Introduction
Pesticides are chemicals used in agriculture to combat pests. Although the use of pesticides can provide benefits such as increased inventory and quality of fruits and vegetables, their excessive use has negative environmental effects and can also affects human health (1). Because of this, different national governments have set maximum residue levels (MRLs) for pesticides in various food products.
Napa cabbage, also known as Chinese cabbage, is a common route of exposure of East Asian populations to pesticides. Various insecticides and fungicides are used in Napa cabbage farming, including diazinon, chlorfenapyr, and lufenuron.
Diazinon (O,O-diethyl O-[6-methyl-2-(1-methylethyl)-4-pyrimidinyl] phosphorothioate) is an organophosphate insecticide-acaricide with contact, stomach, and respiratory action that works by inhibiting cholinesterase (2). The MRL of diazinon as set by the Environmental Protection Agency (EPA) of the United States for brassicas is 0.7 mg/kg (3).
Chlorfenapyr (4-bromo-2-(4-chlorophenyl)-1-ethoxymethyl-5-(trifluoromethyl) pyrrole-3-carbonitrile) is a broad spectrum pyrrole insecticide-acaricide with the appearance of a white-to-tan powder. Chlorfenapyr is actually a pro-insecticide that is converted into an active toxin when ingested by pests, and appears to have little effect on pest predators (4). The MRL of chlorfenapyr as set by the EPA is 1 mg/kg (3).
Lufenuron (N-((2,5-dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy) phenyl)carbamoyl)-2,6-difluorobenzamide) is a benzoylphenylurea insecticide that inhibits chitin synthesis in insects and is used for agricultural and veterinary applications (5). In some countries, lufenuron is only allowed for certain applications; it is allowed in the US in termite baits but not in crops (3).
Multiresidue pesticide analysis is challenging because different pesticides have different solubilities, polarities, and volatilities. Such methods generally require several partitioning steps followed by chromatography, and as such they are time-consuming and require a substantial volume of solvents. Therefore, the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method was developed in the early 2000s to meet this need (6). The QuEChERS method is a microscale extraction in which pesticides are isolated using a minimal amount of acetonitrile and the extract is cleaned by dispersive solid phase extraction (d-SPE). As such, this method allows for considerable savings of both time and materials, while providing high and reproducible recoveries for a wide range of pesticides in various food products (7). The obtained extract can then be analyzed by HPLC-UV, HPLC-FLV, GC-MS or LC-MS. The advantage of using HPLC is that polar and thermally labile compounds can be analyzed without derivatization, whereas GC analysis may require such a procedure in certain cases. Moreover, HPLC methods tend to be more robust that GC-based ones, and HPLC equipment is more widespread and less expensive than LC-MS (4,8).
The aim of this study was to develop a modified QuEChERS method for quantifying diazinon, chlorfenapyr, and lufenuron residues in Napa cabbage, using HPLC-UV. These three pesticides were selected because they represent three chemical classes of pesticides that are commonly used in Napa cabbage in Korea. HPLC-UV was selected as a low cost and widespread analytical technique suitable for determining most compounds.
Materials and methods
Pesticide-free (organic) Napa cabbages were purchased from local shops in Daegu, Korea, and used for validation and recovery experiments. Conventional Napa cabbages were purchased from local markets in Daegu, and analyzed to determine the presence of pesticides.
All solvents used were HPLC-grade. Methanol was obtained from Fisher Scientific (Seoul, Korea) and acetonitrile was obtained from Daejung Chemicals (Siheung, Korea). The water used was from a Milli-Q water purification system (Millipore, Bedford, MA, USA).
Primary secondary amine (PSA) was purchased from Agilent (Little Falls, DE, USA), graphitized carbon black (GCB) and NaCl were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA), and anhydrous MgSO4 was obtained from Duksan Pure Chemicals (Ansan, Korea).
Pesticide analytical standards of diazinon (98.5%), chlorfenapyr (purity 99.6%), and lufenuron (purity 99.7%) were purchased from Sigma-Aldrich Co..
Stock solutions containing all analytes (1 mg/mL of each) were prepared in acetonitrile and stored in the dark at -9℃. Serial dilutions in acetonitrile were prepared to make working standard solutions at concentrations of 0.001-1 mg/mL.
Organic cabbage leaves were chopped, and 200 g of pooled sample was homogenized in a Waring blender (BL233866, Tefal, Shaoxing, China). A QuEChERS method (6) was applied with modifications. Cabbage homogenate (10 g) was weighed in a 50 mL Teflon centrifuge tube, 10 mL of acetonitrile was added, and the tube was shaken for 1 min. Pesticide standards were added to obtain final concentrations of 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/kg. Subsequently, the sample tubes were refrigerated for 30 min, 4 g of anhydrous MgSO4 and 1 g of NaCl were added, and the tubes were shaken for 1 min and centrifuged at 4,000 rpm and 4℃ for 5 min. An aliquot of 6 mL of the supernatant was transferred into a 15 mL Teflon centrifuge tube containing 150 mg PSA, 50 mg GCB and 600 mg MgSO4, and subsequently shaken for 1 min and centrifuged at 4,000 rpm and 4℃ for 5 min. An aliquot of 1 mL of the supernatant was then transferred into an Eppendorf tube, and 20 mL of this sample was injected into the HPLC instrument.
Calibration samples were also prepared in solvent in the same way, replacing the blank cabbage with acetonitrile. Reported values represent the averages of triplicate measurements.
The recovery of the pesticides was determined by spiking cabbage homogenate (10 g) at concentrations of 0.5, 1, and 5 mg/kg. The spiked homogenates were stored in a refrigerator for 3 h to allow pesticide absorption prior to QuEChERS extraction, which was performed as previously described. Five replicates were used to determine recoveries.
Conventional cabbage samples were chosen randomly from markets in the Daegu area and analyzed to determine the presence of the target pesticides. To prepare the samples for analysis, cabbage leaves were chopped, frozen using liquid nitrogen, and extracted applying the QuEChERS method as previously described.
A Shimadzu LC-20A HPLC (Kyoto, Japan) equipped with a UV-Vis detector and a Prominence autosampler was used for analysis. Data were recorded using the LC solution software (Shimadzu). The column was a Waters C18 (3.9×300 mm, 10 μm; Milford, MA, USA).
The mobile phase was methanol:water (75:25, v/v), pumped in isocratic mode at a rate of 1 mL/min for 20 min. The injection volume was 20 mL. The column was maintained at 30℃ and detection was performed at 220 nm.
The parameters determined were linearity, limit of detection (LOD), limit of quantification (LOQ), precision, accuracy, and recovery, applying previously published equations (9,10).
To determine linearity, the concentrations of the pesticides were determined and the peak areas and heights of the standards were recorded. Calibration equations were obtained by linear regression of the response versus concentration as y=ax+b, where y is the response, a is the slope, x is the concentration, and b is the intercept. Calibration curves were obtained for both pesticides diluted in acetonitrile and matrix-matched standards prepared using cabbage blanks for evaluating the matrix effect. The matrix effect was evaluated applying Eq. 1 (11):
In this study, the LOD and LOQ were defined as twice the baseline noise and 10× the baseline noise, respectively, in a part of the chromatogram close to the retention time of each analyte (9).
The accuracy of the method was evaluated in terms of recovery, which was calculated using Eq. 2 (10):
The intraday and interday precision of the method was evaluated in terms of repeatability (%RSD), and was calculated using Eq. 3:
Results and discussion
The compounds investigated in this study are commonly used pesticides in cabbage farming. Because Koreans frequently eat Napa cabbage (such as in kimchi), cabbage intake may be an important route of exposure to these pesticides in this population. Napa cabbage is also a common component of other East Asian cuisines including those of China and Japan, so this vegetable can also be an important route of pesticide exposure in other Asian populations.
The presence of natural pigments such as chlorophyll complicates the extraction of samples for pesticide analysis. In this study, the use of GCB allowed for the elimination of these interfering pigments, leading to acceptable recoveries. Complete separation of all pesticides was achieved using an isocratic elution with methanol:water (75:25, v/v) as the mobile phase on a C18 column. The separation provided clear resolution after an analysis time of 20 min. Maximum absorption of the pesticides was attained at 220 nm in the UV detector.
The chromatogram of the spiked cabbage sample versus a blank cabbage extract is shown in Fig. 1. The organic cabbage blank provided a clean matrix with no interfering peaks.
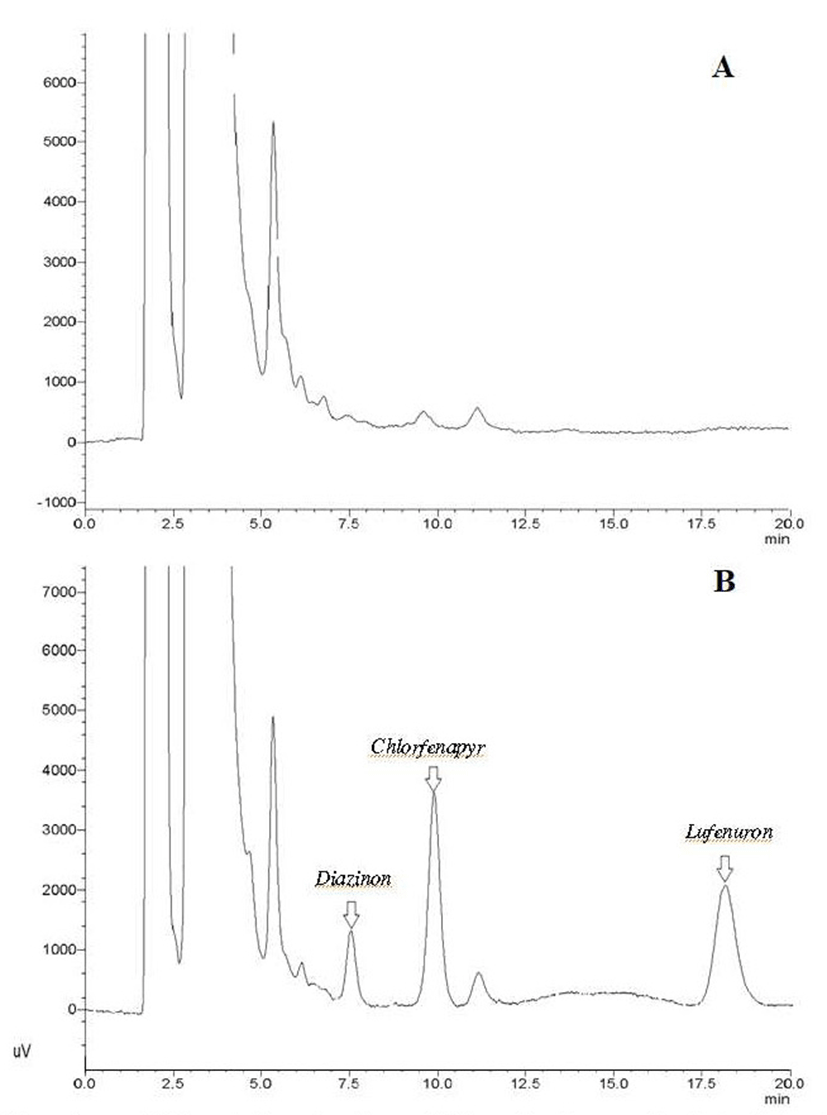
Some studies have reported the application of QuEChERS to determine pesticide contents in cabbage, however, these reported methods require the use of mass spectrometers, making them more expensive and requiring more thorough sample preparation. Zhao et al. (12) used LC-MS/MS to determine 183 pesticides in Chinese cabbage, reporting recoveries in the range of 70-120% with LODs of 0.05-3.06 μg/kg. Nguyen et al. (13) used GC-MS to determine 107 pesticides in cabbage, attaining recoveries of 80-115% with LOQs as low as 0.002 mg/kg.
Chromatographic data analysis involves peak size measurements, which can be achieved using either peak areas or peak heights. Peak areas are more commonly used; when inadequate resolution could be an issue, like in trace analysis, peak height measurements result in less integration error and would be preferable (14). Thus, for constructing the calibration curves, pesticide concentrations of 0.05-3 mg/kg were used and both peak areas and peak heights were integrated. The calibration curves were constructed using standards dissolved in solvent and blank cabbage matrix (Table 1).
Calibration curves obtained using standards dissolved in acetonitrile exhibited higher correlation coefficients (R2) than those obtained in blank cabbage matrix, particularly when using peak heights for quantification. Calibration curves obtained in solvent showed good linearity (>0.99) when using peak heights, however, R2 decreased when using peak areas or blank cabbage matrix (it was higher than 0.97 in all cases). These results suggest interference from the cabbage matrix, requiring further evaluation of the matrix effect.
The matrix effect can appear as suppression or enhancement of the analytical signal because of co-eluting matrix components. While this is a well-known problem in LC-MS method development because the ionization process can be easily disrupted by such compounds, it can also occur in conventional techniques such as HPLC-UV and HPLC-FLV. Because of the matrix effect, an analytical signal obtained in solvent may differ from the same signal obtained in a blank matrix (15,16). The matrix effect can be calculated as the ratio between the peak areas corresponding to the post-extraction spike and solvent standard, expressed as a percentage (10) and it can also be evaluated by comparing the slopes obtained in the calibration curves using matrix-matched standards against those obtained with standards diluted in solvent (11).
Here, the matrix effect was calculated at a concentration of 1 mg/kg using the slopes of the calibration curves obtained by integrating peak heights, and the values were 1.04, 0.95, and 0.49 for diazinon, chlorfenapyr, and lufenuron respectively (Table 2). A value of 1 indicates that there is no matrix effect, whereas values >1 indicate enrichment and values <1 indicate suppression of the analytical signal by the matrix components (11). A matrix effect between 0.9-1.1 can be considered negligible (17). Thus, diazinon and chlorfenapyr exhibited a negligible matrix effect, whereas lufenuron exhibited substantial suppression of the analytical signal.
| Compound | LOD (mg/kg) | LOQ (mg/kg) | Matrix effect |
|---|---|---|---|
| Diazinon | 0.05 | 1 | 1.04 |
| Chlorfenapyr | 0.03 | 0.67 | 0.95 |
| Lufenuron | 0.05 | 1 | 0.49 |
Therefore, we recommend the use of matrix-matched standards for quantification purposes to counteract the observed matrix effect.
The LOD was defined as twice the signal-to-noise ratio and the LOQ was defined as 10× the signal-to-noise ratio (9). The LODs and LOQs were calculated in matrix-matched standards and are summarized in Table 2. The LODs were 0.03 mg/kg for chlorfenapyr and 0.05 mg/kg for both diazinon and lufenuron. The LOQs were 0.67 mg/kg for chlorfenapyr and 1 mg/kg for both diazinon and lufenuron. For the safety of consumers, the Korean government has set maximum residue limits (MRLs) of 0.05, 0.2, and 0.07 mg/kg for diazinon, chlorfenapyr, and lufenuron respectively in Napa cabbage (18). In Japan, the MRLs for diazinon, chlorfenapyr, and lufenuron in Napa cabbage are 0.1, 2, and 1 mg/kg, respectively (19). Thus, the LODs obtained are below the MRLs set in the Korean and Japanese regulations, as well as those set by the EPA for diazinon and chlorfenapyr, although they are higher than those reported by mass spectrometry-based methods (12,13).
The recoveries obtained for cabbage samples spiked with pesticides at three concentration levels (n=5) are shown in Table 3. Diazinon and chlorfenapyr exhibited intraday recoveries of 101-107% and 99-116% respectively, whereas their interday recoveries were both in the range of 101-112%. The intra and interday %RSDs for these two pesticides were lower than 9%. The acceptable recoveries for trace analysis are 70-120% with %RSD ±20% (16), meaning that the values obtained for these two pesticides are adequate. However, in the case of lufenuron, intraday recoveries were in the range of 129-199%, which increased for interday recoveries to 141-213%, even though the %RSD was below 20% in all cases. This result may be due to chromatographic interferences, and suggests that a different clean-up procedure is necessary for the analysis of this particular pesticide (20).
Because abnormal recoveries were obtained for lufenuron, the method was modified to improve the recovery of this particular pesticide. Omitting GCB in the clean-up procedure resulted in better recoveries for this pesticide, although more chlorophyll remained in the extract compared to the one processed with GCB. Nevertheless, when analyzing the presence of this particular pesticide, the presence of chlorophyll is of no concern as it does not interfere with the detection of lufenuron.
GCB has been reported to affect the recovery of planar pesticides (21). Thus, omitting GCB may be necessary when analyzing extracts containing planar pesticides or benzoylphenylureas like lufenuron. The recoveries of lufenuron obtained when omitting GCB are shown in Table 4, and are in the range considered optimal; intraday and interday recoveries were 97-116% and 89-106%, respectively.
| Compound | Concentration (mg/kg) | Recovery 1stday (%) | Recovery 2ndday (%) | RSD 1stday (%) | RSD 2nd day (%) |
|---|---|---|---|---|---|
| Lufenuron | 5 | 97 | 94 | 1.8 | 4.2 |
| 1 | 99 | 89 | 4.4 | 3.1 | |
| 0.5 | 116 | 106 | 8.9 | 9.0 |
The proposed method was applied to the analysis of 9 cabbage samples selected randomly from markets in Daegu, Korea. No residues of the target pesticides were detected in these samples, indicating that the cabbages analyzed were either free from the presence of these compounds or their concentrations were below the LOD of the proposed method.
In conclusion, the proposed method allows for simple and rapid determination of diazinon, chlorfenapyr, and lufenuron in Napa cabbage, using QuEChERS and HPLC-UV. This method allows for fast, easy, and inexpensive pesticide analysis without the need for derivatization. This method could be applied to other leaf vegetables for the analysis of various pesticides. However, if planar pesticides or lufenuron are targets, omitting GCB in the extraction process is advisable.
