies (15-18). 1-heptanol, isoamyl alcohol, 2,3-butanediol, [R-(R*,R*)]-, 2,3-butanediol,[S-(R*,R*)]-, 3-ethyl-1-butanol, phenylethyl alcohol, 1-propanol and isobutyl alcohol were identified in the all red grape wines. Isoamyl alcohol was largest aroma compound in all wine samples without Cabernet Sauvignon, Chanceller and Sauvignon Vert wines. Largest aroma compound of those 3 wines is isobutyl alcohol. 1-butanol and 2-ethyl-1-butanol were detected only in Chanceller and Marchel wines. And, benzyl alcohol was found in several wines, such as Cabernet. The alcohol profile of Chanceller wine was the most diverse in the all wines, containing 14 types of alcohols. However, Cabernet Sauvignon wine was shown in highest concentration of alcohol component. Franc, Cabernet Sauvignon, Chanceller, Marchel and Merlot wines. Studies performed by Tao et al. (19), Cheng et al. (20) and Vilanova et al. (21) reported high values for higher alcohols and ethyl esters in Cabernet Sauvignon wines.
Volatile acid compounds are mainly produced during fermentation and their concentration has been reported to depend on the initial composition of the must and fermentation conditions. The low concentration of these compounds had a positive contribution to the quality of the wine by increasing aroma complexity. However, at levels beyond 20 mg/L, these acids have been associated with negative odors (22). In present study, the total volatile acid concentration was less than 1.1 mg/L in all red wines.
Ester and acetate were identified largest number of the aroma component in 11 red wines, and this result is consistent with previous data for different red wine varieties (17,18). Ethyl esters of the fatty acid were formed from ethanolysis of acyl-CoA during fatty acid synthesis or degradation and appeared mainly at the first phase of the alcoholic fermentation of fatty acid (23,24). In the present study, octanoic acid ethyl ester, hexanoic acid ethyl ester and ethyl acetate were major esters identified in the aroma compounds of the 11 red wines. Ethyl acetate and Lactic acid ethyl ester increases its concentration with the malolactic fermentation (25). This compound was the most abundant ester in 11 redwines, in agreement with published data for Cabernet Sauvignon and Merlot wines (19-21) and new wine grape cultivar Meili (26). While, lactic acid ethyl ester was detected in 10 red wines, with the exception of Cabernet Sauvignon wine. Other ethyl ester, such as butanoic acid ethyl ester, heptanoic acid ethyl ester decanoic acid ethyl ester, dodecanoic acid ethyl ester and hexadecanoic acid ethyl ester were presented in some wines. Dodecanoic acid ethyl ester was found in 10 red wines, with the exception of Cabernet Sauvignon wine. In previous study, dodecanoic acid was found in only Pinot noir among 4 red wines (21). Butanoic acid ethyl ester was shown with high concentration in the Marchel, Narsha and Sangiovetto wines. Only two C6-compounds were identified from 11 red wines. 1-hexanol was found in every red wines, while (Z)-3-hexenol was found Cabernet Franc, MBA and Narsha wines. These C6-compounds were derived from fatty acid by the action of grape-derived lipoxygenase (LOX) and hydroperoxide lyase (HPL), and associated with negative green and herbaceous odors when their concentration is above their odor threshold values (27-28).
Benzaldehyde and acetaldehyde were only detected in aldehyde group. Acetaldehyde was shown in 11 red wines, however, benzaldehyde was detected only in Chanceller and MBA wines. Acetone aroma compound of ketone group was identified in 9 red wines without MBA and Sauvignon Vert wines. Terpene compounds belong to the secondary plant constituents, of which biosynthesis begins with acetyl-coenzyme A (Co A) (29). In this study, α-terpineol and furfural were detected only MBA wine. C13-norisoprenods have low odor threshold values and these compounds play on important role in the varietal typicity of wines (8) in this study, α-ionene, ionene and TDN were detected only Sangiovetto wine. MBA wine was shown only ionene.
The aroma compounds of wine depend on both concentration and threshold. However, only a limited number of aroma compounds can be found at concentration high enough to be perceived (OAV≥1) and considered as flavor contributors as well as active odorants (30). The OAV values for the 14 aroma compounds with OAV≥1 are shown in Table 3. Alcohol, aldehyde, ester and C6-compounds were the main contributors to aroma in 11 red wines. Phenylethyl alcohol obtained from 11 red wines was contributed to the floral odorant. While, isoamyl alcohol and isobutyl alcohol were contributed to sweet and fatty odorant in wines, the aroma of wines contributed by these compounds was week, because they have a high odor threshold. Acetaldehyde was detected only Cabernet Sauvignon and Chaceller wines. Ester and acetate were contributed to fruity, floral and sweet odorant in wines. Octanoic acid ethyl ester and hexanoic acid ethyl ester were most active esters, and they contributed to the fruity, floral and sweet odorant of all red wines. 1-hexanol was detected all wines, it was contributed to green odorant. C13-norisoprenoids and terpene were detected only Sangiovetto and MBA wines, respectively. Although present at low concentrations C13-norisoprenoids and terpene made significant contributions to the aroma of wines due to their low thresholds.
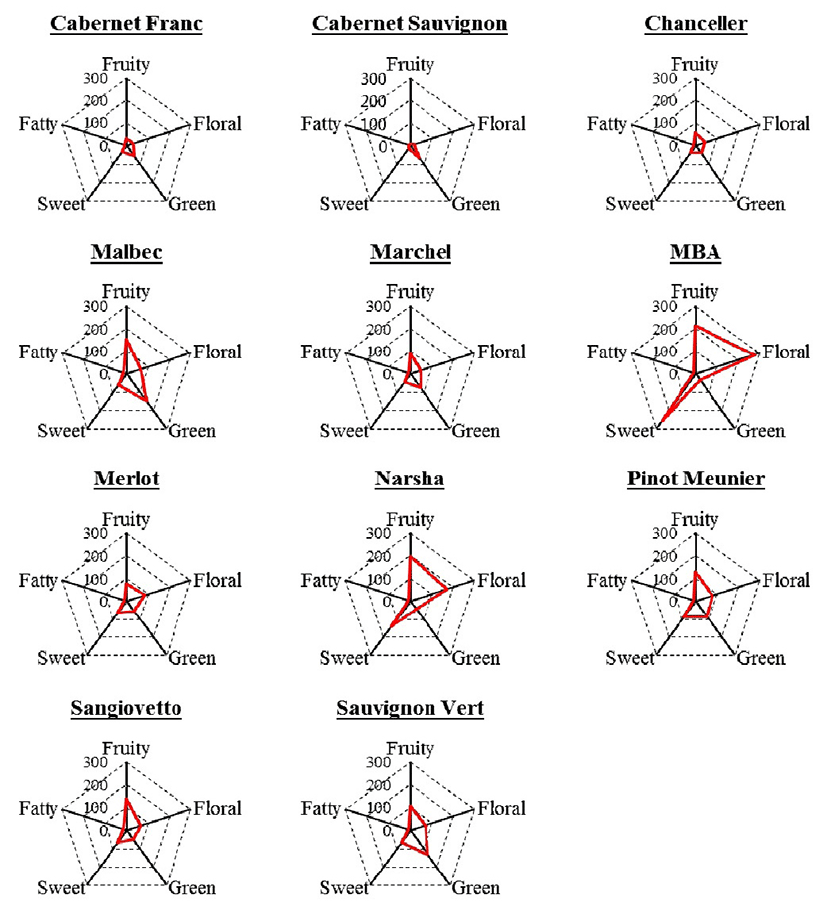
To evaluate the global fermentative aroma of the wines, all aroma compounds were grouped into five aroma series and each compound was assigned to one or more aromatic series based on their similar odor descriptors. Aroma series were represented the main constituents of the aroma profile of the wine: 1-fruity, 2-floral, 3-green, 4-sweet, 5-fatty odors bearing in mind their descriptions in previous papers (31-33). The total intensities for each aromatic series were calculated as the sum of the OAV of each of the compounds assigned to this series and the results are shown in Fig. 1. The highest aroma contribution in Chanceller, Malbec, Marchel, Narsha, Pinot Meunier and Sangiovetto wines were fruity series. While, the green series was present in the highest level in Cabernet Franc, Cabernet Sauvignon and Sauvignon Vert wines. The floral series were higher in MBA and Narsha wines, probably it is due to the high quantity of ester and acetate compounds in these wines. In present study, fruity series were major aroma categories in all red wines, and similar result was reported in previous studies (30,34).
Based on the results of these studies, aroma component analysis could be used as a selection criterion developing wine cultivars.
요 약
본 연구는 완주에서 재배된 세계 주요 11개 적포도 품종으로 제조된 적포도주의 향기 성분을 headspace-solid phase microextraction 분석법으로 확인하였다. 향기성분은 총 75종이 확인되었다. 아로마화합물은 그들의 OAV 값에 의해 5 그룹으로 나뉘었다. 알콜, 알데하이드, 에스테르, C6화합물이 11개 적포도주의 주요한 향기성분이었다. Isoamy alcohol 알콜과 phenylethyl 알콜은 11개 포도주에서 공통적으로 꽃향기, 달콤한 향을 나타내는데 중요한 물질이었다. Octanoic acid, ethyl ester, hexanoic acid ethylester은 모든 레드와인에서 과실향과 꽃향, 달콤한 향을 내는 중요한 성분이었다. 1-Hexanol은 모든 포도주에서 분석되었으나 풀향을 나타내는 향으로 나타났다. Chanceller, Malbec, marchel, Nsrsha, Pinot Meunier, Sangiovetto 포도주의 주요 향기 성분은 과실 향인 것으로 나타났으며 Cabernet Franc, Cabernet Sauvignon, Sauvignon Vert 포도주의 주요 향기 성분은 풀향인 것으로 조사되었다. 또한, MBA와 Narsha 포도주의 경우 꽃향이 주요 향인 것으로 조사되었다. 본 연구 결과를 바탕으로 적포도주용 품종을 육성할 때 선발 기준으로 향기성분 분석을 활용할 수 있을 것으로 판단되었다.