Introduction
Aging is an inevitable process for all living organisms (1). Infertility, growth arrest, hypoactivity, skin atrophy, premature thymic involution, arteriosclerosis, osteoporosis, and pulmonary emphysema are all associated with human aging (2). Bone mass is maintained by a homeostatic balance between the activities of osteoclasts, which resorb bone, and those of osteoblasts, which make bone. The balance between both cells is controlled by various hormones and cytokines. Excessive osteoclast activity leads to bone loss in pathologic or inflammatory conditions, such as rheumatoid arthritis and postmenopausal osteoporosis (3,4). Osteoporosis is a disease which is an increasing problem in our aging society (5). When bone metabolism is homeostatic, normal bone is maintained (6). When the balance is broken due to various diseases, aging, smoking, stress, or sudden estrogen depletion of women, bone absorption is increased relative to bone formation, resulting in bone loss (6,7). Osteoporosis can easily cause fractures, even in the case of mild impacts, and the bone mass per unit volume decreases without significant changes in the chemical composition of the bone (8). Osteoclasts are differentiated and activated by the receptor activator of nuclear factor kappa-B ligand (RANKL) that is secreted by osteoblasts. Activated osteoclasts form an actin ring composed of a fibrous actin sequence between the cell membrane and bone matrix. They secrete proteases and protons, thereby absorbing bone matrix and reducing bone density (9). To inhibit bone resorption, studies are under way to inhibit the early stage of differentiation that blocks the conversion from osteoclast precursor cells to osteoclasts, or to induce the death of differentiated osteoclasts, thereby inhibiting the bone resorption process (10,11). Recently, there is growing concern about the relationship between oxidative stress and osteoporosis (12). In women with osteoporosis, blood concentrations of antioxidants are decreased (13), and bone mineral density is increased due to antioxidant vitamin intake (14).
Corni Fructus is the fruit of Cornus officinalis Sieb. Et Zucc, which is part of the Cornaceae family, commonly known as dogwoods (15). Clinically, it is one of the most popular and widely used herbal medicines in the world and can be used in medicine, food sanitation, and cosmetics (16,17). Corni Fructus contains gallic acid, malic acid, tartaric acid, ursolic acid, 5-hydroxymethylfurfural, morroniside, loganin, and sweroside (18-20). This herb has been used traditionally for mitigating tinnitus, improving impotence, and decreasing excessive urination (19). Corni Fructus also has antioxidant (21), antidiabetic (22), and antineoplastic effects (23).
Our previous study showed that treating Corni Fructus with pressurized steam significantly changed the total extractable phenolic and bioactive compounds (i.e., gallic acid, 5-hydroxymethylfurfural, loganic acid, morroniside, and loganin) (24). Also, the protective effects against H2O2-induced cytotoxicity in L132 cells treated with pressurized steam-treated Corni fructus (PSC) extract increased with increasing treatment times. Thus, the current study was designed to examine the effects of the PSC extract on osteoblast differentiation and osteoclast formation.
Materials and methods
Corni Fructus without seeds was purchased from a local supermarket (Uiseong, Korea). The samples were treated by hot air-drying at 50℃ for 24 h, which was terminated when the moisture content, measured using an infrared moisture analyzer (MB45, Ohaus Inc., Pine Brook, NJ, USA), reached 10%. The treated samples were used for the pressurized-steam treatment.
Dried Corni Fructus (100 g) was soaked with distilled water (1:2, w/v) for 1 h at 25℃, drained, and treated with pressurized steam (121℃, 1.2 kgf/cm2 for 2 h). The PSC sample was hot-air dried at 50℃ for 12 h to adjust the moisture content to 10% as measured using an infrared moisture analyzer (MB45, Ohaus Inc.). The dried sample was ground using a Lab Use grinder (RT-04, Mill Powder Tech, Tainan hsien, Taiwan) at 25,000 rpm for 5 min at 25℃ and used for the experiments. PSC (15 g) was reflux extracted with 80% ethanol (1:20, w/v) for 3 h at 80℃. The extract was then filtered and then concentrated (N-1N, Eyela, Tokyo, Japan) under reduced pressure. The concentrate was lyophilzed (Free Zone 2.5, Labconco, Kansas, MO, USA) to yield a powder for use.
The newborn mouse calvaria preosteoblast cell line, MC3T3-E1 (ATCC CRL 2593), was obtained from the American Type Culture Collection (Rockville, MD, USA). Complete culture medium was α-minimum essential medium (Welgene, Gyeongsan, Korea) containing 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA), and 2% penicillin-streptomycin (Gibco BRL). Cells were plated at a density of 1.0×105 cells/well in a 24-well plate, and maintained at 37℃ in a humidified incubator (MCO-18AIC, Sanyo, Sakata, Japan) with a 5% CO2 atmosphere.
For measuring effects on proliferation and differentiation, MC3T3-E1 cells were maintained in culture medium overnight. The cells were then treated with the PSC extract and differentiation culture medium, containing 10 mM β-glycerophosphate (Sigma-Aldrich, St. Louis, MO, USA) and 50 μg/mL L-ascorbic acid (Sigma-Aldrich). The medium was changed every 3 days and cells were harvested at 1, 7, 14, and 21 days. The following parameters were utilized to monitor of the effects of the PSC extract on the differentiation of mouse preosteoblasts: cellular alkaline phosphatase (ALP) activity, cell matrix ALP staining, Alizarin Red S staining, and von Kossa staining.
The mouse monocyte cell line, RAW264.7 (KCLB 40071), was obtained from the Korean Cell Line Bank (Seoul, Korea). Complete culture medium for RAW264.7 cells was the same as for MC3T3-E1 cells. Cells were plated at a density of 5.0×105 cells/well in a 24-well plate and maintained at 37℃ in a humidified incubator with a 5% CO2 atmosphere.
To assess effects on differentiation, RAW264.7 cells were maintained in culture medium overnight. The cells were then treated with the PSC extract, in differentiation culture medium containing 50 ng/mL soluble RANKL (PeproTech, Rocky Hill, NJ, USA) and 50 ng/mL macrophage colony stimulating factor (M-CSF, PeproTech). The medium was changed every 3 days and cells were harvested after 7 days. The following parameters were utilized to assess the effects of the PSC extract on the differentiation of mouse osteoclast cells: cellular tartrate-resistant acid phosphatase (TRAP) activity, and cell matrix TRAP staining.
Cytotoxicity was determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method, as described by Denizot and Lang (25). Osteoblast cells were measured at 3 days, and differentiated osteoclast cell were measured at 7 days, respectively. After treatments, the MTT solution in phosphate-buffered saline (PBS) (1 mg/mL, Sigma-Aldrich) was added to each well at 1:10 v/v of medium. The cells were incubated for 4 h at 37℃, and dimethyl sulfoxide was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using an Asys microplate reader (Biochrome, Cambridge, UK). Cytotoxicity was calculated as follows: cytotoxicity (%)=(absorbance sample/absorbance control)×100.
Cellular ALP activity was determined by the method described by Ozeki et al. (26), using p-nitrophenyl phosphate (Sigma-Aldrich) as the substrate. At 1, 7, 14, and 21 days of differentiation, cells were rinsed twice with PBS, then 0.1% TritonTMX-100 in 10 mM sodium phosphate buffer (pH 6.8) was added. After mixing gently and heating at 37℃ for 30 min, glycine (0.1 M, Sigma-Aldrich) and p-nitrophenyl phosphate (100 mM) were added. After mixing gently and heating at 37℃ for 30 min, 1 N sodium hydroxide was added to stop the reaction. The reaction mixture was transferred to a 96-well plate, and the absorbance was measured at 405 nm using an Asys microplate reader. ALP activity was calculated as follows: activity (%)=(absorbance sample/absorbance control)×100.
At 14 and 21 days of differentiation, ALP staining was performed using the Leukocyte Alkaline Phosphatase Kit (86R, Sigma-Aldrich). Staining was visualized by light microscopy (TS100, Nikon Eclipse, Tokyo, Japan) and images were acquired with an i-Solution digital camera (IMTcam3, Canon, Tokyo, Japan). Cells with ALP activity were stained red as an indicator of bone nodule areas.
To measure extracellular matrix calcium deposits indicative of bone nodule formation, the cellular matrix was stained using Alizarin Red S dye (Sigma-Aldrich), which combines with calcium in the matrix, as described previously (27). At 14 and 21 days of differentiation, cells were rinsed twice with PBS and then fixed with 2% formaldehyde. Cells were stained with Alizarin Red S solution (40 mM, pH 4.4) for 40 min at 25℃, and rinsed twice with distilled water. Staining was visualized by light microscopy and images were acquired with an i-Solution digital camera. After photographing, 10% cetylpyridinium chloride (Sigma-Aldrich) in 10 mM sodium phosphate buffer (pH 7.0) was added. The liquid containing dissolved Alizarin Red S was transferred to a 96-well plate, and the absorbance was measured at 562 nm using an Asys microplate reader. The mineralization value was calculated as follows: mineralization (%)=(absorbance sample/absorbance control)×100. The concentration of Alizarin Red S staining in the samples was determined by comparing the absorbance values with those obtained from Alizarin Red S standards.
Calcium coprecipitates with phosphate ions in the matrix. Thus, von Kossa staining (which stains phosphate ions) was also used to assess mineralization in the cultures, as described by Kwon et al (28). At 14 and 21 days of differentiation, cells were rinsed twice with PBS and then fixed with 2% formaldehyde. After rinsing twice with distilled water, cells were treated under ultraviolet light with 3% silver nitrate solution (Sigma-Aldrich) for 1 h at 25℃. After rinsing twice with distilled water, staining was visualized by light microscopy and images were acquired with an i-Solution digital camera.
Cellular TRAP activity was determined using the method described by Woo et al. (29). At 7 days of differentiation, cells were rinsed twice with PBS, and 0.2% TritonTM X-100 solution was added. After mixing gently and heating at 37℃ for 30 min, TRAP solution (1.36 mg/mL 4-nitrophenyl phosphate disodium salt (Sigma-Aldrich) and 10 mM tartrate in 50 mM citrate buffer, pH 4.6) were added. After mixing gently and heating at 37℃ for 30 min, 0.1 N sodium hydroxide was added to stop the reaction. The reaction mixture was transferred to a 96-well plate, and absorbance was measured at 405 nm using an Asys microplate reader. TRAP activity was calculated as follows: activity (%)=(absorbance sample/absorbance control)×100.
At 7 days of differentiation, cell matrix TRAP staining was measured using the TRACP & ALP Double-Stain Kit (MK300, Takara Bio, Shiga, Japan). Staining was visualized by light microscopy and images were acquired with an i-Solution digital camera. Products of TRAP activity were stained red, indicating bone nodule areas.
All experiments were carried out in triplicate, and data are reported as means±SD. Duncan’s multiple-range test was used to adjust for multiple comparisons, and null hypotheses were rejected at the 0.05 level. All data were analyzed using SPSS/Windows software (Version 19.0, IBM, Chicago, IL, USA).
Results and discussion
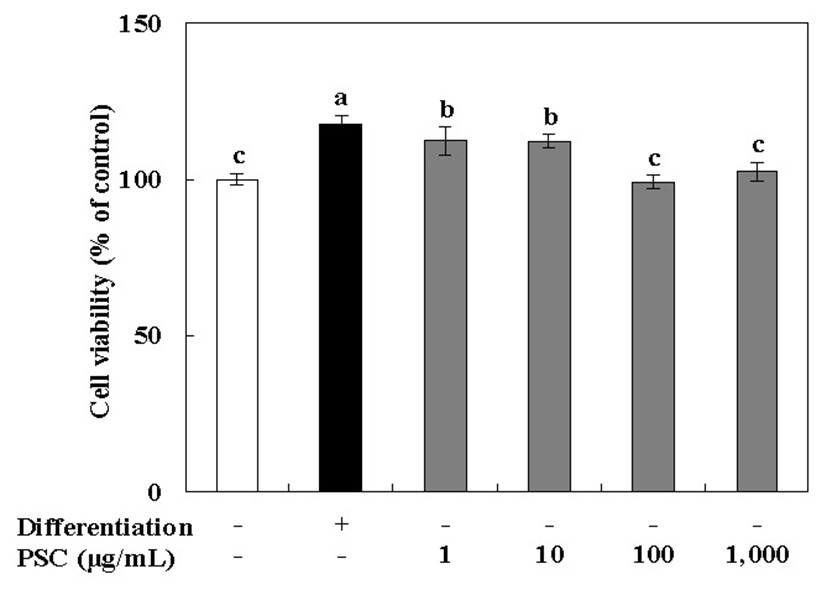
The effect of the PSC extract on the viability of MC3T3-E1 osteoblast cells was determined using the MTT assay. MC3T3-E1 cells are derived from mouse calvaria and have metabolic characteristics similar to osteoblasts such as proliferation, differentiation, and calcification in vivo (30). In particular, MC3T3-E1 cells have a glycoprotein, ALP, in the cell membrane, that is useful in studies related to bone formation (30,31). MC3T3-E1 osteoblast cells were cultured under osteogenic conditions, and treated with 1-1,000 μg/mL of the PSC extract. Our results showed that there was no cytotoxic effect on these cells at the tested concentrations (Fig. 1). After MC3T3-E1 osteoblast cells were cultured for 72 h, the PSC extract at concentrations of 1 and 10 μg/mL showed the most proliferation-stimulating activity (112% at both concentrations as compared with untreated cells). It is noteworthy that the PSC extract did not show strong activity on MC3T3-E1 osteoblast cells, and cell viability was not promoted in a concentration-dependent manner. In fact, low concentrations had the most significant effects. Therefore, the PSC extract at 10 μg/mL was selected for subsequent experiments.
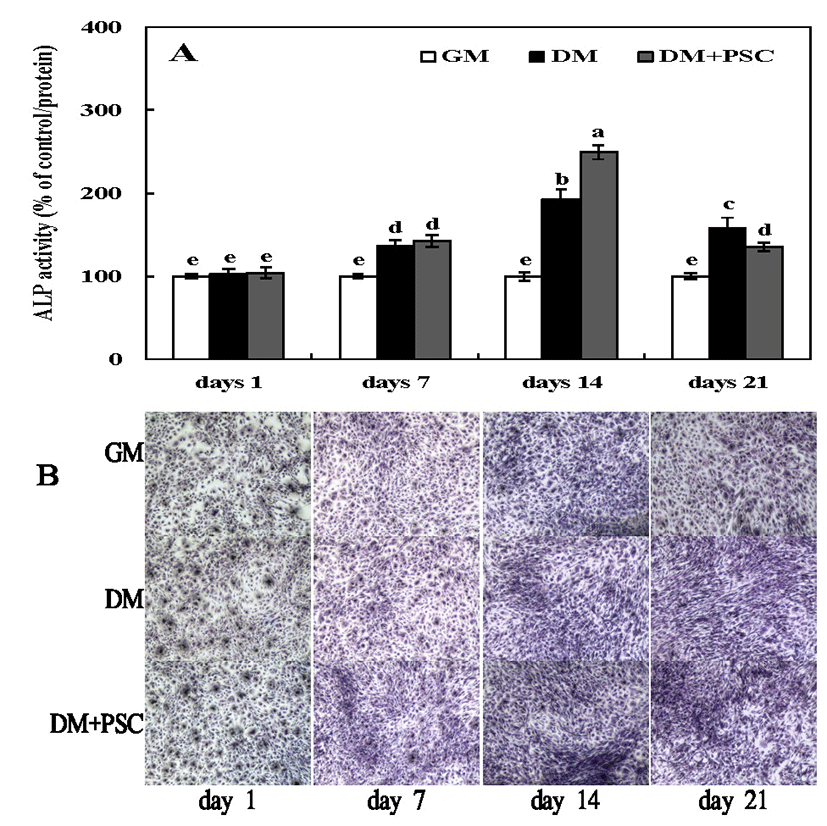
ALP is present in the cell membranes of osteoblasts, and is found at high concentrations in the outer membrane of these cells and calcified tissue (32). ALP is marker of osteoblastic activity, and acts as a regulator of transport of inorganic phosphoric acid, and cell division or differentiation during calcification (31,32). To evaluate the effect of the PSC extract on the differentiation of MC3T3E1 osteoblast-like cells, ALP activity was evaluated during 1 to 21 days of culturing. MC3T3E1 cells were cultured in differentiation medium (β-glycerophosphate and ascorbic acid) and treated with 10 μg/mL of the PSC extract. Differentiation was confirmed with ALP staining (Fig. 2). On day 1, ALP activity did not differ significantly between non-treated and PSC-treated cultures, whereas on days 7 and 14, there were significant increases in ALP activity. This was especially true in the group treated for 14 days with the PSC extract compared with the control. According to Ji et al. (33), the ALP activity was significantly increased in MC3T3E1 cells after 3 days of treatment with a Petasites japonicus extract. ALP activity reached a maximum at 14 days of culturing and decreased thereafter. The results of our study are consistent with these results. Because ALP is secreted as well as being membrane-bound, extracellular ALP activity was also assessed. The influence of PSC extract treatment on ALP activity in the extracellular matrix was measured by ALP staining at 1, 7, 14, and 21 days (Fig. 2B). In contrast to the control, ALP activity showed a time-dependent stimulation in response to PSC extract treatment.
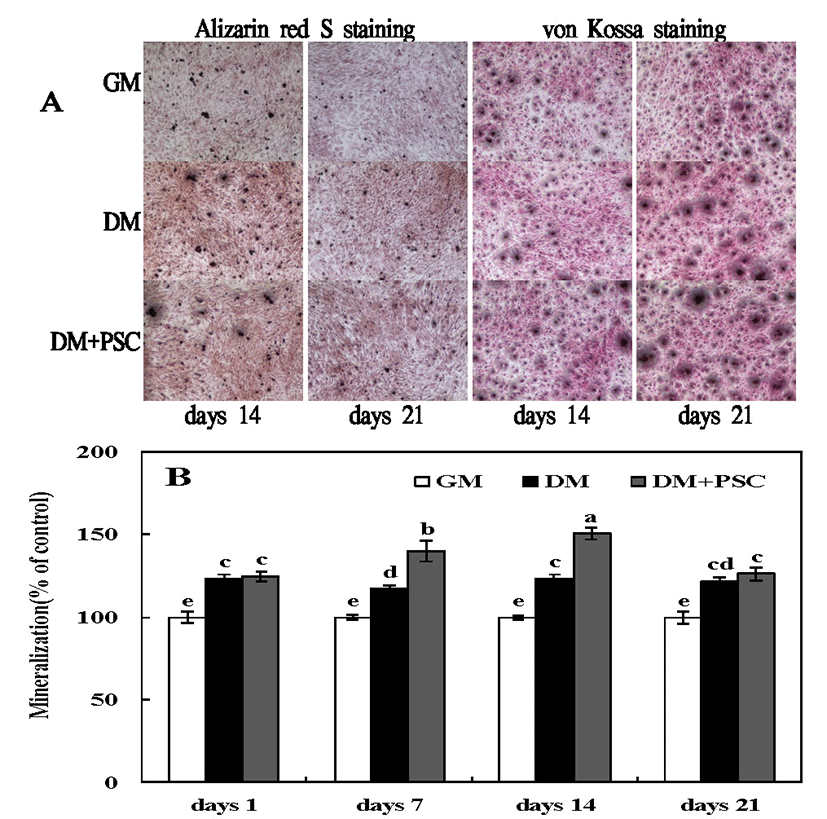
Bone cell culture systems are essential tools for investigating of the molecular mechanisms that regulate mineralization of the extracellular matrix (34). Such studies provide fundamental information on biomineralization that is important to understanding basic skeletal (and dental) biology, and to managing mineralization-related pathologies, such as osteomalacia and ectopic calcification. Data on mineralization mechanisms obtained from cell culture models are also valuable for the rational design of bone repair strategies and tissue engineering constructs, and for biomaterial innovation (35). We verified the effects of the PSC extract on osteoblastic differentiation. MC3T3-E1 cells were cultured under osteoblastic conditions and treated with the extract for 12 and 21 days. PSC extract treatment significantly increased mineralization. Specifically, both Alizarin Red S and von Kossa staining revealed an increase in the volume of mineralized nodules following PSC extract treatment (Fig. 3). These results confirm that the PSC extract facilitates osteoblastic differentiation.
Monocyte/macrophagic RAW264.7 cells have been used previously as a model for osteoclast formation (36). In view of their potential utility for elucidating both essential elements of osteoclast differentiation as well as the mechanism through which the PSC extract might inhibit osteoclast formation, we studied RANKL-induced osteoclast formation.
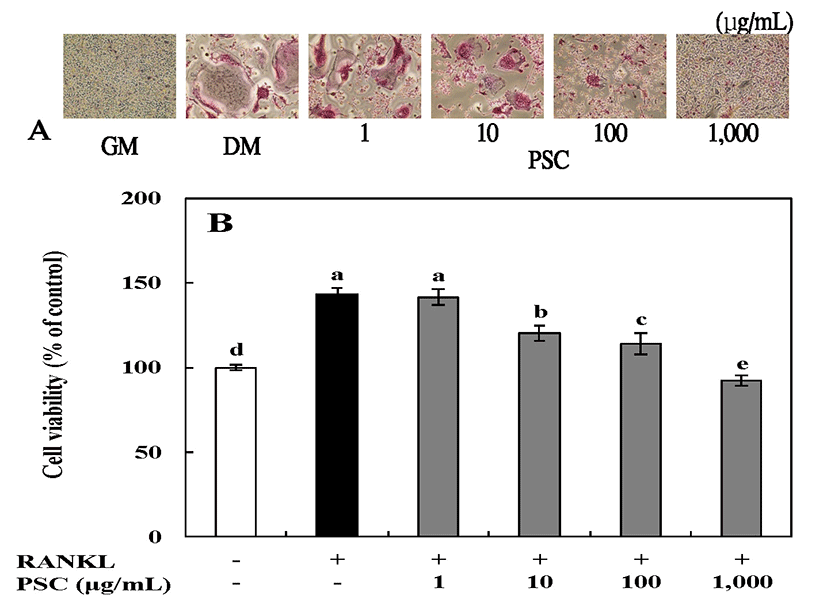
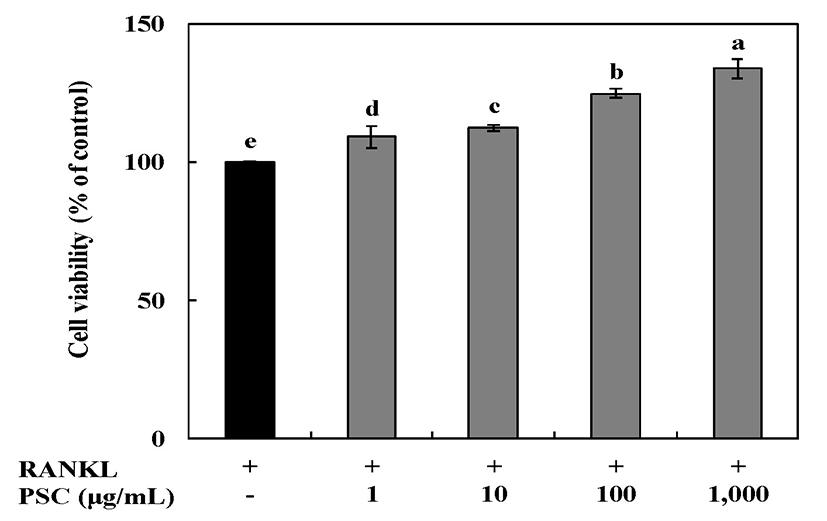
Cytotoxicity of RANKL-induced RAW264.7 osteoclast cells treated with 1-1,000 μg/mL of the PSC extract for 7 days was assessed using the MTT assay (Fig. 4). Cells treated with 1,000 μg/mL of the PSC extract showed the greatest viability (134% of untreated cells). These results showed that there was no cytotoxic effect on RANKL-induced RAW264.7 osteoclast cells at the tested concentrations of the PSC extract. Therefore, concentrations of the PSC extract from 1 to 1,000 μg/mL were selected for subsequent experiments.
Osteoclasts are derived from hematopoietic precursors and differentiate from mononuclear macrophages. Differentiation is generally regulated by two cytokines, M-CSF and tumor necrosis factor related activation-induced cytokine (TRANCE, RANKL or ODF) (37). When osteoclasts are differentiated, they form mononuclear proliferating osteocytes (30). But, when cells are fused, they form multi-nucleated mature osteoclasts, which attach to the bone surface and absorb bone (30,38). In addition, osteoclasts have TRAP and calcitonin receptors, and are active in acid production, and TRAP is widely used as a marker for osteoclasts (38,39). we treated RAW264.7 cells with RANKL in the absence or presence of PSC and assessed the TRAP-positive cells present on day 7. Treatment of RAW264.7 cells with soluble human RANKL for 7 days led to a profound differentiation of this monocyte-macrophage cell line into multinucleated, TRAP-positive, osteoclast-like cells when compared with cells without RANKL treatment (Fig. 5A). The extent of staining decreased as the PSC extract concentration increased. As shown in Fig. 5B, cellular TRAP activity with RANKL alone was 148% of control, and decreased in a concentration-dependent manner: with PSC extract treatment to 142, 120, and 114 and 92% of untreated cells at 1, 10, 100, and 1,000 μg/mL, respectively. Lee et al.(40) reported that, besides their microbicidal properties, reactive oxygen species produced in macrophages and monocytes through RANKL-TRAF6-Rac1-NADPH oxidase-dependent pathways may be involved in the mediation of osteoclast differentiation. In our previous study, the PSC extract showed protective effects against oxidative cell death induced by H2O2. Collectively, these findings indicate that the PSC extract plays a key linkage role in the productions of reactive oxygen species by RANKL. Overall, in this study, the PSC extract showed physiological activities on osteoblast differentiation and osteoclast formation without cytotoxicity. This indicates that the PSC extract could be developed as a health functional food and medicinal agent to prevent bone-related diseases derived from natural materials.