Introduction
Persimmon (Diospyros kaki) is a member of the Ebenaceae family and is cultivated all over Eastern Asia. The fruit of the persimmon is eaten either fresh or dried, while the leaves are a staple ingredient in pharmaceuticals, cosmetics, and beverages. The leaf is commonly used to make a tea, effects for ailments including frostbite and, paralysis, while it is also used to treat bleeding and burns (1).
Persimmon leaf contains large amounts of bioactive compounds such as flavonoids, phenols, tannins, caffeine, chlorophyll, and ascorbic acid (vitamin C) (1,2). Ascorbic acid is an odorless white crystalline material which is soluble in water due to its polar characteristics. Generally, ascorbic acid is found in fruits, vegetables, as well as teas, and has long been associated with the prevention of diseases such as scurvy. Ascorbic acid is easily degraded, however, as a result of several factors such as temperature, pH, light intensity, oxygen, enzymes, metallic catalyzers, relative humidity, physical damage, frost damage, adverse handling, and storage conditions (3,4).
Moreover, it is important to recognize the seasonal changes in the mineral contents of the leaves and identify the optimum harvesting time. Leaf-nutrient analysis plays an important role in evaluating future fertilization requirements (5) and also plays a significant part in the growth and development of plants and fruit quality. Thus, it is important to be aware of the fluctuations in the mineral contents of persimmon leaves in order to determine the optimum harvesting time. Not least, macro-and micro-nutrients play an active role in the prevention and treatment of atherosclerosis as well as coronary heart diseases (6,7).
The drying method can affect the quantity and quality of the final product. Drying is necessary to ensure the physical, chemical, and microbiological stability of the harvested persimmon leaves. Any drying process should be capable of preserving the nutrient content, while facilitating the easy storage and transportation of the leaves. Hot-air drying and freeze-drying are the most common drying techniques used in food preservation. Freeze-drying is the optimum drying method for preserving e-nutritional value as well as color. However, both methods affect the quality and availability of the minerals, vitamins, and other nutrients to different degrees. Therefore, it is important to identify the optimum drying technique to ensure the retention of the ascorbic acid as well as the mineral content.
To the best of our knowledge, only a limited number of studies have examined the physicochemical properties of persimmon leaves. The present study set out to determine the moisture, color, vitamin C, and mineral contents of Korean persimmon leaves, the effect of different drying techniques (hot-air drying at 100℃ for 30 min and freeze drying), differences between the cultivars (‘Sangju-dungsi’, ‘Sangam-dungsi’, ‘Cheongdobansi’, ‘Gabjubaekmok,’ and ‘Suhong’), and the optimum harvest time (25th May vs. 25th June).
Materials and methods
L-ascorbic acid and meta-phosphoric acid were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA), 2, 4-dinitrophenyl hydrazine was obtained from TCI Co., Ltd. (Tokyo, Japan), sulfuric acid and acetic acid were purchased from Duksan Pure Chemicals Co., Ltd. (Ansan, Korea), and nitric acid was acquired from Daejung Chemical & Metal Co., Ltd. (Siheung, Korea). All the other chemicals were of analytical grade.
For each cultivar, 30 trees were selected, and leaf samples were collected. The leaves of four cultivars (Sangju-dungsi’, ‘Sangam-dungsi’, ‘Gabjubaekmok’, and ‘Suhong’) of persimmon were collected from the Sangju Persimmon Experiment Station, South Korea on 25th May (flowering stage) and again on the 25th June (fruiting stage). At the same time, ‘Cheongdobansi’ persimmon leaves were harvested in Cheongdo, Korea.
The persimmon leaves were blanched at 100℃ for 2 min and were then dried using either of two drying methods: (i) Hot-air drying at 100℃ for 30 min; (ii) Freeze-drying for 72 h in a vacuum freeze dryer (8).
The color was measured using a color-difference meter (UltraScan Pro Spectrophotometer, HunterLab Inc., Reston, VA, USA). Black and white tiles were used as standards to calibrate the device before the analysis. Dried persimmon leaf powder was then transferred to a measuring cell and lightness (L*), redness (a*, red-green), and yellowness (b*, yellow-blue) were determined using the computerized system.
The vitamin C content was determined using the method devised by Khan et al. (10) with slight modifications. Briefly, 5 g of sample were homogenized in 25 mL of 3% metaphosphoric acid (MPA) 8% acetic acid (AA) solution. The resulting solution was then transferred to a, 50 mL volumetric jar and shaken gently to homogenize the solution. It was then diluted up to the 50 mL mark by adding more of the same MPA-AA solution. The resulting solution was filtered and then 4-5 drops of bromine water were added until the solution developed a color. Next, a few drops of 10% thiourea (CH4N2S) solution were added to eliminate the extra bromine, thus producing a clear solution. This was coupled with 2, 4-dinitrophenyl hydrazine for 1 h and then treated with 85% sulfuric acid. Then, the absorbance was measured using a spectrophotometer (T60U, PG Instruments, Leicestershire, UK) at 521 nm. Standard crystalline ascorbic acid within a concentration array of 0-500 ppm was used to plot the standard curve for the vitamin C content.
The mineral content was evaluated according to the method devised by Bond et al. (11) with some modifications. First, 2 g of each sample were mixed with 20 mL of concentrated nitric acid and placed on a hot-plate at 90℃ overnight to volatilize the nitric acid. After cooling, the mixture was diluted to a final volume of 50 mL by adding distilled water. Then, the samples were examined using an ICP spectrometer (iCAP7600 Duo, Themo Scientific, Shanghai, China) to determine the mineral content. The instrument was calibrated using known standards for each mineral.
The data were reported as the mean±SD of three measurements. Analysis of variance (ANOVA) was performed to estimate the effect of the process variables (harvest time, drying method, and leaf variety). Any significant difference (p<0.05) between means was analyzed by using the Statistical Analysis System (SAS 9.3) program.
Results and discussion
Moisture plays a key role in the growth and development of leaf pests and bacteria. Leaves can be made safe for consumption by eliminating moisture and thus minimizing the effects of pathogens.
Therefore, it is important to reduce the moisture content to the desired level. The moisture content of persimmon leaf powder was found to be highly dependent on the drying treatments (Table 1). Fresh persimmon leaves have a moisture content of around 75-80% whereas the powdered dried leaves, treated by hot-air and freeze-drying, have moisture contents of ca. 10% and 4%, respectively. Freeze-drying resulted in a lower moisture content than hot-air drying (p<0.05). In contrast, powdered ‘Suhong’ leaf had the highest moisture content of all the cultivars, regardless of the harvesting time. Moreover, a similar moisture content was detected in the other analyzed cultivars collected at both harvesting times, although there were variations in the moisture content according to the cultivar.
There are many pigments such as chlorophyll, anthocyanins, carotene, and xanthophyll which are responsible for the change in the color of the leaves during the growing season. The color, described in terms of the Hunter L*, a*, and b* values, of the dried persimmon leaves is presented in Table 2. No statistical differences in the L* and b* values were observed between the flowering (late May) and fruiting (late June) stages, whereas the highest a* value was obtained in the flowering stage.
The L* value of the freeze-dried leaves was the highest, while the leaves dried with hot air produced the lowest L* value (p<0.05). Similar trends were observed for the b* value, with the freeze-dried samples revealing the highest b* value, while the opposite trend was noted for those samples dried with hot air. Previous studies have revealed that the most common causes of color damage in the drying process are an excessively high temperature as well as an overly long drying time (12-14). However, those leaves dried with hot air produced a significantly higher a* value, while those which had been freeze-dried produced a lower a* value (p<0.05). Youssef and Mokhtar (15) demonstrated that the hot-air-dried purslane leaves exhibited a higher a* value than freeze-dried samples. On average, the ‘Gabjubaekmok’ persimmon leaf powder exhibited slightly higher color values (lightness, redness, and yellowness) than the other cultivars.
The effect of drying on the vitamin C content of the persimmon leaves is clearly shown in Figs. 1 and 2. There was no difference in the final vitamin C content regardless of whether the leaves were hot-air dried or freeze-dried, or whether they were collected in the flowering (late May) or fruiting (late June) stages. Matsuura et al. (16) noted that drying led to a reduction in the vitamin C content , with fresh persimmon leaves having a relatively high vitamin C content. The loss of vitamin C as a result of the drying depends on the type of the drying that is applied and the physical properties of the products (17). Although, freeze-drying is regarded as being one of the best drying techniques for maintaining the characteristics of the samples, in the present study loss was not an issue. Michalska et al. (18) found that freeze-dried samples had a lower amount of vitamin C than those dried using another means. It is possible that increased enzymatic activity during the freeze drying and upon thawing could lead to degradation of the ascorbic acid. Also, vitamin C retention may be adversely affected by the extended drying duration (72 h) compared to hot-air drying (30 min). Hence, in one respect it can be said that hot-air drying is superior in terms of retaining the vitamin C content in persimmon leaves. Furthermore, freeze drying always regarded as being the more expensive method.
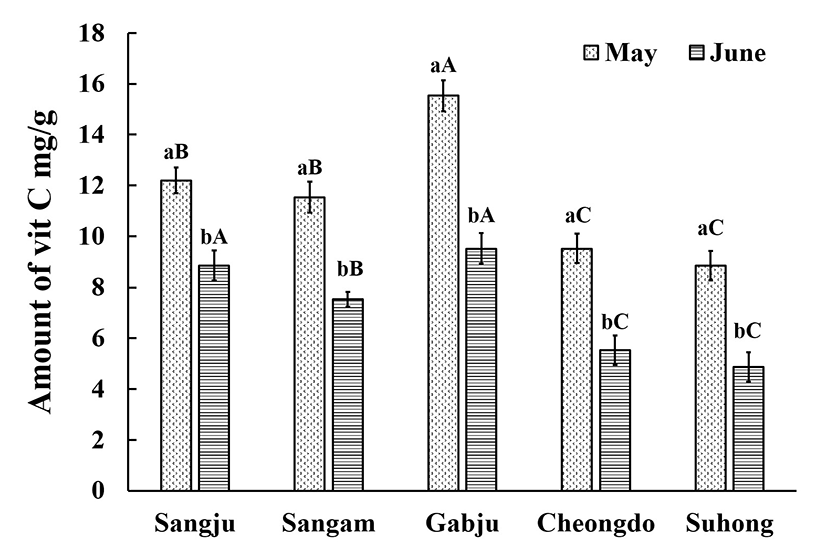
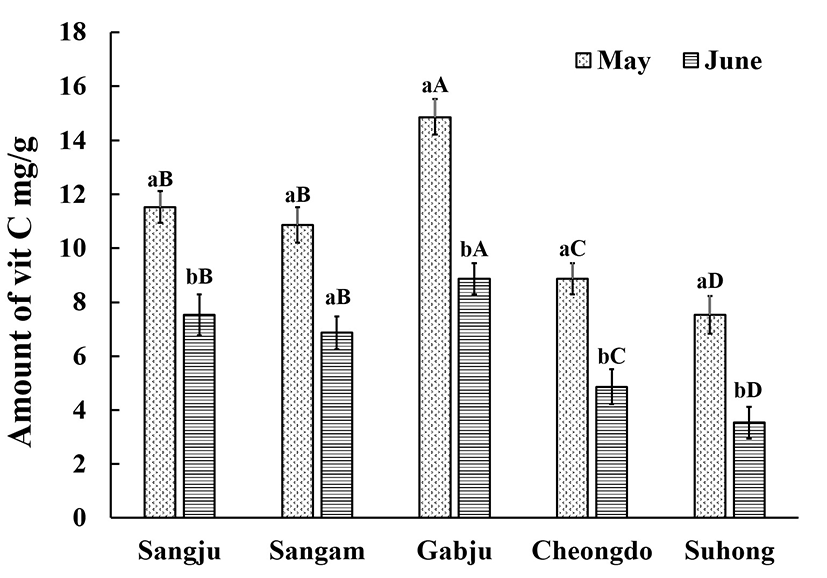
However, the amount of vitamin C was highest in the ‘Gabjubaekmok’ persimmon leaves while the lowest vitamin C content was observed in ‘Suhong’ persimmon leaves, regardless of the harvesting stage (p<0.05). A similar vitamin C content was observed for the ‘Cheongdobansi’ and ‘Suhong’ leaves that had been hot-air dried, regardless of the harvesting time. After freeze drying, the vitamin C content could be ranked, in descending order, as ‘Gabjubaekmok’ > ‘Sangju-dungsi’ > ‘Sangam-dungsi’ > ‘Cheongdobansi’ > ‘Suhong’. Meanwhile, higher levels of vitamin C were noted in the flowering phase compared to the fruiting phase. Kim et al. (19) and Chung et al. (20) reported that the vitamin C content of the persimmon leaves began to fall after 20thJune. However, this finding was not confirmed in the present study. A possible reason for this effect could be the movement of the vitamin C from the leaves to the fruits at the fruiting stage. Another possible reason for the differences between harvesting times can be associated with the plant physiological characteristics, light intensity, environmental conditions and the amount of nitrogen present in the soil (4).
Changes in the mineral contents of the persimmon leaves treated with different drying methods are listed in Table 3. The hot-air and freeze-drying both had a significant effect on the mineral concentration, in that the reduction in the moisture content as a result of the drying greatly increased the concentration of the nutrients (21). These results pointed to freeze-drying being the most efficient whereas hot-air drying was the least efficient with respect to the mineral content (p<0.05). This was most likely due to the absence of heat and photoreactions during the freeze-drying process. Ladan et al. (22) reported that the application of heat decreases the nutrient content of tomatoes. Our findings were in good agreement with those of Umar et al. (23), who reported that freeze-dried moringa leaf has a higher mineral content than leaves dried using other techniques.
It was determined that the levels of Na, Fe, Cu, and Zn decreased from May to June. Similar results, again for persimmon leaves, were reported by Clark and Smith (24) and Yildiz and Kaplankiran (25), They went on to state that the contents of these minerals decreased throughout the growing season. Possible reasons for this trend could be the movement of the minerals from the leaves to the shoots and, finally, into the fruits. Yildiz and Kaplankiran (25) stated that the amount of Na, Fe, Cu, and Zn in persimmon shoots increased after June, whereas Clark and Smith (26) showed that the nutrient level (Fe, Cu, and Zn) in persimmon fruits increased during fruiting. Moreover, the decline in the Fe content is affected by other factors including soil moisture, salinity, carbonate levels in the soil, and decreasing temperature, whereas the level of Cu and Zn uptake is influenced by the plant species as well as the cultivar (27).
Persimmon leaves are noted for their high K content, although the amounts of Cu and Zn are not so high. The highest Na, Fe, and Zn levels were recorded for the ‘Sangam-dungsi’ leaf, regardless of whether it had been hot-air or freeze-dried, relative to the other cultivars during the flowering (late May) stage. However, the Na and Fe levels varied depending on the cultivars during the fruiting (late June) stage, with the ‘Sangam-dungsi’ cultivar being superior in terms of Zn content, regardless of the harvesting stage. Moreover, the level of Cu was the highest in the ‘Sangju-dungi’ leaves collected at the flowering stage and treated by both types of drying, while this figure varied at the fruiting stage. The ‘Sangju-dungi’ and ‘Gabjubaekmok’ cultivars proved to have poor Na and Cu contents, respectively.
It was found that the Mg, Ca, K, Mn, and Al levels increased from May to June. These findings were in good agreement with the findings of Clark and Smith (24) and Yildiz and Kaplankiran (25), who stated that the mineral (Mg, Ca, and Mn) contents of persimmon leaves increased during the growing season. Moreover, Liu and Wang (28), Rehalia and Sandhu (29), and Fernandez-Escobar et al. (30) reported that the amount of Mg and Ca in persimmon leaves increased throughout the growing season as a result of the transpiration stream carrying water and nutrients to the leaves throughout the growing season, such that the Mg content steadily increased (29). Trees take up calcium and magnesium from the soil at rates that vary with the temperature. However, Fernandez-Escobar et al. (30) reported that the amount of Mn and K in olive leaves increased steadily from the beginning of the season, given that the manganese concentration in the leaves tends to rise over time in deciduous fruit plants (31,32) and plays an important role in photosynthesis. Also, the amount of Mn increases during the growing season due to the increase in the soil temperature. Potassium is a highly mobile macronutrient which plays a vital role in protein synthesis, carbohydrate translocation, osmoregulation, enzyme activation, stomatal movement, and cell turgidity (33). However, Yildiz and Kaplankiran (25) showed that the amount of K declined during the growing season whereas the opposite trend was observed in the present study. This can probably be attributed to differences in the soil types, fertilizers, management, and climate. Tanaka et al. (34) stated that, to fulfill the requirements of fruit development, large amounts of potassium would need to be transferred from other parts of the plant.
The highest amounts of Mg, K, and Ca were observed in ‘Sangam-dungsi’ persimmon leaves collected at the flowering stage, regardless of the drying technique, while these figures varied during the fruiting stage. On average, ‘Suhong’ persimmon leaves have come to be regarded as an inferior cultivar in terms of the Mg, K, and Ca content. Furthermore, the ‘Cheongdobansi’ and ‘Suhong’ cultivars are superior and inferior, respectively, in terms of Mn content, regardless of whether they are collected in the flowering or fruiting stages, and regardless of the drying method that is applied.
Conclusion
We observed significant variations in the color and moisture, vitamin C, and mineral contents of leaves collected from five persimmon cultivars. It was found that freeze-drying was superior to hot-air drying in terms of preserving the mineral content and color. However, hot-air drying proved to be superior in terms of maintaining the vitamin C content due to the short drying time, which also reduced the energy consumption, although very similar results could be attained by freeze-drying. Our results suggest that ‘Gabjubaekmok’ is the superior cultivar, while late May is the optimum harvesting time, with respect to the vitamin C content. On the other hand, the amounts of most of the macronutrients increased from May to June, while the opposite was true for the micronutrients.
The amounts of copper and zinc in the persimmon leaves were lower than those of the other elements. Therefore, farmers should apply basic copper-and zinc-based fertilizer during the growing season. Our results would help persimmon growers to identify a cultivar and harvest time that would allow them to produce better-quality persimmon leaves. Further work is required to optimize the potential differences in the mineral contents of persimmon leaves collected from different cultivars and at different harvest times.
